1. Kumar SK, Rajkumar SV, Dispenzieri A, Lacy MQ, Hayman SR, Buadi FK, et al. Improved survival in multiple myeloma and the impact of novel therapies. Blood. 2008; 111:2516–20.

2. Palumbo A, Anderson K. Multiple myeloma. N Engl J Med. 2011; 364:1046–60.

3. Katodritou E, Papadaki S, Konstantinidou P, Terpos E. Is it possible to cure myeloma without allogeneic transplantation? Transfus Apher Sci. 2016; 54:63–70.

4. Lokhorst H, Einsele H, Vesole D, Bruno B, San Miguel J, PerezSimon JA, et al. International Myeloma Working Group consensus statement regarding the current status of allogeneic stem-cell transplantation for multiple myeloma. J Clin Oncol. 2010; 28:4521–30.

5. Barlogie B, Shaughnessy J, Tricot G, Jacobson J, Zangari M, Anaissie E, et al. Treatment of multiple myeloma. Blood. 2004; 103:20–32.

6. Kim HT, Armand P. Clinical endpoints in allogeneic hematopoietic stem cell transplantation studies: the cost of freedom. Biol Blood Marrow Transplant. 2013; 19:860–6.

7. Giralt S, Garderet L, Durie B, Cook G, Gahrton G, Bruno B, et al. American Society of Blood and Marrow Transplantation, European Society of Blood and Marrow Transplantation, Blood and Marrow Transplant Clinical Trials Network, and International Myeloma Working Group consensus conference on salvage hematopoietic cell transplantation in patients with relapsed multiple myeloma. Biol Blood Marrow Transplant. 2015; 21:2039–51.
8. Gahrton G, Svensson H, Cavo M, Apperly J, Bacigalupo A, Bjorkstrand B, et al. Progress in allogenic bone marrow and peripheral blood stem cell transplantation for multiple myeloma: a comparison between transplants performed 1983-93 and 1994-8 at European Group for Blood and Marrow Transplantation centres. Br J Haematol. 2001; 113:209–16.

9. Glucksberg H, Storb R, Fefer A, Buckner CD, Neiman PE, Clift RA, et al. Clinical manifestations of graft-versus-host disease in human recipients of marrow from HL-A-matched sibling donors. Transplantation. 1974; 18:295–304.

10. Durie BG, Harousseau JL, Miguel JS, Blade J, Barlogie B, Anderson K, et al. International uniform response criteria for multiple myeloma. Leukemia. 2006; 20:1467–73.

11. Scott EC, Hari P, Sharma M, Le-Rademacher J, Huang J, Vogl D, et al. Post-transplant outcomes in high-risk compared with non-high-risk multiple myeloma: a CIBMTR analysis. Biol Blood Marrow Transplant. 2016; 22:1893–9.

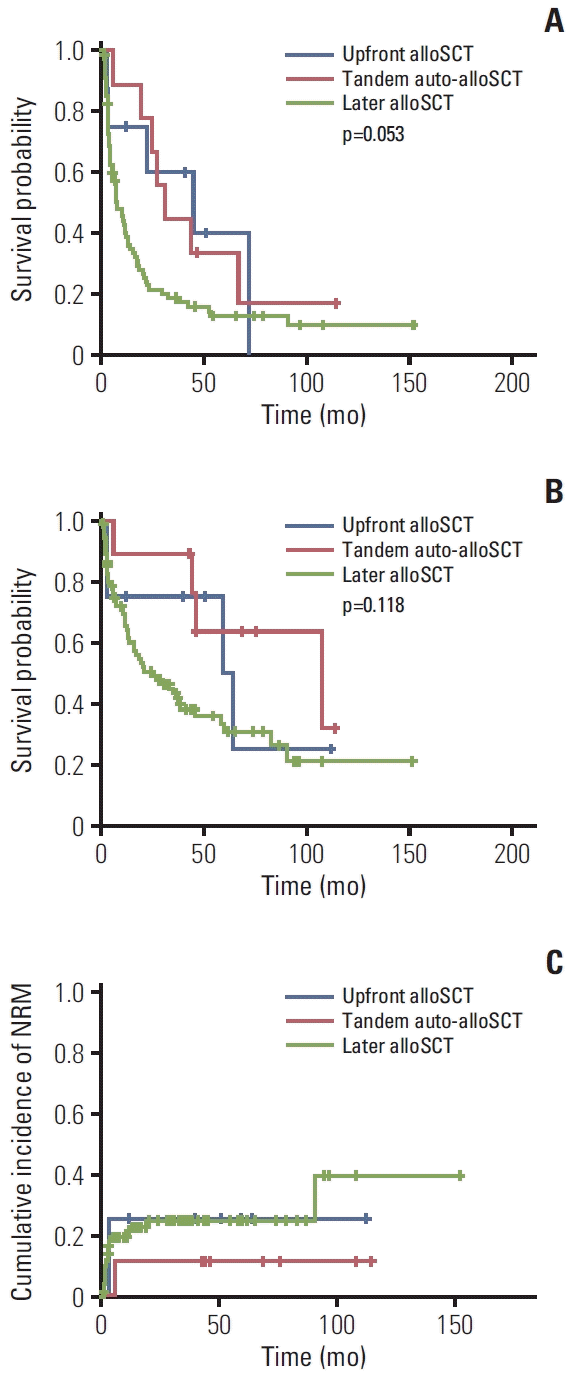
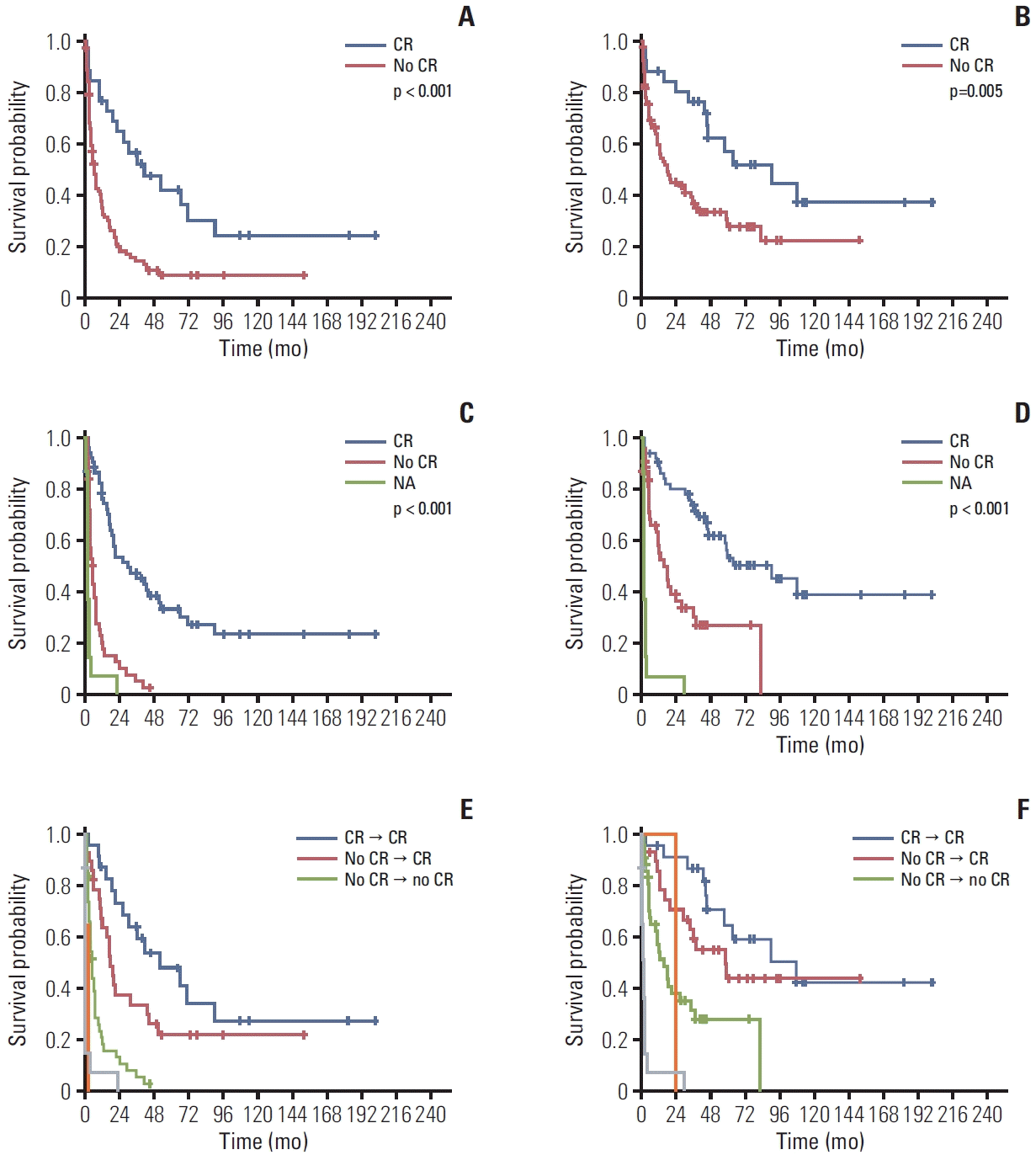
12. Iacobelli S, de Wreede LC, Schonland S, Bjorkstrand B, Hegenbart U, Gruber A, et al. Impact of CR before and after allogeneic and autologous transplantation in multiple myeloma: results from the EBMT NMAM2000 prospective trial. Bone Marrow Transplant. 2015; 50:505–10.

13. Ocio EM, Richardson PG, Rajkumar SV, Palumbo A, Mateos MV, Orlowski R, et al. New drugs and novel mechanisms of action in multiple myeloma in 2013: a report from the International Myeloma Working Group (IMWG). Leukemia. 2014; 28:525–42.

14. Qazilbash MH, Saliba R, De Lima M, Hosing C, Couriel D, Aleman A, et al. Second autologous or allogeneic transplantation after the failure of first autograft in patients with multiple myeloma. Cancer. 2006; 106:1084–9.

15. Auner HW, Szydlo R, van Biezen A, Iacobelli S, Gahrton G, Milpied N, et al. Reduced intensity-conditioned allogeneic stem cell transplantation for multiple myeloma relapsing or progressing after autologous transplantation: a study by the European Group for Blood and Marrow Transplantation. Bone Marrow Transplant. 2013; 48:1395–400.

16. Freytes CO, Vesole DH, LeRademacher J, Zhong X, Gale RP, Kyle RA, et al. Second transplants for multiple myeloma relapsing after a previous autotransplant-reduced-intensity allogeneic vs autologous transplantation. Bone Marrow Transplant. 2014; 49:416–21.

17. Patriarca F, Einsele H, Spina F, Bruno B, Isola M, Nozzoli C, et al. Allogeneic stem cell transplantation in multiple myeloma relapsed after autograft: a multicenter retrospective study based on donor availability. Biol Blood Marrow Transplant. 2012; 18:617–26.

18. de Lavallade H, El-Cheikh J, Faucher C, Furst S, Stoppa AM, Coso D, et al. Reduced-intensity conditioning allogeneic SCT as salvage treatment for relapsed multiple myeloma. Bone Marrow Transplant. 2008; 41:953–60.

19. Rotta M, Storer BE, Sahebi F, Shizuru JA, Bruno B, Lange T, et al. Long-term outcome of patients with multiple myeloma after autologous hematopoietic cell transplantation and nonmyeloablative allografting. Blood. 2009; 113:3383–91.

20. Rosinol L, Perez-Simon JA, Sureda A, de la Rubia J, de Arriba F, Lahuerta JJ, et al. A prospective PETHEMA study of tandem autologous transplantation versus autograft followed by reduced-intensity conditioning allogeneic transplantation in newly diagnosed multiple myeloma. Blood. 2008; 112:3591–3.

21. Crawley C, Szydlo R, Lalancette M, Bacigalupo A, Lange A, Brune M, et al. Outcomes of reduced-intensity transplantation for chronic myeloid leukemia: an analysis of prognostic factors from the Chronic Leukemia Working Party of the EBMT. Blood. 2005; 106:2969–76.

22. Kim I, Lee KH, Choi Y, Keam B, Koo NH, Yoon SS, et al. Allogeneic stem cell transplantation for patients with advanced hematological malignancies: comparison of fludarabine-based reduced intensity conditioning versus myeloablative conditioning. J Korean Med Sci. 2007; 22:227–34.

23. Kawamura K, Tsukada N, Kanda Y, Ikeda T, Yoshida A, Ueda Y, et al. The role of allogeneic transplantation for multiple myeloma in the era of novel agents: a study from the Japanese Society of Myeloma. Biol Blood Marrow Transplant. 2018; 24:1392–8.

24. Montefusco V, Mussetti A, Rezzonico F, Maura F, Pennisi M, de Philippis C, et al. Allogeneic stem cell transplantation and subsequent treatments as a comprehensive strategy for long-term survival of multiple myeloma patients. Bone Marrow Transplantation. 2017; 52:1602–8.

25. Khaled Y, Mellacheruvu S, Reddy P, Peres E, Mineishi S. Long-term outcomes following myeloablative allogeneic transplantation for multiple myeloma compared to autologous transplantation and the impact of graft-versus-myeloma effect. Bone Marrow Transplant. 2009; 44:325–6.

26. Kuruvilla J, Shepherd JD, Sutherland HJ, Nevill TJ, Nitta J, Le A, et al. Long-term outcome of myeloablative allogeneic stem cell transplantation for multiple myeloma. Biol Blood Marrow Transplant. 2007; 13:925–31.

27. Gahrton G, Iacobelli S, Bandini G, Bjorkstrand B, Corradini P, Crawley C, et al. Peripheral blood or bone marrow cells in reduced-intensity or myeloablative conditioning allogeneic HLA identical sibling donor transplantation for multiple myeloma. Haematologica. 2007; 92:1513–8.

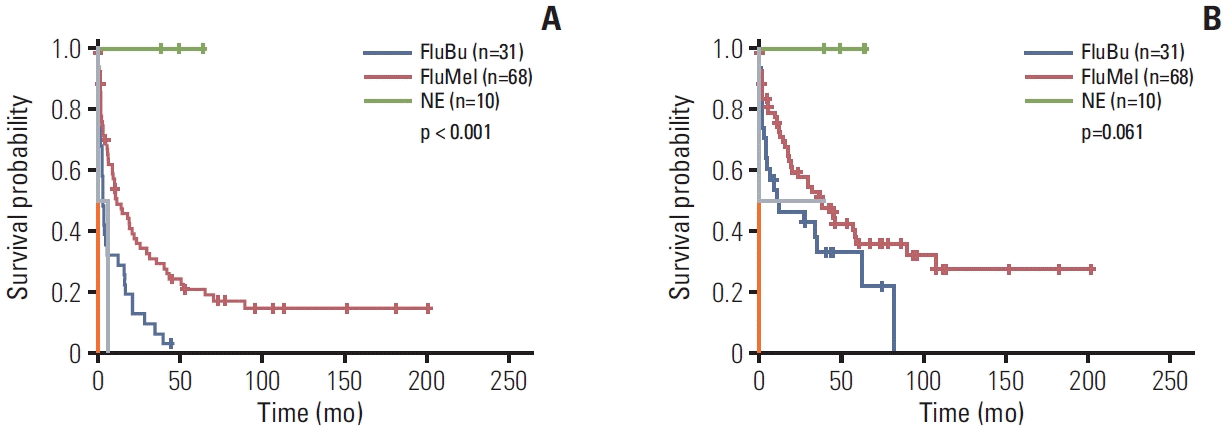
28. Bashir Q, Khan H, Thall PF, Liu P, Shah N, Kebriaei P, et al. A randomized phase II trial of fludarabine/melphalan 100 versus fludarabine/melphalan 140 followed by allogeneic hematopoietic stem cell transplantation for patients with multiple myeloma. Biol Blood Marrow Transplant. 2013; 19:1453–8.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download