2. Yoon SH, Bae J, Yoon S, Na KJ, Lee HJ. 2023; Correlation between pain intensity and quality of recovery after video-assisted thoracic surgery for lung cancer resection. J Pain Res. 16:3343–52. DOI:
10.2147/JPR.S426570. PMID:
37808464. PMCID:
PMC10558582.

4. van Boekel RLM, Warlé MC, Nielen RGC, Vissers KCP, van der Sande R, Bronkhorst EM, et al. 2019; Relationship between postoperative pain and overall 30-day complications in a broad surgical population: an observational study. Ann Surg. 269:856–65. DOI:
10.1097/SLA.0000000000002583. PMID:
29135493.
5. Kim BR, Yoon SH, Lee HJ. 2023; Practical strategies for the prevention and management of chronic postsurgical pain. Korean J Pain. 36:149–62. DOI:
10.3344/kjp.23080. PMID:
36973967. PMCID:
PMC10043790.

6. Myles PS, Boney O, Botti M, Cyna AM, Gan TJ, Jensen MP, et al. 2018; Systematic review and consensus definitions for the Standardised Endpoints in Perioperative Medicine (StEP) initiative: patient comfort. Br J Anaesth. 120:705–11. DOI:
10.1016/j.bja.2017.12.037. PMID:
29576111.

7. Chou R, Gordon DB, de Leon-Casasola OA, Rosenberg JM, Bickler S, Brennan T, et al. 2016; Management of postoperative pain: a clinical practice guideline from the American Pain Society, the American Society of Regional Anesthesia and Pain Medicine, and the American Society of Anesthesiologists' Committee on Regional Anesthesia, Executive Committee, and Administrative Council. J Pain. 17:131–57. Erratum in: J Pain 2016; 17: 508-10. DOI:
10.1016/j.jpain.2015.12.008. PMID:
26827847.
8. Gilron I, Lao N, Carley M, Camiré D, Kehlet H, Brennan TJ, et al. 2024; Movement-evoked pain versus pain at rest in postsurgical clinical trials and in meta-analyses: an updated systematic review. Anesthesiology. 140:442–9. DOI:
10.1097/ALN.0000000000004850. PMID:
38011045.
9. Aubrun F, Nouette-Gaulain K, Fletcher D, Belbachir A, Beloeil H, Carles M, et al. 2019; Revision of expert panel's guidelines on postoperative pain management. Anaesth Crit Care Pain Med. 38:405–11. DOI:
10.1016/j.accpm.2019.02.011. PMID:
30822542.
10. Malviya S, Voepel-Lewis T, Burke C, Merkel S, Tait AR. 2006; The revised FLACC observational pain tool: improved reliability and validity for pain assessment in children with cognitive impairment. Paediatr Anaesth. 16:258–65. DOI:
10.1111/j.1460-9592.2005.01773.x. PMID:
16490089.

11. Rat P, Jouve E, Pickering G, Donnarel L, Nguyen L, Michel M, et al. 2011; Validation of an acute pain-behavior scale for older persons with inability to communicate verbally: Algoplus. Eur J Pain. 15:198.e1–e10. DOI:
10.1016/j.ejpain.2010.06.012. PMID:
20638878.
12. Schug SA, Palmer GM, Scott DA, Alcock M, Halliwell R, Mott JF. APM:SE Working Group of the Australian and New Zealand College of Anaesthetists and Faculty of Pain Medicine. 2020. Acute pain management: scientific evidence. 5th ed. ANZCA & FPM;p. 62–71.
13. Mariano ER, Dickerson DM, Szokol JW, Harned M, Mueller JT, Philip BK, et al. 2022; A multisociety organizational consensus process to define guiding principles for acute perioperative pain management. Reg Anesth Pain Med. 47:118–27. DOI:
10.1136/rapm-2021-103083. PMID:
34552003.

14. Baamer RM, Iqbal A, Lobo DN, Knaggs RD, Levy NA, Toh LS. 2022; Utility of unidimensional and functional pain assessment tools in adult postoperative patients: a systematic review. Br J Anaesth. 128:874–88. DOI:
10.1016/j.bja.2021.11.032. PMID:
34996588. PMCID:
PMC9074792.
15. Todd KH, Funk KG, Funk JP, Bonacci R. 1996; Clinical significance of reported changes in pain severity. Ann Emerg Med. 27:485–9. DOI:
10.1016/S0196-0644(96)70238-X. PMID:
8604867.

17. Bond MR, Pilowsky I. 1966; Subjective assessment of pain and its relationship to the administration of analgesics in patients with advanced cancer. J Psychosom Res. 10:203–8. DOI:
10.1016/0022-3999(66)90064-X. PMID:
4165548.

19. Heller GZ, Manuguerra M, Chow R. 2016; How to analyze the Visual Analogue Scale: myths, truths and clinical relevance. Scand J Pain. 13:67–75. DOI:
10.1016/j.sjpain.2016.06.012. PMID:
28850536.

21. Ogon M, Krismer M, Söllner W, Kantner-Rumplmair W, Lampe A. 1996; Chronic low back pain measurement with visual analogue scales in different settings. Pain. 64:425–8. DOI:
10.1016/0304-3959(95)00208-1. PMID:
8783305.
24. Collins SL, Moore RA, McQuay HJ. 1997; The visual analogue pain intensity scale: what is moderate pain in millimetres? Pain. 72:95–7. DOI:
10.1016/S0304-3959(97)00005-5. PMID:
9272792.

25. Moore RA, Clephas PRD, Straube S, Wertli MM, Ireson-Paige J, Heesen M. 2024; Comparing pain intensity rating scales in acute postoperative pain: boundary values and category disagreements. Anaesthesia. 79:139–46. DOI:
10.1111/anae.16186. PMID:
38058028.

26. Myles PS, Myles DB, Galagher W, Boyd D, Chew C, MacDonald N, et al. 2017; Measuring acute postoperative pain using the visual analog scale: the minimal clinically important difference and patient acceptable symptom state. Br J Anaesth. 118:424–9. DOI:
10.1093/bja/aew466. PMID:
28186223.

27. Briggs M, Closs JS. 1999; A descriptive study of the use of visual analogue scales and verbal rating scales for the assessment of postoperative pain in orthopedic patients. J Pain Symptom Manage. 18:438–46. DOI:
10.1016/S0885-3924(99)00092-5. PMID:
10641470.
28. González-Fernández M, Ghosh N, Ellison T, McLeod JC, Pelletier CA, Williams K. 2014; Moving beyond the limitations of the visual analog scale for measuring pain: novel use of the general labeled magnitude scale in a clinical setting. Am J Phys Med Rehabil. 93:75–81. DOI:
10.1097/PHM.0b013e31829e76f7. PMID:
23900013.

29. Hawker GA, Mian S, Kendzerska T, French M. 2011; Measures of adult pain: Visual Analog Scale for Pain (VAS Pain), Numeric Rating Scale for Pain (NRS Pain), McGill Pain Questionnaire (MPQ), Short-Form McGill Pain Questionnaire (SF-MPQ), Chronic Pain Grade Scale (CPGS), Short Form-36 Bodily Pain Scale (SF-36 BPS), and Measure of Intermittent and Constant Osteoarthritis Pain (ICOAP). Arthritis Care Res (Hoboken). 63 Suppl 11:S240–52. DOI:
10.1002/acr.20543. PMID:
22588748.

32. Karcioglu O, Topacoglu H, Dikme O, Dikme O. 2018; A systematic review of the pain scales in adults: which to use? Am J Emerg Med. 36:707–14. DOI:
10.1016/j.ajem.2018.01.008. PMID:
29321111.
33. Gerbershagen HJ, Rothaug J, Kalkman CJ, Meissner W. 2011; Determination of moderate-to-severe postoperative pain on the numeric rating scale: a cut-off point analysis applying four different methods. Br J Anaesth. 107:619–26. DOI:
10.1093/bja/aer195. PMID:
21724620.

35. Herr KA, Spratt K, Mobily PR, Richardson G. 2004; Pain intensity assessment in older adults: use of experimental pain to compare psychometric properties and usability of selected pain scales with younger adults. Clin J Pain. 20:207–19. DOI:
10.1097/00002508-200407000-00002. PMID:
15218405.
36. Tomlinson D, von Baeyer CL, Stinson JN, Sung L. 2010; A systematic review of faces scales for the self-report of pain intensity in children. Pediatrics. 126:e1168–98. DOI:
10.1542/peds.2010-1609. PMID:
20921070.

38. Van Giang N, Chiu HY, Thai DH, Kuo SY, Tsai PS. 2015; Validity, sensitivity, and responsiveness of the 11-Face Faces Pain Scale to postoperative pain in adult orthopedic surgery patients. Pain Manag Nurs. 16:678–84. DOI:
10.1016/j.pmn.2015.02.002. PMID:
25962544.
39. Kawamura H, Homma S, Yokota R, Watarai H, Yokota K, Kondo Y. 2009; Assessment of pain by face scales after gastrectomy: comparison of laparoscopically assisted gastrectomy and open gastrectomy. Surg Endosc. 23:991–5. DOI:
10.1007/s00464-008-0090-y. PMID:
18806941.

40. Adeboye A, Hart R, Senapathi SH, Ali N, Holman L, Thomas HW. 2021; Assessment of functional pain score by comparing to traditional pain scores. Cureus. 13:e16847. DOI:
10.7759/cureus.16847. PMID:
34522490. PMCID:
PMC8425136.

41. Manz BD, Mosier R, Nusser-Gerlach MA, Bergstrom N, Agrawal S. 2000; Pain assessment in the cognitively impaired and unimpaired elderly. Pain Manag Nurs. 1:106–15. DOI:
10.1053/jpmn.2000.19332. PMID:
11709864.

43. Gagliese L, Weizblit N, Ellis W, Chan VWS. 2005; The measurement of postoperative pain: a comparison of intensity scales in younger and older surgical patients. Pain. 117:412–20. DOI:
10.1016/j.pain.2005.07.004. PMID:
16153776.
44. Hølen JC, Saltvedt I, Fayers PM, Hjermstad MJ, Loge JH, Kaasa S. 2007; Doloplus-2, a valid tool for behavioural pain assessment? BMC Geriatr. 7:29. DOI:
10.1186/1471-2318-7-29. PMID:
18093294. PMCID:
PMC2234400.

45. Chibnall JT, Tait RC. 2001; Pain assessment in cognitively impaired and unimpaired older adults: a comparison of four scales. Pain. 92:173–86. DOI:
10.1016/S0304-3959(00)00485-1. PMID:
11323138.

46. DeLoach LJ, Higgins MS, Caplan AB, Stiff JL. 1998; The visual analog scale in the immediate postoperative period: intrasubject variability and correlation with a numeric scale. Anesth Analg. 86:102–6. DOI:
10.1213/00000539-199801000-00020.
47. Machata AM, Kabon B, Willschke H, Fässler K, Gustorff B, Marhofer P, et al. 2009; A new instrument for pain assessment in the immediate postoperative period. Anaesthesia. 64:392–8. DOI:
10.1111/j.1365-2044.2008.05798.x. PMID:
19317704.

48. Lee HJ, Cho Y, Joo H, Jeon JY, Jang YE, Kim JT. 2021; Comparative study of verbal rating scale and numerical rating scale to assess postoperative pain intensity in the post anesthesia care unit: a prospective observational cohort study. Medicine (Baltimore). 100:e24314. DOI:
10.1097/MD.0000000000024314. PMID:
33578527. PMCID:
PMC10545085.
50. Rogger R, Bello C, Romero CS, Urman RD, Luedi MM, Filipovic MG. 2023; Cultural framing and the impact on acute pain and pain services. Curr Pain Headache Rep. 27:429–36. DOI:
10.1007/s11916-023-01125-2. PMID:
37405553. PMCID:
PMC10462520.

51. Givler A, Bhatt H, Maani-Fogelman PA. 2024. The importance of cultural competence in pain and palliative care [Internet]. StatPearls Publishing;Treasure Island (FL): Available at:
https://www.ncbi.nlm.nih.gov/books/NBK493154/.
52. Perry M, Baumbauer K, Young EE, Dorsey SG, Taylor JY, Starkweather AR. 2019; The influence of race, ethnicity and genetic variants on postoperative pain intensity: an integrative literature review. Pain Manag Nurs. 20:198–206. DOI:
10.1016/j.pmn.2018.11.002. PMID:
31080143. PMCID:
PMC7841600.

53. Bartley EJ, Fillingim RB. 2013; Sex differences in pain: a brief review of clinical and experimental findings. Br J Anaesth. 111:52–8. DOI:
10.1093/bja/aet127. PMID:
23794645. PMCID:
PMC3690315.
54. Craft RM, Mogil JS, Aloisi AM. 2004; Sex differences in pain and analgesia: the role of gonadal hormones. Eur J Pain. 8:397–411. DOI:
10.1016/j.ejpain.2004.01.003. PMID:
15324772.

55. Derbyshire SW, Nichols TE, Firestone L, Townsend DW, Jones AK. 2002; Gender differences in patterns of cerebral activation during equal experience of painful laser stimulation. J Pain. 3:401–11. DOI:
10.1054/jpai.2002.126788. PMID:
14622744.

56. Robinson ME, Riley JL 3rd, Myers CD, Papas RK, Wise EA, Waxenberg LB, et al. 2001; Gender role expectations of pain: relationship to sex differences in pain. J Pain. 2:251–7. DOI:
10.1054/jpai.2001.24551. PMID:
14622803.

57. Bond LM, Flickinger D, Aytes L, Bateman B, Chalk MB, Aysse P. 2005; Effects of preoperative teaching of the use of a pain scale with patients in the PACU. J Perianesth Nurs. 20:333–40. DOI:
10.1016/j.jopan.2005.08.002. PMID:
16246810.

58. Wikström L, Eriksson K, Årestedt K, Fridlund B, Broström A. 2014; Healthcare professionals' perceptions of the use of pain scales in postoperative pain assessments. Appl Nurs Res. 27:53–8. DOI:
10.1016/j.apnr.2013.11.001. PMID:
24387871.
59. McGlothlin AE, Lewis RJ. 2014; Minimal clinically important difference: defining what really matters to patients. JAMA. 312:1342–3. DOI:
10.1001/jama.2014.13128. PMID:
25268441.
60. Muñoz-Leyva F, El-Boghdadly K, Chan V. 2020; Is the minimal clinically important difference (MCID) in acute pain a good measure of analgesic efficacy in regional anesthesia? Reg Anesth Pain Med. 45:1000–5. DOI:
10.1136/rapm-2020-101670. PMID:
32900985.

61. Olsen MF, Bjerre E, Hansen MD, Hilden J, Landler NE, Tendal B, et al. 2017; Pain relief that matters to patients: systematic review of empirical studies assessing the minimum clinically important difference in acute pain. BMC Med. 15:35. DOI:
10.1186/s12916-016-0775-3. PMID:
28215182. PMCID:
PMC5317055.

62. Cepeda MS, Africano JM, Polo R, Alcala R, Carr DB. 2003; What decline in pain intensity is meaningful to patients with acute pain? Pain. 105:151–7. DOI:
10.1016/S0304-3959(03)00176-3. PMID:
14499431.

63. Danoff JR, Goel R, Sutton R, Maltenfort MG, Austin MS. 2018; How much pain is significant? Defining the minimal clinically important difference for the visual analog scale for pain after total joint arthroplasty. J Arthroplasty. 33:S71–5.e2. DOI:
10.1016/j.arth.2018.02.029. PMID:
29567002.
64. Laigaard J, Pedersen C, Rønsbo TN, Mathiesen O, Karlsen APH. 2021; Minimal clinically important differences in randomised clinical trials on pain management after total hip and knee arthroplasty: a systematic review. Br J Anaesth. 126:1029–37. DOI:
10.1016/j.bja.2021.01.021. PMID:
33678402.

66. Vasilopoulos T, Wardhan R, Rashidi P, Fillingim RB, Wallace MR, et al. Crispen PL; Temporal Postoperative Pain Signatures (TEMPOS) Group. 2021; Patient and procedural determinants of postoperative pain trajectories. Anesthesiology. 134:421–34. DOI:
10.1097/ALN.0000000000003681. PMID:
33449996. PMCID:
PMC8726009.

67. Panzenbeck P, von Keudell A, Joshi GP, Xu CX, Vlassakov K, Schreiber KL, et al. 2021; Procedure-specific acute pain trajectory after elective total hip arthroplasty: systematic review and data synthesis. Br J Anaesth. 127:110–32. DOI:
10.1016/j.bja.2021.02.036. PMID:
34147158.

68. Kelly I, Fields K, Sarin P, Pang A, Sigurdsson MI, Shernan SK, et al. 2022; Identifying patients vulnerable to inadequate pain resolution after cardiac surgery. Semin Thorac Cardiovasc Surg. doi: 10.1053/j.semtcvs.2022.08.010. DOI:
10.1053/j.semtcvs.2022.08.010. PMID:
36084862.
70. Lapkin S, Ellwood L, Diwan A, Fernandez R. 2021; Reliability, validity, and responsiveness of multidimensional pain assessment tools used in postoperative adult patients: a systematic review of measurement properties. JBI Evid Synth. 19:284–307. DOI:
10.11124/JBISRIR-D-19-00407. PMID:
32833789.

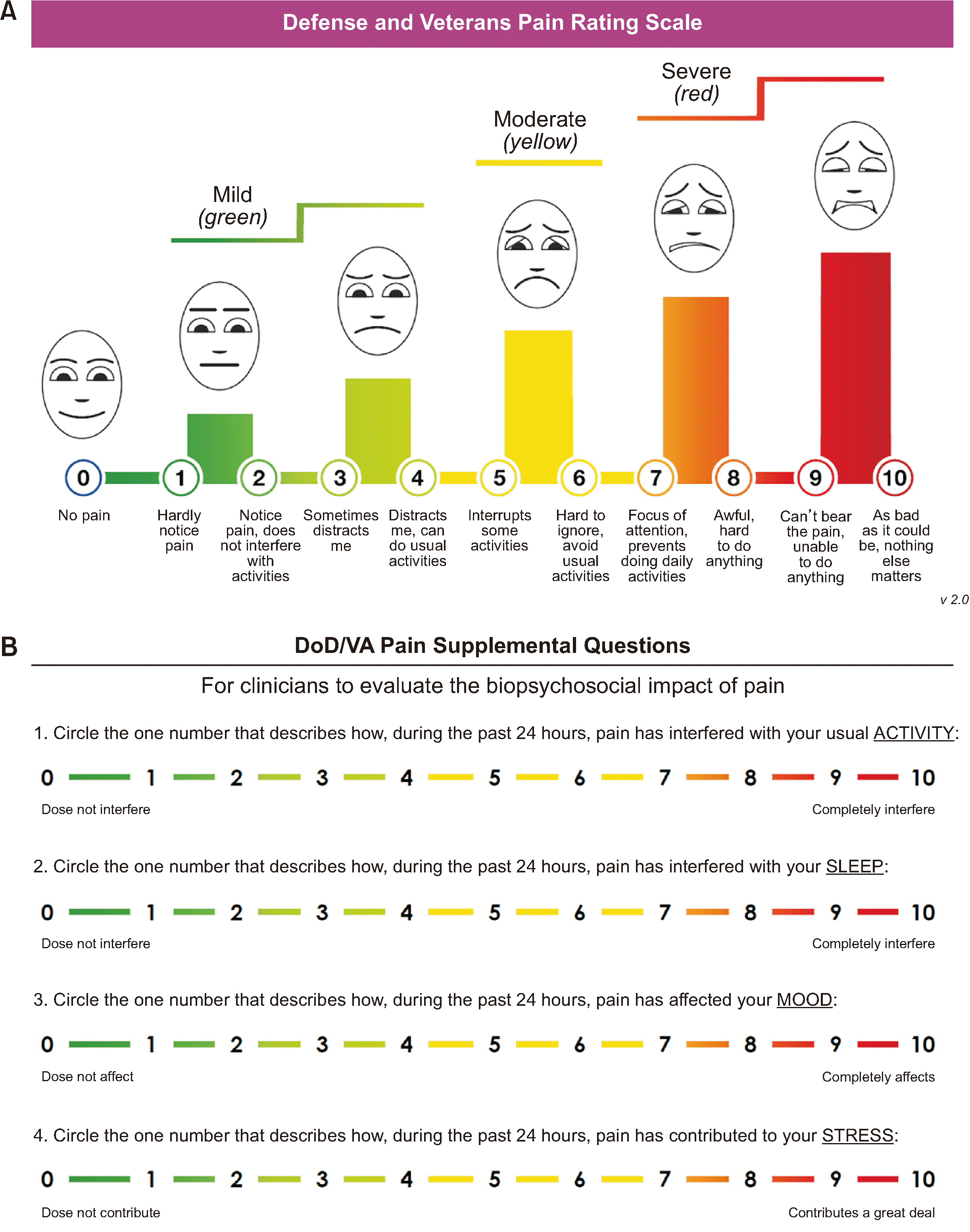
71. Buckenmaier CC 3rd, Galloway KT, Polomano RC, McDuffie M, Kwon N, Gallagher RM. 2013; Preliminary validation of the Defense and Veterans Pain Rating Scale (DVPRS) in a military population. Pain Med. 14:110–23. DOI:
10.1111/j.1526-4637.2012.01516.x. PMID:
23137169.

72. Nassif TH, Hull A, Holliday SB, Sullivan P, Sandbrink F. 2015; Concurrent validity of the defense and veterans pain rating scale in VA outpatients. Pain Med. 16:2152–61. DOI:
10.1111/pme.12866. PMID:
26257151.

73. Cho S, Kim YJ, Lee M, Woo JH, Lee HJ. 2021; Cut-off points between pain intensities of the postoperative pain using receiver operating characteristic (ROC) curves. BMC Anesthesiol. 21:29. Erratum in: BMC Anesthesiol 2021; 21: 191. DOI:
10.1186/s12871-021-01245-5. PMID:
33494704. PMCID:
PMC7831264.
74. van Dijk JF, van Wijck AJ, Kappen TH, Peelen LM, Kalkman CJ, Schuurmans MJ. 2012; Postoperative pain assessment based on numeric ratings is not the same for patients and professionals: a cross-sectional study. Int J Nurs Stud. 49:65–71. DOI:
10.1016/j.ijnurstu.2011.07.009. PMID:
21840522.





 PDF
PDF Citation
Citation Print
Print




 XML Download
XML Download