Abstract
Notes
Ethics Statement
The Institutional Review Board at National Defense Medical College approved publication of this article (Registration number, 4802). Informed consent from the participant has been waived by Institutional Review Board.
Availability of Data and Material
The datasets generated or analyzed during the study are available from the corresponding author on reasonable request.
Author Contributions
Conceptualization: NI, KM. Data curation: NI, KM. Formal analysis: KM. Investigation: MT, MS, TS, SK, FK. Methodology: KM, MT, SO. Project administration: KM. Resources: SO, SM. Supervision: SK, SO, FK, SM. Visualization: KM. Writing—original draft: NI, KM. Writing—review & editing: MT, MS, SK, SO, FK, SM. Approval of final manuscript: all authors.
ACKNOWLEDGMENTS
References
Fig. 1.
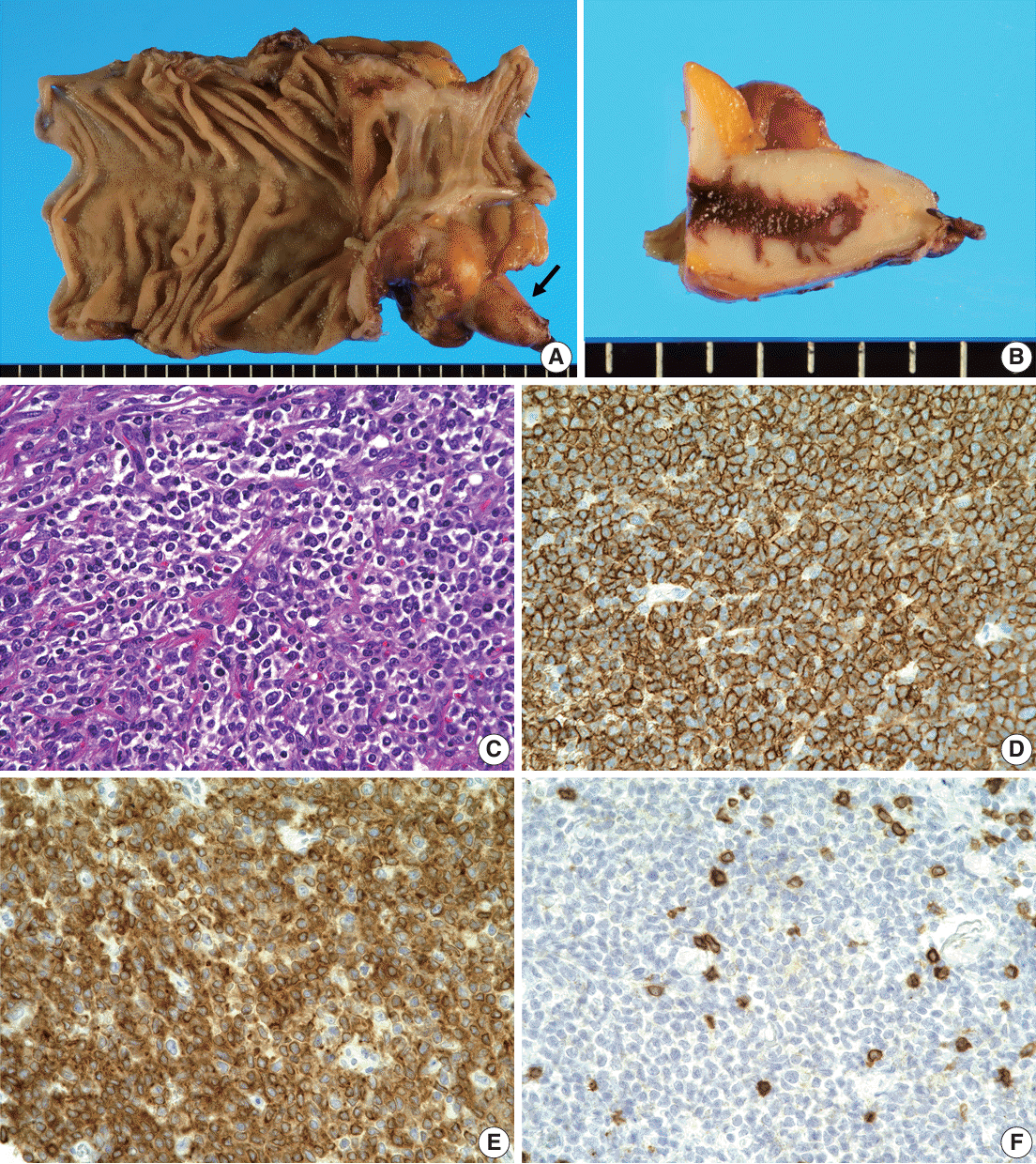
Fig. 2.
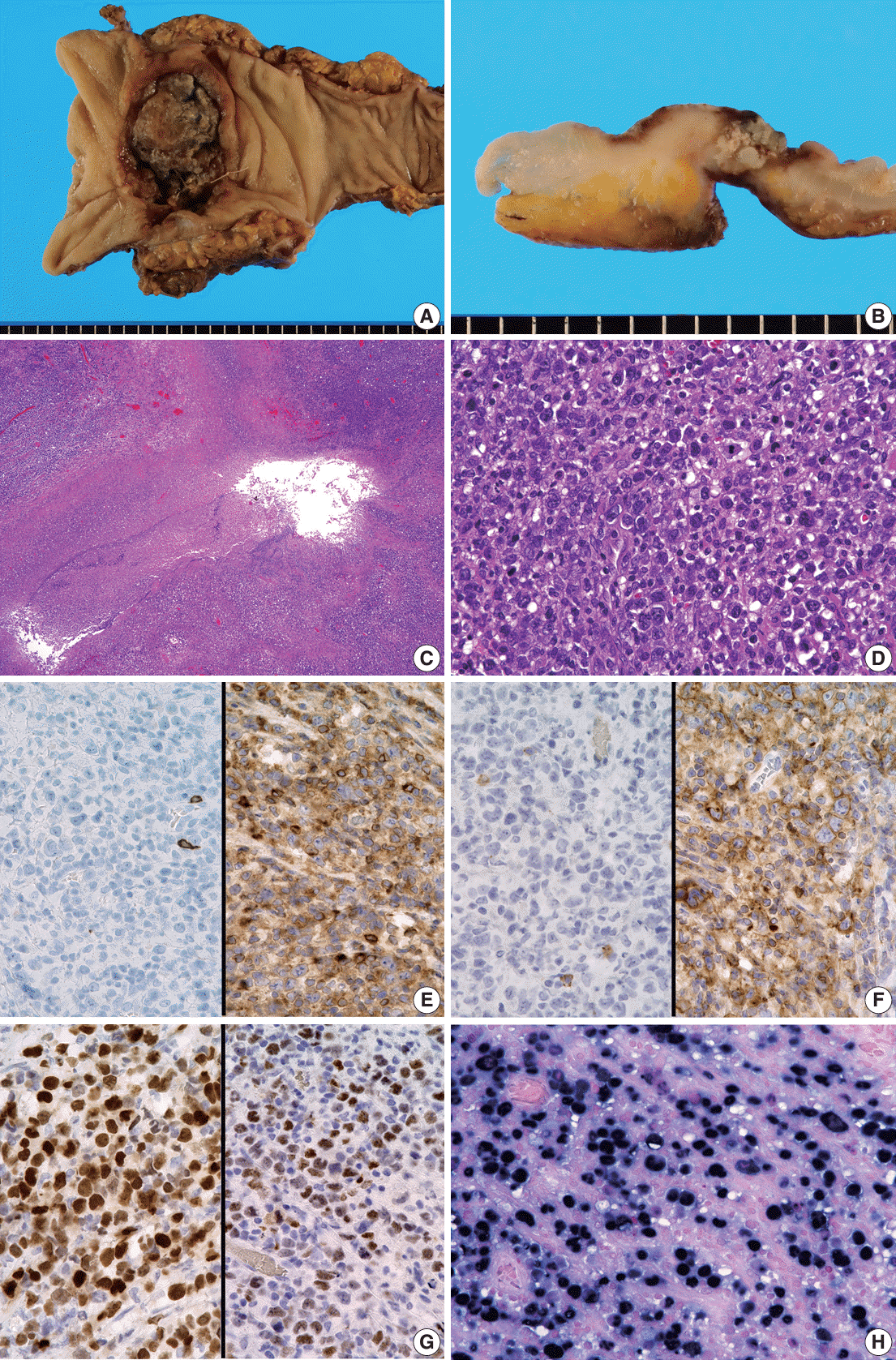
Fig. 3.
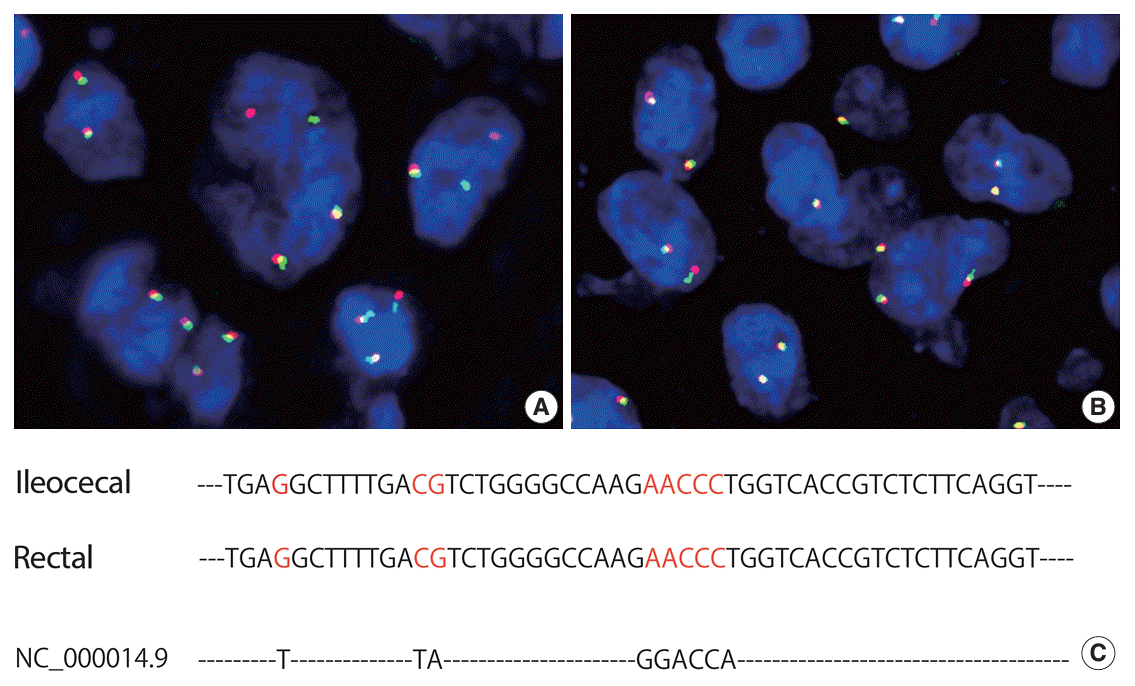
Table 1.
| Case No. | Age (yr)/Sex | Immunological status | Location of PBL | Type and location of other tumors | Same clonality of lymphomas | Chemoradiotherapy |
Immunohistochemical analysis of PBL |
Outcome, post diagnosis of PBL | Study | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CD20 | CD79a | CD138 | CD38 | MUM1 | MYC | EBER | |||||||||
| 1 | 67/M | Immunocompetent, HIV (–) | Ileocecal valve, left humerus | CLL, bone marrow | N/A | Bortezomib and dexamethasone | - | + | + | + | + | N/A | + | DOD, 2.5 mo | Hatzimichael et al. [10] |
| 2 | 61/M | Immunocompetent, HIV (–), EBV (–), HHV-8 (–) | Lymph node | SLL, the same lymph node as PBL | Detected | Hyper-C-VAD | - | N/A | + | N/A | + | N/A | - | DOD, a couple of months | Ronchi et al. [11] |
| 3 | 48/M | Immunocompetent, HIV (–) | Left supraclavicular area, duodenum | CLL, bone marrow | N/A | R-CHOP, bortezomib, ifosfamide, etoposide, carboplatin, mesna, brentuximab vedotin, and radiation to the neck lesion | N/A | + | + | + | + | N/A | N/A | DOD, N/A | Holderness et al. [12] |
| 4 | 57/F | Postchemotherapy for CLL, HIV (–), CMV IgM (–), CMV IgG (+) | Mandible, bone marrow | CLL, bone marrow | Not detected (different clones) | VAD and CHOP | - | N/A | N/A | N/A | N/A | N/A | N/A | DOD | Robak et al. [13] |
| 6 mo | |||||||||||||||
| 5 | 69/M | Postchemotherapy for CLL, EBV (+) | Nasopharynx | CLL, bone marrow; cHL, left cervical lymph node | Not detected (CLL and PBL) | R-CHOP and radiation to the nasopharynx | - | - | + | N/A | + | N/A | + | DOD | Foo et al. [14] |
| N/A (cHL and PBL) | 9 mo | ||||||||||||||
| 6 | 37/M | EBV (+) | Urinary bladder | DLBCL, right nasal cavity | Detected | R-CHOP, intrathecal infusions of methotrexate, cytarabine, and hydrocortisone | - | - | + | N/A | N/A | + | N/A | Recurrence of DLBCL, 4 yr | Hashimoto et al. [9] |
| 7 | 84/F | Immunocompetent | Rectum | DLBCL, cecum and appendix vermiformis | Detected | R-CHOP | - | + | - | + | + | + | + | No recurrence, 11 mo | Present case |
PBL, plasmablastic lymphoma; EBER, Epstein-Barr virus–encoded RNA; M, male; HIV, human immunodeficiency virus; CLL, chronic lymphocytic leukemia; N/A, not available; DOD, death of disease; EBV, Epstein-Barr virus; HHV-8, human herpes virus-8; SLL, small lymphocytic lymphoma; Hyper-C-PAD, cyclophosphamide, vincristine, adriamycin, and dexamethasone; R-CHOP, rituximab, cyclophosphamide, doxorubicin, oncovin, and prednisolone; CMV, cytomegarovirus; VAD, vincristine, adriamycin, and dexamethasone; cHL, classical Hodgkin lymphoma; DLBCL, diffuse large B-cell lymphoma.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download