Abstract
Background/Aims
Methods
Results
Notes
Funding Source
The authors received no financial support for the research, authorship, and/or publication of this article.
Author Contributions
Conceptualization: Shimizu H, Aonuma Y. Data curation: Shimizu H, Aonuma Y, Hibiya S. Formal analysis: Shimizu H, Aonuma Y, Hibiya S. Investigation: Aonuma Y. Methodology: Shimizu H, Aonuma Y. Project administration: Shimizu H. Resources: Shimizu H. Supervision: Takenaka K, Fujii T, Saito E, Nagahori M, Ohtsuka K, Okamoto R. Validation: Shimizu H, Aonuma Y, Hibiya S. Visualization: Shimizu H. Writing - original draft: Shimizu H. Writing - review & editing: Kawamoto A. Approval of final manuscript: all authors.
Supplementary Material
Supplementary Table 1.
Supplementary Table 2.
Supplementary Table 3.
Supplementary Table 4.
Supplementary Fig. 1.
REFERENCES
Fig. 1.
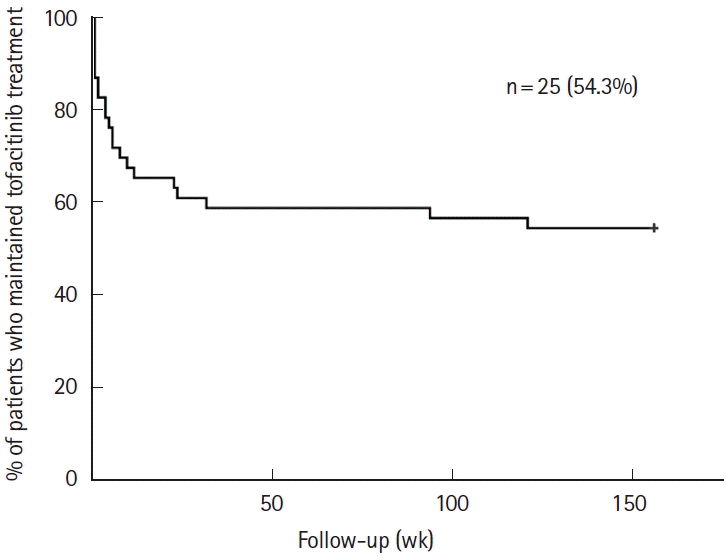
Fig. 2.
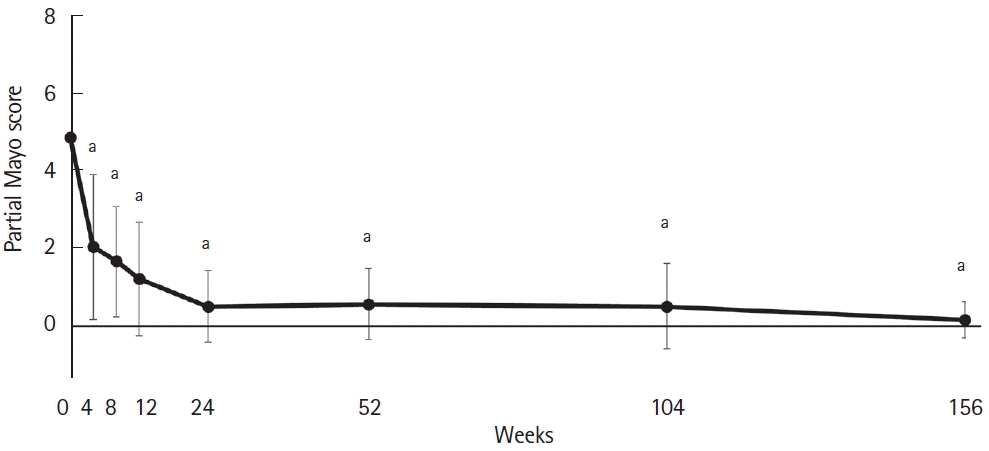
Fig. 3.
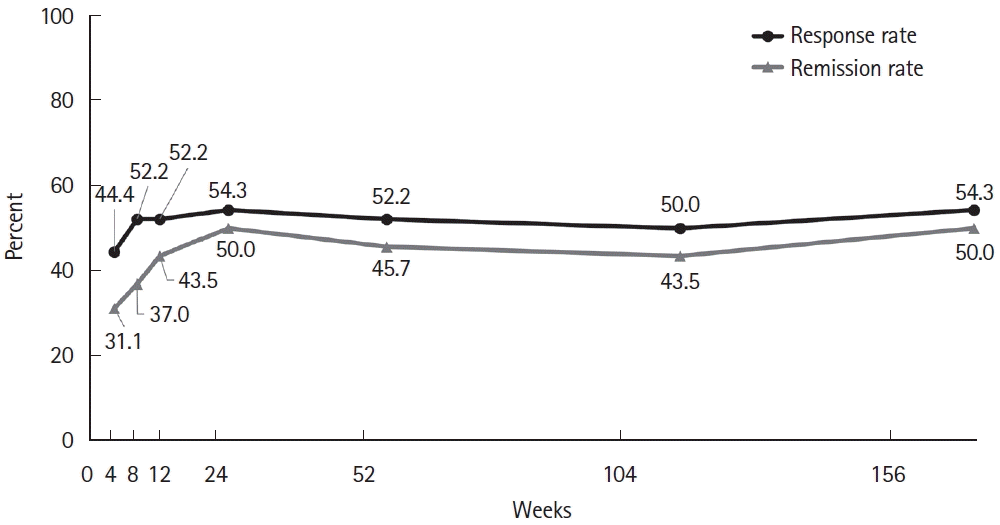
Table 1.
Table 2.
| Outcome | Value (n = 46) |
|---|---|
| Tofacitinib retention rate at 156 wk | 25 (54.3) |
| Short-term efficacy | |
| Remission at 4 wk | 14 (31.1) |
| Remission at 8 wk | 17 (37.0) |
| Remission at 12 wk | 20 (43.5) |
| Response at 4 wk | 20 (44.4) |
| Response at 8 wk | 24 (52.2) |
| Response at 12 wk | 24 (52.2) |
| Long-term efficacy | |
| Remission at 52 wk | 21 (45.7) |
| Remission at 104 wk | 20 (43.5) |
| Remission at 156 wk | 23 (50.0) |
| Response at 52 wk | 24 (52.2) |
| Response at 104 wk | 23 (50.0) |
| Response at 156 wk | 25 (54.3) |
| Dose optimization study in responsive patients at 8 wk (n = 24)a | |
| Continue 10 mg b.d. | 1 (4.2) |
| Reduced 5 mg b.d. after induction | 23 (95.8) |
| Median period to reduction after induction (wk) | 14.0 (9.0–19.0) |
| Continued 5 mg b.d. | 10 (41.7) |
| Loss of response | 13 (54.2) |
| Increased 10 mg b.d. after reduction | 12 (50.0) |
| Median period to increase (wk) | 39.5 (14.5–84.0) |
| Reduced 5 mg b.d. again | 5 (20.8) |
| Median period to reduction (wk) | 11.0 (9.0–18.0) |
| Continued 10 mg b.d. | 5 (20.8) |
| Repeat reduce and increase | 2 (8.3) |
| Adverse events | 23 (50.0) |
Table 3.
| Variable | Non-failure (n = 25) | Failure (n = 21)a | P-value |
|---|---|---|---|
| Sex | |||
| Male | 15 (60.0) | 11 (52.4) | 0.412 |
| Female | 10 (40.0) | 10 (47.6) | |
| Age (yr) | 33.5 (29.0–53.0) | 29.0 (18.5–39.5) | 0.164 |
| Disease duration (yr) | 8.9 (5.4–12.4) | 5.9 (1.9–9.9) | 0.045 |
| Extent of disease | |||
| Extensive | 16 (64.0) | 13 (61.9) | 1.000 |
| Left-sided | 9 (36.0) | 8 (38.1) | 1.000 |
| Severity | |||
| Severe | 1 (4.0) | 2 (9.5) | 0.585 |
| Moderate | 22 (88.0) | 19 (90.5) | 1.000 |
| Mild | 2 (8.0) | 0 | |
| Partial Mayo score | 5 (3–7) | 6 (5–7) | 0.109 |
| UCEIS (n = 16) | 5.0 (3.8–6.3) | 5.0 (4.1–5.9) | 1.000 |
| White blood cells (/μL) | 5,600 (3,700–7,500) | 6,400 (4,775–8,025) | 0.251 |
| Hemoglobin (g/dL) | 13.2 (11.9–14.5) | 12.2 (10.9–14.5) | 0.076 |
| Albumin (g/dL) | 3.9 (3.5–4.3) | 3.9 (3.4–4.4) | 0.674 |
| CRP (mg/L) | 1.9 (0–4.2) | 1.9 (0–8.9) | 0.938 |
| Previous medication use | |||
| Oral aminosalicylate | 12 (48.0) | 9 (42.9) | 0.774 |
| Corticosteroid | |||
| Refractory | 8 (32.0) | 8 (38.1) | 0.760 |
| Dependent | 16 (64.0) | 13 (61.9) | 1.000 |
| Never used | 1 (4.0) | 0 | |
| Immunomodulator | 21 (84.0) | 19 (90.5) | 0.673 |
| Calcineurin inhibitor | 4 (16.0) | 9 (42.9) | 0.056 |
| Biological agent | |||
| Naïve | 4 (16.0) | 0 | 0.114 |
| 1 Agent | 8 (32.0) | 10 (47.6) | 0.367 |
| 2 Agents | 9 (36.0) | 9 (42.9) | 0.764 |
| 3 Or more agents | 4 (16.0) | 2 (9.5) | 0.673 |
Values are presented as number (percent) or median (interquartile range). Differences in medians between the 2 groups were compared by non-parametric test (Mann-Whitney test or Wilcoxon test), and comparison between categorical variables were performed using chi-square test.
a Failure was defined as tofacitinib discontinuation due to any of the following: relapse following remission, nonresponse to tofacitinib treatment, or adverse events. Twenty-five patients continued tofacitinib until 156 weeks after initiation, and 21 patients discontinued tofacitinib before 156 weeks.
Table 4.
| Adverse eventsa | No. (%) |
|---|---|
| Infectious adverse event | |
| Nasopharyngitis | 8 (17.4) |
| Herpes zoster | 3 (6.5) |
| Paranasal sinusitis | 3 (6.5) |
| Serpigo | 1 (2.2) |
| External otitis | 1 (2.2) |
| Herpangina | 1 (2.2) |
| COVID-19 | 4 (8.7) |
| Dyslipidemia | 8 (17.4) |
| Acne | 5 (10.9) |
| Abnormal liver function tests | 1 (2.2) |
| Dyspnea | 1 (2.2) |
| Cancer | 0 |
| Venous thromboembolism | 0 |




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download