Abstract
Due to increased life expectancy and lifestyle changes, the prevalence of diabetes among the elderly in Korea is continuously rising, as is the associated public health burden. Diabetes management in elderly patients is complicated by age-related physiological changes, sarcopenia characterized by loss of muscle mass and function, comorbidities, and varying levels of functional, cognitive, and mobility abilities that lead to frailty. Moreover, elderly patients with diabetes frequently face multiple chronic conditions that elevate their risk of cardiovascular diseases, cancer, and mortality; they are also prone to complications such as hyperglycemic hyperosmolar state, diabetic ketoacidosis, and severe hypoglycemia. This review examines the characteristics of and management approaches for diabetes in the elderly, and advocates for a comprehensive yet personalized strategy.
• In elderly diabetes patients, glucose and insulin metabolism are impaired.
• Sarcopenia, frailty, and multimorbidity complicate managing diabetes in the elderly.
• Elderly patients with diabetes face high risks of severe hyperglycemia and hypoglycemia.
• Management should focus on glucose-, cardiorenal-, and weight-centric approaches.
• Evidence-based studies are needed to refine diabetes care in the elderly.
The elderly population is rapidly increasing worldwide; South Korea especially is among the countries expected to see life expectancy increase significantly [1]. As of 2024, those aged 65 years and older accounted for 19.2% of the country’s total population [2]. The speed of this aging is the fastest in the world. While Korea’s aging population reflects significant social progress, being due to medical advancements and improved living standards, it also brings to the forefront various public health issues, such as the increasing burden of chronic diseases, including type 2 diabetes mellitus (T2DM); this burden has become a major concern globally. Diabetes in particular is a significant cause of death, accounting for an estimated 11.3% of deaths worldwide in 2019 [3]. As of 2020, 5.27 million Korean adults over 30 years of age were diagnosed with diabetes; 3 million of those patients are over 60 years of age, making up more than half of the total diabetes population [4]. Looked at from the other direction, the prevalence of diabetes among Korean seniors over 65 years of age is 30.1% [4]; moreover, the prevalence of pre-diabetes in this age group is alarmingly high at 50.4%. Given the rising trend in obesity among the elderly, it is anticipated that the risk of diabetes in this population will continue to increase in the foreseeable future [5]. The increasing trend of diabetes prevalence in the elderly varies by region and race; prevalences are relatively low in European countries, but particularly pronounced in high-income East Asian countries like Korea. This strong increase is attributed to changes in lifestyle habits, including diet, and to rapid changes in socio-economical factors in East Asia, in addition to pathophysiological characteristics [6].
The dual burden of T2DM and geriatric-specific syndromes in the elderly leads to these patients having multifaceted needs, which a comprehensive approach is needed to address [7]. Current management strategies are still largely based on evidence from younger populations; more tailored approaches for elderly T2DM are currently lacking and emphatically required. Nonetheless, establishing an optimal management strategy for elderly diabetes is challenging for several reasons. Disease expression in the elderly is often ambiguous, complicating diagnosis and management; in particular, unlike younger adults, elderly adults with T2DM exhibit considerable variability due to factors such as age-related physiological changes, multiple chronic diseases, and varying levels of functional, cognitive, and mobility abilities [8]. Elderly individuals also have a high incidence of cardiovascular diseases and cancer, which conditions significantly increase the risk of death. Acute complications of diabetes that are particularly dangerous in the elderly include hyperglycemic hyperosmolar state (HHS), diabetic ketoacidosis (DKA), severe hypoglycemia, and severe acute infections, which also elevate mortality risk [9]. Even non-lifethreatening microvascular complications can lead to declines in function and quality of life, potentially resulting in loss of independence. Neurocognitive impairment is also more frequent in patients with diabetes, and constrains their ability to manage glycemic levels and respond to hyperglycemia and hypoglycemia [10]. Moreover, recent socioeconomic changes have led to an increase in obesity, which, in addition to being often seen in younger diabetes, is now also appearing as sarcopenic obesity in elderly T2DM [11]. Finally, polypharmacy is common among the elderly, and increases the risk of drug interactions and side effects [12]. Given all of these complicating factors, it is necessary to develop an integrated strategy for diabetes care in the elderly that reflects the complex and heterogeneous individual characteristics of this age group and takes into account their distinctive lifestyle habits, socioeconomic factors, and support systems.
In this review, we will explore the multifaceted characteristics of T2DM in the elderly and approaches to its treatment. By enumerating these factors, we aim to highlight the importance of individualized treatment and the need for treatment strategies that can address the diverse needs of elderly patients with T2DM.
T2DM in the elderly involves complex age-related changes in numerous physiological processes associated with glucose and insulin metabolism. Although the action of insulin does not itself change significantly, insulin secretion from β-cells is reduced and insulin sensitivity in peripheral tissues is decreased as part of the aging process [13]. Reduction of the rate of insulin secretion in response to glucose is particularly pronounced in lean elderly individuals [14]. Generally, aging is associated with decreased skeletal muscle mass and function; this is accompanied by an increase in abdominal visceral fat, another major factor leading to increased insulin resistance [15]. Notably, in Korea, the prevalence of obesity among individuals over 60 years of age is 40% as of 2021, with the abdominal obesity rate exceeding 30% [5]. In the very elderly, those over 70 and even 80 years of age, the abdominal obesity rate is as high as 38%. Other factors contributing to increased insulin resistance in elderly T2DM include decreased physical activity, increased fat accumulation in muscles and liver, dysfunction of muscle mitochondria, and an increased inflammatory response. A sedentary lifestyle leads to increased obesity and insulin resistance; obesity increases low-grade inflammation, which can inhibit the insulin signaling system and lead to hyperglycemia [16,17].
In the elderly, not only are the mechanisms that regulate hyperglycemia compromised, but also those that protect against hypoglycemia [14,18]. There is a diminished response of glucose counter-regulatory hormones, such as glucagon and catecholamines, which are normally secreted in response to declining blood glucose levels. Additionally, in the elderly, the hypoglycemia-associated autonomic function involved in the detection and response to hypoglycemia are impaired. Functional impairments, cognitive decline, and mobility problems commonly associated with geriatric syndromes further impede the ability to recognize and respond to recurrent hypoglycemic events, increasing the risk of severe hypoglycemic episodes [19].
Sarcopenia is defined as progressive loss of skeletal muscle mass and function. In the elderly, it is caused by decreased metabolic rate due to aging, inhibition of protein synthesis, and impairment of muscle repair and regeneration capabilities. Muscle mass typically decreases at a rate of 1% to 2% per year starting at age 50, increasing to 3% per year after age 60, and then accelerating rapidly after age 75 [20,21]. In men, sarcopenia may correlate with a decrease in testosterone levels. As age increases, changes in the balance of hormone axes linked to the adrenal glands, sex hormones, insulin-like growth factor-1 (IGF-1), or IGF receptors can further contribute to muscle strength loss [22].
In elderly T2DM, multiple mechanisms cause skeletal muscle mass to be lost at a faster rate than in healthy elderly individuals [21]. Accordingly, the risk of developing sarcopenia is two to three times higher compared to those without T2DM [21,23]. The prevalence of sarcopenia in diabetes varies across studies, with meta-analyses reporting rates ranging from 5% to 50% [24]. Mechanistically, insulin resistance in T2DM results in inefficient utilization of glucose and amino acids, leading to nutritional deficiencies that cause muscle weakness and sarcopenia. Meanwhile, hyperglycemia leads to accumulation of advanced glycation end-products in muscle tissue, which weakens muscle contractility. Additionally, the decrease in activity of antioxidant enzymes and increase in reactive oxygen species associated with diabetes damage muscle mitochondria, resulting in abnormal mitochondrial function. This is linked to altered lipid oxidation, elevated lipid levels in muscle cells, and insulin resistance, all of which contribute to sarcopenia [25]. The chronic systemic pro-inflammatory state common in diabetes, characterized by increased levels of inflammatory cytokines such as tumor necrosis factor-alpha (TNF-α), interleukin 5 (IL-5), interleukin 6 (IL-6), and C-reactive protein and decreased levels of anti-inflammatory cytokines, may promote muscle damage and breakdown [26]. Finally, sarcopenia itself interferes with metabolic processes and negatively impacts glucose metabolism, further worsening the progression of diabetes and its complications, contributing to additional muscle loss and dysfunction, and ultimately leading to a vicious cycle that increases the risk of frailty. Some medications related to diabetes treatment may also affect muscle mass and performance in elderly patients with T2DM [27].
Frailty, characterized by decreased physiological and homeostatic reserves and increased vulnerability to stressors, is closely linked to the onset and progression of T2DM in the elderly. Fried’s frailty criteria are often used as a clinical phenotype and definition of frailty. According to this rubric, an elderly person can be diagnosed with frailty if they exhibit three or more of the following: (1) weakness or poor handgrip strength, (2) slow gait speed, (3) low physical activity, (4) exhaustion, and (5) unintentional weight loss [28]. When frailty coexists with T2DM in elderly individuals, the risk of hospitalization and mortality increases, and the prognosis upon hospitalization is generally worse [29]. Lack of physical activity, loss of appetite, and reduced food intake are major contributing factors to frailty, and diabetes itself can also be a risk factor for the progression of frailty. Notably, in elderly individuals with diabetes, it has been observed that frailty increases with both lower and higher blood glucose levels, suggesting a U-shaped relationship with glycemic control. Therefore, it is crucial to avoid significant fluctuations that can lead to either hypoglycemia or hyperglycemia [30].
Since frailty is a multifaceted syndrome influenced by various physiological, psychological, and social factors, multifactorial intervention is crucial in delaying its progression. However, evidence supporting this approach is still limited [31,32]. The Sarcopenia and Physical Frailty IN older people: multicomponent Treatment strategies (SPRINTT) project was a clinical study conducted to determine the effectiveness of multifactorial intervention therapy on 1,519 community residents aged 70 or older with frailty and sarcopenia. In that study, the intervention therapy consisted of personalized moderate-intensity physical activity, customized nutritional counseling, and technological support. After a 3-year follow-up, the customized intervention was found to significantly improve mobility [33].
As individuals age, the impact of single diseases such as diabetes on the risk of death decreases. However, diabetes is networked with the onset and progression of numerous major diseases, and in the elderly, it often coexists with multiple chronic conditions; the interaction between diabetes and these conditions then increases risk of death. The diversity of potential multi-morbidities highlights the complex heterogeneity of diabetes in the elderly population. Managing diabetes requires an individualized treatment approach tailored to the characteristics of major comorbidities and maintaining quality of life.
Concerning the specifics of multimorbidity, it has been reported that as many as 50% to 90% of elderly people with diabetes have at least one additional chronic disease [34], and that 40% have four or more comorbidities [35]. Both comorbidities and the medications used to treat them can affect insulin secretion and sensitivity. For example, steroids used in the treatment of many combined diseases facilitate hyperglycemia by promoting hepatic glucose production and contribute to insulin resistance by increasing visceral fat, breaking down proteins and fats, producing free fatty acids, and causing hepatic fat accumulation [36]. When conditions such as depression and arthritis co-occur with diabetes complications, they lead to decreased physical activity, increased disability, lower quality of life, and significantly higher use of medical services [37]. Diabetes patients are subject to greatly increased risk of severe major comorbidities such as heart failure, renal and liver diseases, and dementia. These diseases increase glycemic variability, reduce exercise ability, and impair cognitive function, making glucose management difficult and hindering the achievement of treatment goals. As a result, elderly diabetes patients with these comorbidities face greater risk of hyperglycemic crises or severe hypoglycemia and of mortality [38,39].
In diabetes patients, the incidence of hypoglycemia increases with age. In 2019, we reported that compared to patients in their 30s, Korean diabetes patients in their 70s had 1.4 times higher risk of hypoglycemia, and patients in their 80s, 1.9 times higher risk [40]. Fortunately, this shows a trend of decreasing risk of hypoglycemia in the elderly compared to 10 years prior. In addition to age itself, hypoglycemia risk is increased by aging-related factors such as decreased renal function, changes in the pharmacokinetics of insulin or insulin secretagogues, cognitive decline, and low body weight [41,42]. As mentioned earlier, a defective glucose counter-regulatory system, hypoglycemia-associated autonomic dysfunction, and diminished awareness of hypoglycemia in the elderly all contribute to the frequent occurrence of hypoglycemia; thus, in a vicious cycle, frequent experiences of hypoglycemia further impair the ability to recognize and respond to low blood glucose levels, thereby increasing the risk of severe hypoglycemic events [43]. Notably, the glycemic range at which adrenergic and cholinergic symptoms appear before neuroglycopenic symptoms (which can lead to decreased consciousness) is narrower in elderly individuals than in young adults [18,44]. Moreover, the symptoms of hypoglycemia in the elderly are often atypical, leading to unclear and delayed diagnosis. Elderly patients may also have poor physical performance, making it difficult to manage hypoglycemia even if they know how to respond, particularly for individuals living alone. Severe hypoglycemia, which often manifests as decreased consciousness, can result in falls, fractures, cognitive impairment, cardiovascular disease, malignant arrhythmias, renal dysfunction, dementia, and even death [41].
Hyperglycemia-related complications in elderly individuals can also be fatal. Particularly, HHS or DKA with uncontrolled diabetes have demonstrated higher mortality, and commonly co-occur with severe infection in elderly T2DM. With the recent increase in use of sodium glucose co-transporter 2 (SGLT2) inhibitors, there is especially a need for caution regarding development of DKA. Euglycemic DKA occurs even without an absolute deficiency of insulin and is caused by excess glucosuria from SGLT2 inhibitor use, dehydration from polyuria, and excessive use of ketones as an alternative energy source due to glucose starvation. In the elderly, impaired insulin secretion, decreased renal function, and mitigated glucose control, along with acute conditions such as nutritional deficiencies, disorders of the thirst center, concurrent use of diuretics, and infections, can all be risk factors for euglycemic DKA, significantly increasing the vulnerability of this population [45]. Fig. 1 summarizes the various factors contributing to the onset and progression of diabetes in the elderly.
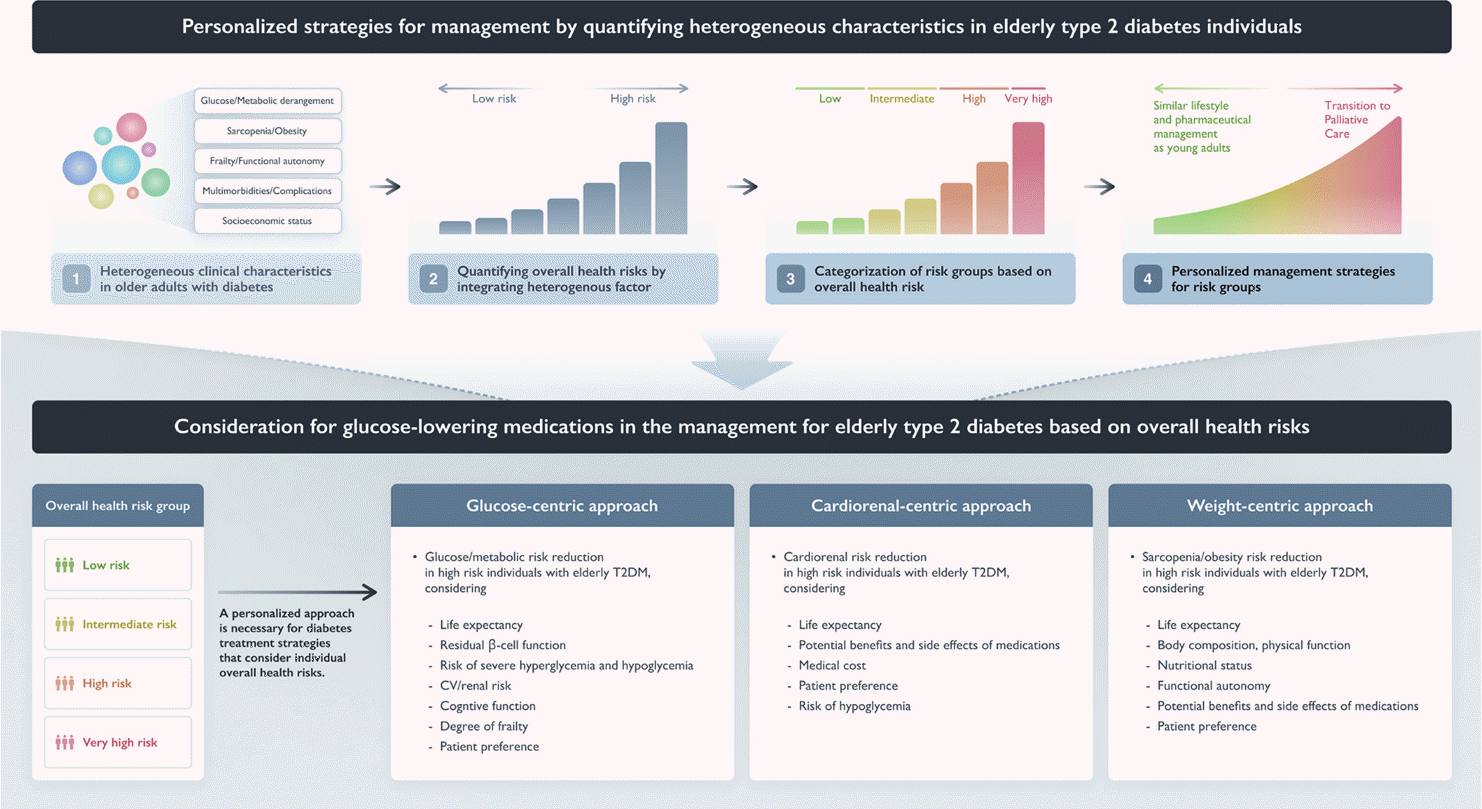
In devising treatment strategies, it is crucial to both target factors related to the pathophysiological pathways of diabetes and address risk factors that can significantly impact the patient’s ability to manage their condition effectively. This approach includes the development of tools that synthesize the characteristics of the heterogeneous elderly population-based on the main risk factors for diabetes in this group. Additionally, there is a need to reorganize existing diabetes management approaches for the general adult population to consider the individual overall health status of elderly patients, particularly those in high-risk health groups. However, evidence supporting treatment strategies for elderly patients with diabetes who are highly vulnerable to complications remains limited.
Effectively managing glycemic care in elderly diabetes patients involves controlling hyperglycemia to suppress the development of acute and chronic complications, reducing the risk of hypoglycemic events, improving quality of life, and preserving function. The balance of these factors must be appropriately adjusted according to the characteristics of each patient. Among Korean elderly diabetes patients, the recognition rate is 76%, the treatment rate is 73%, and the control rate is 77% based on achieving glycosylated hemoglobin (HbA1c) levels below 7.5%. These rates are higher than those in the general adult population [46]. In addition to balancing treatment goals, healthcare professionals managing diabetes in the elderly must consider the heterogeneity of this patient population when setting goals and priorities [47]. Factors to consider include overall health risk and life expectancy, the function of remaining β-cells, the risk of severe hyperglycemia and hypoglycemia, cardiovascular risk, renal function, cognitive function, use of multiple pharmaceuticals, degree of frailty, knowledge of diabetes care, desired treatment intensity, and patient preferences [48,49].
In general, healthy elderly patients without comorbidities that limit life expectancy can benefit from intensive glycemic control, which helps prevent various complications. However, as life expectancy decreases and comorbidities develop, the benefits of intensive glycemic control diminish. Therefore, multiple guidelines emphasize setting individualized HbA1c level targets based on individual health conditions such as life expectancy, duration of diabetes, and the presence of complex comorbidities; such targets may range from 6.5% to 9.0%. At the same time, the standards for the various guidelines are heterogeneous and ambiguous, and most of the evidence is based on expert opinions rather than concrete research findings (Table 1) [49-55].
In real-world clinical practice, assessing diabetes status in the elderly and determining the ideal extent of treatment remains ambiguous. Establishing an optimal glucose-centered treatment strategy is challenging due to the difficulty of including very elderly or frail patients in large-scale clinical trials [7]. Most glucose-lowering medications do not directly address age-related functional decline in glycemic control. In many elderly patients, attenuated defense mechanisms against hypoglycemia and impaired mechanisms for suppressing hyperglycemia result in significant glycemic variability and a narrow optimal glycemic control range. Additionally, the necessity for individualized glycemic control plans makes it challenging to classify elderly people with diabetes into appropriate subgroups. Recently, attempts have been made to evaluate vulnerability and mortality risk in the elderly using frailty indicators, clinical factors, and multiple disease indices [56-58]. Using data from 275,000 veterans with diabetes aged 65 years or older in the United States, researchers estimated 5- and 10-year mortality; the respective C-index statistics of 0.74 and 0.76 indicated excellent performance [39]. In another study, the Life Expectancy Estimator for Older Adults with Diabetes (LEAD) tool was developed based on electronic health records data and achieved C-index values of 0.78 to 0.81, representing excellent discriminative performance [59]. These indicators can help objectively quantify and assess life expectancy and vulnerability to diabetes in the elderly, and thereby facilitate development of individualized treatment goals that balance the benefits and risks of treatment intensification. In particular, these tools can serve as a useful starting point for clinical decisions about selecting less stringent glycemic targets for vulnerable patients [49]. However, research has not yet advanced to the point of developing tools that support clinical decision-making for setting optimal glycemic control targets in elderly diabetes patients. Additionally, the tools that currently exist were developed for Western populations and may not be applicable to Asian populations due to racial, regional, and social differences. Therefore, there is yet a need for the development of new tools tailored to different populations.
The latest trend in managing diabetes in the general adult population emphasizes reducing the risk of death by prioritizing risk management among patients who have cardiovascular or renal disease or are in a high-risk group. Given that many elderly T2DM patients have a high-risk of cardiovascular disease, including renal dysfunction, treatment strategies aimed at managing cardiovascular disease risk factors must not be overlooked for this population. In elderly diabetes patients, factors to consider for reducing the risk of cardiovascular disease include overall health risk, life expectancy, potential benefits and side effects of medications, medical costs, and patient preference. Hypoglycemic events in T2DM patients can contribute to the progression of cardiovascular or renal deterioration; therefore, minimizing risk of hypoglycemia is of paramount importance in elderly T2DM patients [60,61].
SGLT2 inhibitors and GLP-1 receptor agonists (GLP-1 RAs) have demonstrated excellent benefits for cardiovascular and renal diseases in numerous clinical trials; furthermore, there is continuously increasing evidence that those benefits extend to individuals over 65 years of age. In a meta-analysis confirming the effectiveness and safety of SGLT2 inhibitors and GLP-1 RAs in elderly diabetes, 9.6% of included subjects were over 75 years of age. The analysis found that, similar to those over 65 years of age, treatment of these over 75 patients with GLP-1 RAs and SGLT2 inhibitors significantly reduced major adverse cardiovascular events, heart failure, and composite renal outcomes [62]. Indeed, the cardiorenal benefits from these medications may be more pronounced in the elderly because the absolute incidence of cardiovascular and kidney diseases increases with age [63]. Additionally, the included clinical studies found no evidence of safety concerns specifically in the elderly.
However, there are many considerations when applying these treatments to the elderly in the real world. For example, the diuretic effects of SGLT2 inhibitors may make elderly patients vulnerable to polyuria, frequent urination, dehydration, and orthostatic hypotension. This vulnerability may be further increased in individuals at risk for heart failure, especially patients taking concomitant medications that contain diuretics. Severe nocturia can lead to sleep deprivation, decreased quality of life, and an increased risk of falls [64]. Additionally, some patients may have difficulty expressing these symptoms properly. The increased risk of genital and urinary tract infections (UTIs) in the elderly should also be considered. It is well known that elderly diabetes patients are vulnerable to asymptomatic bacteriuria and recurrent UTIs, which can lead to increased risk of hospitalization due to sepsis as the UTI and acute pyelonephritis progress. In addition, the U.S. Food and Drug Administration warns that Fournier’s syndrome may rarely occur after the use of SGLT2 inhibitors, it is important to emphasize hygiene management after urination when prescribing these medications. As mentioned earlier, caution is needed concerning DKA, which can occur in elderly patients, necessitating appropriate nutritional support and early prevention of risk factors [65]. A meta-analysis of the side effects of six clinical studies using SGLT2 inhibitors, published in 2023, found these medications to lower the risk of acute kidney injury and to be neutral in terms of risk of fractures, UTIs, dehydration, and DKA. However, there was an increased risk of genital tract infections [66]. It must be taken into account that elderly individuals aged 75 years or older may have been under-represented and under-reported in the relevant clinical studies. Furthermore, evaluations focusing on major cardiovascular and renal endpoints often overlooked other outcomes important in the elderly, such as disability, frailty, and quality of life.
GLP-1 RAs are administered as injectable medications, for which it is necessary that the patient maintain appropriate cognitive and functional status. With regard to GLP-1 RAs, clinical research reports indicate that 40% of patients initially experience nausea, vomiting, and diarrhea, and some may experience severe gastrointestinal disorders, making them a less preferred treatment for frail elderly patients [67]. A meta-analysis found no significant difference between elderly and young patients in either overall adverse events or serious adverse events. However, the rate of discontinuation due to adverse events was high among the elderly [68]. Therefore, when starting GLP-1 RA treatment in an elderly patient, it is important to understand patient factors and carefully monitor and manage the occurrence of side effects. In an elderly patient, it is important to comprehensively determine whether cardiovascular and renal benefits can be sufficiently secured, taking into account overall frailty, functional status, and comorbidities, and to carefully monitor and manage the occurrence of side effects.
In elderly people with diabetes, muscle mass and weight are crucial treatment factors that affect frailty, quality of life, and clinical outcomes. Factors to consider for managing weight and sarcopenia in elderly diabetes patients include body composition, physical functions, functional autonomy, nutritional status, medical costs, and potential benefits and side effects of medications for weight management. Even in the elderly, obesity worsens insulin resistance and is associated with the onset and progression of various cardiovascular and other major diseases. Generally, SGLT2 inhibitors and GLP-1 RAs may be more suitable drugs for obese elderly diabetes patients.
In the Semaglutide Effects on Cardiovascular Outcomes in People with Overweight or Obesity (SELECT) trial, which included subjects without overt diabetes (including pre-diabetes) aged 61.6 years on average, use of semaglutide by obese subjects of significantly older age resulted in a 10% weight loss, improvement in metabolic indicators, and significantly reduced risk of cardiovascular disease [69]. In the recently announced Semaglutide Treatment Effect in People with Obesity and Heart Failure with Preserved Ejection Fraction and Diabetes Mellitus (STEP-HFpEF DM) trial, use of semaglutide in subjects with an average age of 70 improved heart failure-related symptoms and physical limitations, and further induced a weight reduction of 6.4 kg compared to placebo [70]. A subanalysis of clinical studies, including the Study of Tirzepatide versus Semaglutide Once Weekly as Add-on Therapy to Metformin in Participants with Type 2 Diabetes (SURPASS) trial in East Asians, found that use of tirzepatide in diabetes subjects aged 65 years or older reduced body mass index by an average of 2 to 4 kg/m², regardless of whether they were obese. However, among subjects receiving a high dose of 10 to 15 mg, the rate of reaching underweight was significantly higher; accordingly, dose adjustment in elderly East Asian patients should be considered to prevent progression to underweight [71]. The rate of obesity and abdominal obesity in the Korean elderly population is quite high, but underweight and sarcopenia may have greater impacts on mortality and functional maintenance in those with diabetes [39,72]. There is still insufficient evidence from clinical studies regarding the effects of SGLT2 inhibitors and GLP-1 RAs on muscle. However, if muscle is lost along with body fat due to insufficient energy intake or excessive catabolism, the aging process may be accelerated, resulting in a loss that outweighs the benefits gained from drug use [73]. Losing weight at the expense of lean body mass can be counterproductive in patients with sarcopenia and is undesirable in frail elders with anorexia and malnutrition. Finally, although the once-weekly administration of drugs in the GLP-1 RA class is convenient, their high cost may limit their use in socio-economically vulnerable elderly people.
Despite ongoing research efforts, many challenges remain in solving elderly diabetes in the elderly. First, to fully understand the status of diabetes in the elderly, it is necessary to conduct a sufficiently large-scale, population-based status analysis using available health and medical big data. In addition to prevalence, awareness rate, and control rate, this analysis should include factors such as body composition indicators (e.g., sarcopenia, sarcopenic obesity, and underweight), cognitive function, mental health, physical function, lifestyle habits, income level, the status of socio-economically vulnerable subjects, excessive medical use including polypharmacy, and major concomitant diseases. Second, a tool is needed to classify the heterogeneous characteristics of elderly diabetes patients into appropriate subgroups (Fig. 2). This classification should consider frailty status, disease severity, residual pancreatic function, cardiorenal risk, cognitive function, medication use, and socioeconomic factors. However, no large-scale, comprehensive dataset measuring all these variables currently exists. An integrated healthcare big data platform utilizing linked data from the Korea Disease Control and Prevention Agency, Statistics Korea, Health Insurance Review and Assessment Service, National Health Insurance Service, and individual hospitals can be used to develop a health risk score that objectifies the vulnerability of the elderly. If a more advanced dataset can be built in the future, it can be used to strengthen the health risk prediction and classification system. For elderly patients with advanced diabetes, the intensity of drug treatment should be gradually reduced, and treatment focus should shift to caregiving to improve quality of life for the remainder of their time. It is important to determine the optimal ranges of blood glucose, blood pressure, and lipid levels that maintain function and quality of life while minimizing damage from hyperglycemia and hypoglycemia. Moreover, risks and functional vulnerabilities for cardiovascular and renal disease must be identified, and optimal metabolic control goals and drug use strategies tailored to those conditions must be established. Quality of life can be further improved by reducing polypharmacy as much as possible and simplifying overly complex treatment regimens. Elderly diabetes patients with multiple comorbidities are at very high-risk of frequent hospitalizations due to acute illnesses. Individualized strategies for inpatient and aftercare are needed for these patients, focusing on glycemic management, polypharmacy, delirium, and fall prevention. In addition, there is a need to develop multifactorial interventions tailored to overcoming frailty, such as exercise, nutrition, and support interventions. Lastly, although many Western research results have been published, these findings cannot be directly applied to Koreans due to racial, regional, and social differences. Therefore, efforts will be needed to establish and analyze data on diabetes in the elderly in Korea to determine optimal management strategies for this population.
The increasing prevalence of elderly T2DM highlights increases in risk factors such as previous obesity and inactivity, as well as improvements in general healthcare that extend life expectancy. The diverse phenotypes of T2DM in the elderly are often accompanied by frailty, necessitating a highly individualized management approach. Comorbidities prevalent in this population may limit treatment options, and drug contraindications must be carefully considered. As a result, treatment goals for the elderly are often less stringent, intended to prevent hypoglycemia and minimize lifestyle changes that may not provide significant benefit within the expected lifespan. The presence of frailty, sarcopenia, severe life-limiting conditions, cognitive decline, and functional impairment greatly influences management strategies. Therefore, individualized treatment plans must balance the benefits and risks of intensive glycemic control with the goal of preventing complications of hyperglycemia and hypoglycemia while maintaining quality of life. Additionally, developing effective and tailored exercise and nutritional interventions is essential to combat frailty in this population. Lastly, it is important to build and analyze data tailored to the Korean population to optimize diabetes management strategies for the specific needs of the country’s elderly population.
Notes
ACKNOWLEDGMENTS
We would like to express our gratitude to Minji Kim for her invaluable assistance with the creation of the figures and the graphical abstract.
REFERENCES
1. Kontis V, Bennett JE, Mathers CD, Li G, Foreman K, Ezzati M. Future life expectancy in 35 industrialised countries: projections with a Bayesian model ensemble. Lancet. 2017; 389:1323–35.

2. Statistics Korea. 2024 Statistics Korea. Available from: http://kostat.go.kr (cited 2024 Jul 11).
3. GBD 2019 viewpoint collaborators. Five insights from the global burden of disease study 2019. Lancet. 2020; 396:1135–59.
4. Bae JH, Han KD, Ko SH, Yang YS, Choi JH, Choi KM, et al. Diabetes fact sheet in Korea 2021. Diabetes Metab J. 2022; 46:417–26.

5. Jeong SM, Jung JH, Yang YS, Kim W, Cho IY, Lee YB, et al. 2023 Obesity fact sheet: prevalence of obesity and abdominal obesity in adults, adolescents, and children in Korea from 2012 to 2021. J Obes Metab Syndr. 2024; 33:27–35.

6. Cho NH, Shaw JE, Karuranga S, Huang Y, da Rocha Fernandes JD, Ohlrogge AW, et al. IDF Diabetes Atlas: global estimates of diabetes prevalence for 2017 and projections for 2045. Diabetes Res Clin Pract. 2018; 138:271–81.

7. Bellary S, Kyrou I, Brown JE, Bailey CJ. Type 2 diabetes mellitus in older adults: clinical considerations and management. Nat Rev Endocrinol. 2021; 17:534–48.
8. Miklavcic JJ, Fraser KD, Ploeg J, Markle-Reid M, Fisher K, Gafni A, et al. Effectiveness of a community program for older adults with type 2 diabetes and multimorbidity: a pragmatic randomized controlled trial. BMC Geriatr. 2020; 20:174.

9. Huang ES, Laiteerapong N, Liu JY, John PM, Moffet HH, Karter AJ. Rates of complications and mortality in older patients with diabetes mellitus: the diabetes and aging study. JAMA Intern Med. 2014; 174:251–8.

10. Sebastian MJ, Khan SK, Pappachan JM, Jeeyavudeen MS. Diabetes and cognitive function: an evidence-based current perspective. World J Diabetes. 2023; 14:92–109.

11. Batsis JA, Villareal DT. Sarcopenic obesity in older adults: aetiology, epidemiology and treatment strategies. Nat Rev Endocrinol. 2018; 14:513–37.

12. Remelli F, Ceresini MG, Trevisan C, Noale M, Volpato S. Prevalence and impact of polypharmacy in older patients with type 2 diabetes. Aging Clin Exp Res. 2022; 34:1969–83.
13. Basu R, Breda E, Oberg AL, Powell CC, Dalla Man C, Basu A, et al. Mechanisms of the age-associated deterioration in glucose tolerance: contribution of alterations in insulin secretion, action, and clearance. Diabetes. 2003; 52:1738–48.
14. Meneilly GS, Elahi D. Metabolic alterations in middle-aged and elderly lean patients with type 2 diabetes. Diabetes Care. 2005; 28:1498–9.

15. Kanaya AM, Harris T, Goodpaster BH, Tylavsky F, Cummings SR; Health, Aging, and Body Composition (ABC) Study. Adipocytokines attenuate the association between visceral adiposity and diabetes in older adults. Diabetes Care. 2004; 27:1375–80.

16. Ferriolli E, Pessanha FP, Marchesi JC. Diabetes and exercise in the elderly. Med Sport Sci. 2014; 60:122–9.

17. Hotamisligil GS. Inflammation and metabolic disorders. Nature. 2006; 444:860–7.
18. Matyka K, Evans M, Lomas J, Cranston I, Macdonald I, Amiel SA. Altered hierarchy of protective responses against severe hypoglycemia in normal aging in healthy men. Diabetes Care. 1997; 20:135–41.

19. Hope SV, Taylor PJ, Shields BM, Hattersley AT, Hamilton W. Are we missing hypoglycaemia?: elderly patients with insulin-treated diabetes present to primary care frequently with nonspecific symptoms associated with hypoglycaemia. Prim Care Diabetes. 2018; 12:139–46.

20. Vellas B, Fielding R, Bhasin S, Cerreta F, Goodpaster B, Guralnik JM, et al. Sarcopenia trials in specific diseases: report by the International Conference on Frailty and Sarcopenia Research Task Force. J Frailty Aging. 2016; 5:194–200.

21. Kalyani RR, Corriere M, Ferrucci L. Age-related and disease-related muscle loss: the effect of diabetes, obesity, and other diseases. Lancet Diabetes Endocrinol. 2014; 2:819–29.

22. Rolland Y, Czerwinski S, Abellan Van Kan G, Morley JE, Cesari M, Onder G, et al. Sarcopenia: its assessment, etiology, pathogenesis, consequences and future perspectives. J Nutr Health Aging. 2008; 12:433–50.
23. Chung SM, Moon JS, Chang MC. Prevalence of sarcopenia and its association with diabetes: a meta-analysis of community-dwelling Asian population. Front Med (Lausanne). 2021; 8:681232.

24. Anagnostis P, Gkekas NK, Achilla C, Pananastasiou G, Taouxidou P, Mitsiou M, et al. Type 2 diabetes mellitus is associated with increased risk of sarcopenia: a systematic review and meta-analysis. Calcif Tissue Int. 2020; 107:453–63.

25. Sinclair AJ, Rodriguez-Manas L. Diabetes and frailty: two converging conditions? Can J Diabetes. 2016; 40:77–83.

26. Wilson D, Jackson T, Sapey E, Lord JM. Frailty and sarcopenia: the potential role of an aged immune system. Ageing Res Rev. 2017; 36:1–10.

27. Sanz-Canovas J, Lopez-Sampalo A, Cobos-Palacios L, Ricci M, Hernandez-Negrin H, Mancebo-Sevilla JJ, et al. Management of type 2 diabetes mellitus in elderly patients with frailty and/or sarcopenia. Int J Environ Res Public Health. 2022; 19:8677.
28. Fried LP, Tangen CM, Walston J, Newman AB, Hirsch C, Gottdiener J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001; 56:M146–56.

29. Ida S, Kaneko R, Imataka K, Murata K. Relationship between frailty and mortality, hospitalization, and cardiovascular diseases in diabetes: a systematic review and meta-analysis. Cardiovasc Diabetol. 2019; 18:81.
30. Nishikawa H, Fukunishi S, Asai A, Yokohama K, Ohama H, Nishiguchi S, et al. Sarcopenia, frailty and type 2 diabetes mellitus (Review). Mol Med Rep. 2021; 24:854.
31. Hopman P, de Bruin SR, Forjaz MJ, Rodriguez-Blazquez C, Tonnara G, Lemmens LC, et al. Effectiveness of comprehensive care programs for patients with multiple chronic conditions or frailty: a systematic literature review. Health Policy. 2016; 120:818–32.

32. Hoogendijk EO. How effective is integrated care for community-dwelling frail older people?: the case of the Netherlands. Age Ageing. 2016; 45:585–8.
33. Bernabei R, Landi F, Calvani R, Cesari M, Del Signore S, Anker SD, et al. Multicomponent intervention to prevent mobility disability in frail older adults: randomised controlled trial (SPRINTT project). BMJ. 2022; 377:e068788.

34. Melis R, Marengoni A, Angleman S, Fratiglioni L. Incidence and predictors of multimorbidity in the elderly: a populationbased longitudinal study. PLoS One. 2014; 9:e103120.

35. Wolff JL, Starfield B, Anderson G. Prevalence, expenditures, and complications of multiple chronic conditions in the elderly. Arch Intern Med. 2002; 162:2269–76.

36. Mazziotti G, Gazzaruso C, Giustina A. Diabetes in Cushing syndrome: basic and clinical aspects. Trends Endocrinol Metab. 2011; 22:499–506.

37. Fried LP, Bandeen-Roche K, Kasper JD, Guralnik JM. Association of comorbidity with disability in older women: the Women’s Health and Aging Study. J Clin Epidemiol. 1999; 52:27–37.
38. Magnan EM, Palta M, Mahoney JE, Pandhi N, Bolt DM, Fink J, et al. The relationship of individual comorbid chronic conditions to diabetes care quality. BMJ Open Diabetes Res Care. 2015; 3:e000080.

39. Griffith KN, Prentice JC, Mohr DC, Conlin PR. Predicting 5- and 10-year mortality risk in older adults with diabetes. Diabetes Care. 2020; 43:1724–31.

40. Yun JS, Han K, Ko SH. Trends of severe hypoglycemia in patients with type 2 diabetes in Korea: a longitudinal nationwide cohort study. J Diabetes Investig. 2022; 13:1438–43.
41. Yun JS, Ko SH. Risk factors and adverse outcomes of severe hypoglycemia in type 2 diabetes mellitus. Diabetes Metab J. 2016; 40:423–32.

42. Holstein A, Plaschke A, Egberts EH. Clinical characterisation of severe hypoglycaemia: a prospective population-based study. Exp Clin Endocrinol Diabetes. 2003; 111:364–9.
43. Cryer PE. Mechanisms of hypoglycemia-associated autonomic failure in diabetes. N Engl J Med. 2013; 369:362–72.

44. Yun JS, Ko SH. Avoiding or coping with severe hypoglycemia in patients with type 2 diabetes. Korean J Intern Med. 2015; 30:6–16.

46. Ko SH, Han KD, Park YM, Yun JS, Kim K, Bae JH, et al. Diabetes mellitus in the elderly adults in Korea: based on data from the Korea National Health and Nutrition Examination Survey 2019 to 2020. Diabetes Metab J. 2023; 47:643–52.

47. Strain WD, Hope SV, Green A, Kar P, Valabhji J, Sinclair AJ. Type 2 diabetes mellitus in older people: a brief statement of key principles of modern day management including the assessment of frailty: a national collaborative stakeholder initiative. Diabet Med. 2018; 35:838–45.
48. Sinclair A, Morley JE, Rodriguez-Manas L, Paolisso G, Bayer T, Zeyfang A, et al. Diabetes mellitus in older people: position statement on behalf of the International Association of Gerontology and Geriatrics (IAGG), the European Diabetes Working Party for Older People (EDWPOP), and the International Task Force of Experts in Diabetes. J Am Med Dir Assoc. 2012; 13:497–502.

49. American Diabetes Association Professional Practice Committee. 13. Older adults: standards of care in diabetes-2024. Diabetes Care. 2024; 47(Suppl 1):S244–57.
50. Korean Diabetes Association. Clinical practice guidelines for diabetes 2023. 8th ed. Seoul: KDA;2023.
51. Garber AJ, Abrahamson MJ, Barzilay JI, Blonde L, Bloomgarden ZT, Bush MA, et al. Consensus statement by the American Association of Clinical Endocrinologists and American College of Endocrinology on the comprehensive type 2 diabetes management algorithm: 2019 executive summary. Endocr Pract. 2019; 25:69–100.
52. LeRoith D, Biessels GJ, Braithwaite SS, Casanueva FF, Draznin B, Halter JB, et al. Treatment of diabetes in older adults: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2019; 104:1520–74.

53. Bourdel-Marchasson I, Maggi S, Abdelhafiz A, Bellary S, Demurtas J, Forbes A, et al. Essential steps in primary care management of older people with type 2 diabetes: an executive summary on behalf of the European Geriatric Medicine Society (EuGMS) and the European Diabetes Working Party for Older People (EDWPOP) collaboration. Aging Clin Exp Res. 2023; 35:2279–91.

54. Araki E, Goto A, Kondo T, Noda M, Noto H, Origasa H, et al. Japanese clinical practice guideline for diabetes 2019. J Diabetes Investig. 2020; 11:1020–76.
55. Diabetes Canada Clinical Practice Guidelines Expert Committee, Meneilly GS, Knip A, Miller DB, Sherifali D, Tessier D, et al. Diabetes in older people. Can J Diabetes. 2018; 42 Suppl 1:S283–95.

56. Lee AK, Diaz-Ramirez LG, Boscardin WJ, Smith AK, Lee SJ. A comprehensive prognostic tool for older adults: predicting death, ADL disability, and walking disability simultaneously. J Am Geriatr Soc. 2022; 70:2884–94.
57. Deardorff WJ, Barnes DE, Jeon SY, Boscardin WJ, Langa KM, Covinsky KE, et al. Development and external validation of a mortality prediction model for community-dwelling older adults with dementia. JAMA Intern Med. 2022; 182:1161–70.

58. Deardorff WJ, Jeon SY, Barnes DE, Boscardin WJ, Langa KM, Covinsky KE, et al. Development and external validation of models to predict need for nursing home level of care in community-dwelling older adults with dementia. JAMA Intern Med. 2024; 184:81–91.

59. Karter AJ, Parker MM, Moffet HH, Lipska KJ, Laiteerapong N, Grant RW, et al. Development and validation of the Life Expectancy Estimator for Older Adults with Diabetes (LEAD): the diabetes and aging study. J Gen Intern Med. 2023; 38:2860–9.
60. Yun JS, Park YM, Han K, Cha SA, Ahn YB, Ko SH. Severe hypoglycemia and the risk of cardiovascular disease and mortality in type 2 diabetes: a nationwide population-based cohort study. Cardiovasc Diabetol. 2019; 18:103.

61. Yun JS, Park YM, Han K, Kim HW, Cha SA, Ahn YB, et al. Severe hypoglycemia and the risk of end stage renal disease in type 2 diabetes. Sci Rep. 2021; 11:4305.

62. Karagiannis T, Tsapas A, Athanasiadou E, Avgerinos I, Liakos A, Matthews DR, et al. GLP-1 receptor agonists and SGLT2 inhibitors for older people with type 2 diabetes: a systematic review and meta-analysis. Diabetes Res Clin Pract. 2021; 174:108737.

63. Custodio JS Jr, Roriz-Filho J, Cavalcanti CA, Martins A, Salles JE. Use of SGLT2 inhibitors in older adults: scientific evidence and practical aspects. Drugs Aging. 2020; 37:399–409.

64. Kim SY, Bang W, Kim MS, Park B, Kim JH, Choi HG. Nocturia is associated with slipping and falling. PLoS One. 2017; 12:e016–9690.
65. Handelsman Y, Henry RR, Bloomgarden ZT, Dagogo-Jack S, DeFronzo RA, Einhorn D, et al. American Association of Clinical Endocrinologists and American College of Endocrinology position statement on the association of SGLT-2 inhibitors and diabetic ketoacidosis. Endocr Pract. 2016; 22:753–62.

66. Rigato M, Fadini GP, Avogaro A. Safety of sodium-glucose cotransporter 2 inhibitors in elderly patients with type 2 diabetes: a meta-analysis of randomized controlled trials. Diabetes Obes Metab. 2023; 25:2963–9.

67. Frias JP, Davies MJ, Rosenstock J, Perez Manghi FC, Fernandez Lando L, Bergman BK, et al. Tirzepatide versus semaglutide once weekly in patients with type 2 diabetes. N Engl J Med. 2021; 385:503–15.

68. Wang Y, Wang J, Gong Q, Wu H, Yang S, He J, et al. Efficacy and safety of glucagon-like peptide-1 receptor agonists in the elderly versus non-elderly patients with type 2 diabetes mellitus: insights from a systematic review. Endocr J. 2024; 71:571–82.

69. Lincoff AM, Brown-Frandsen K, Colhoun HM, Deanfield J, Emerson SS, Esbjerg S, et al. Semaglutide and cardiovascular outcomes in obesity without diabetes. N Engl J Med. 2023; 389:2221–32.
70. Kosiborod MN, Petrie MC, Borlaug BA, Butler J, Davies MJ, Hovingh GK, et al. Semaglutide in patients with obesity-related heart failure and type 2 diabetes. N Engl J Med. 2024; 390:1394–407.

71. Kiyosue A, Dunn JP, Cui X, Hickey A, Hirase T, Imaoka T, et al. Safety and efficacy analyses across age and body mass index subgroups in East Asian participants with type 2 diabetes in the phase 3 tirzepatide studies (SURPASS programme). Diabetes Obes Metab. 2023; 25:1056–67.
72. Korean Society for the Study of Obesity. Obesity fact sheet in Korea 2023. https://www.kosso.or.kr/popup/obesity_fact_sheet.html (cited 2024 Jul 12).
Fig. 1.
Factors contributing to the onset and worsening of type 2 diabetes mellitus in the elderly. IGF-1, insulin-like growth factor-1; AGE, advanced glycation end product; ROS, reactive oxygen species; ESKD, end-stage kidney disease.
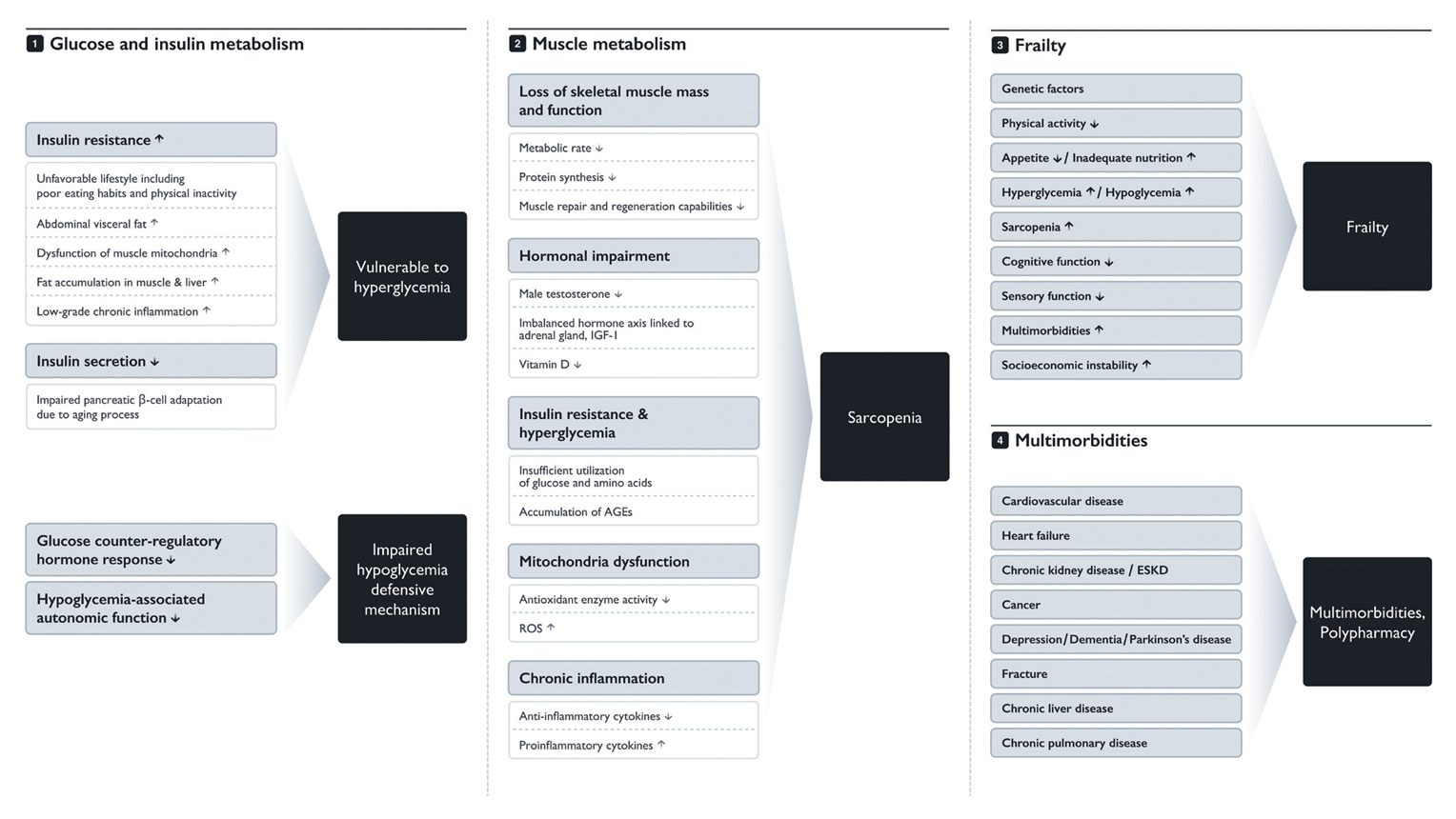
Fig. 2.
Personalized strategies for managing elderly diabetes based on overall health risks and considerations for glucose-lowering medications in the treatment of elderly type 2 diabetes mellitus (T2DM). CV, cardiovascular; SGLT2, sodium glucose co-transporter 2; GLP-1 RA, glucagon-like peptide-1 receptor agonist; UTI, urinary tract infection; GTI, genital tract infection; DKA, diabetic ketoacidosis; DM, diabetes mellitus.
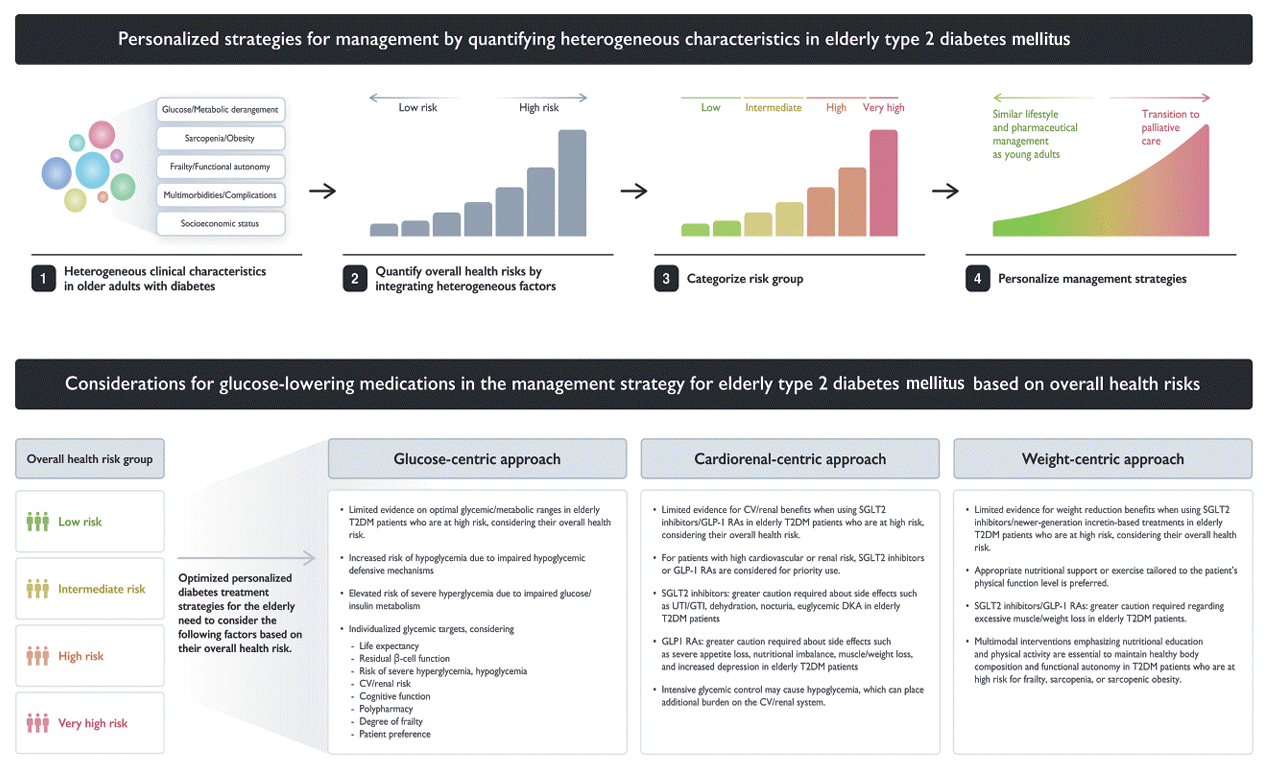
Table 1.
Summary of glycemic target recommendations for elderly individuals with T2DM
| Guideline and subgroups | Group definition | Glycemic target | Clinical consideration |
|---|---|---|---|
| 2023 KDA guideline [50] | |||
| General elderly T2DM | HbA1c <7.5% | Consider individualizing treatment based on health status or frailty level. | |
| Healthy older adults may maintain similar glycemic goals as younger adults. | |||
| In elderly patients with multiple comorbidities and challenging functional impairments, glycemic targets can be tailored. | |||
| For patients nearing the end of life, prioritize minimal treatment focused on managing symptoms caused by hyperglycemia. | |||
| 2024 ADA guideline [49] | |||
| Healthy | Few coexisting chronic illnesses, intact cognitive and functional status | HbA1c <7.0%–7.5% | |
| Fasting or preprandial glucose 80–130 mg/dL | |||
| Bedtime glucose 80–180 mg/dL | |||
| Complex/intermediate | Multiple coexisting chronic illnesses or two or more instrumental ADL impairments or mild to moderate cognitive impairment | HbA1c <8.0% | |
| Fasting or preprandial glucose 90–150 mg/dL | |||
| Bedtime glucose 100–180 mg/dL | |||
| Very complex/poor health | LTC or end-stage chronic illnesses or moderate to severe cognitive impairment or two or more ADL impairments | Avoid reliance on HbA1c | Glucose control decisions should be based on avoiding hypoglycemia and symptomatic hyperglycemia. |
| Fasting or preprandial glucose 100–180 mg/dL | |||
| Bedtime glucose 110–220 mg/dL | |||
| 2023 EuGMS-EDWPOP [53] | |||
| General elderly group | Without frailty or dementia and without significant associated medical comorbidities | HbA1c <7.0%–7.5% | Consider deprescribing antidiabetic medications if the patient’s HbA1c is below 6.5% or below 7.0% in the presence of frailty. |
| Older adults with T2DM, especially those with dementia, moderate to severe frailty, significant renal impairment, or high multimorbidity, may benefit from a deprescribing approach. | |||
| Older adults with T2DM experiencing frequent hypoglycemia on complex insulin regimens should be reassessed for potential deprescribing. | |||
| 2019 Endocrine Society [52] | |||
| Good health | ≤1 IADL impairment and no ADL impairment | Low risk of hypoglycemia | The glucose targets are flexible within each group based on individual circumstances. |
| Intact cognitive status | HbA1c <7.5% | ||
| 0–2 chronic illnesses | Fasting glucose 90–130 mg/dL | Coexisting chronic illnesses include conditions like osteoarthritis, hypertension, chronic kidney disease stages 1–3, or stroke. | |
| Bedtime glucose 90–150 mg/dL | |||
| High risk of hypoglycemia | End-stage conditions include terminal cancer, advanced heart failure, and other serious illnesses. | ||
| HbA1c 7.0%–7.5% | |||
| Fasting glucose 90–150 mg/dL | ADLs include basic activities like bathing, dressing, and eating; IADLs include managing money, shopping, and using the telephone. | ||
| Bedtime glucose 100–180 mg/dL | |||
| Intermediate health | ≥2 IADL impairment | Low risk of hypoglycemia | |
| Mild cognitive impairment or early dementia | HbA1c <8.0% | ||
| Fasting glucose 90–150 mg/dL | |||
| ≥3 chronic illnesses | Bedtime glucose 100–180 mg/dL | ||
| High risk of hypoglycemia | |||
| HbA1c 7.5%–8.0% | |||
| Fasting glucose 100–150 mg/dL | |||
| Bedtime glucose 150–180 mg/dL | |||
| Poor health | ≥2 IADL impairment | Low risk of hypoglycemia | |
| Moderate to severe dementia | HbA1c <8.5% | ||
| End-stage illnesses | Fasting glucose 100–180 mg/dL | ||
| Long-term care | Bedtime glucose 110–200 mg/dL | ||
| High risk of hypoglycemia | |||
| HbA1c 8.0%–8.5% | |||
| Fasting glucose 100–180 mg/dL | |||
| Bedtime glucose 150–250 mg/dL | |||
| 2019 JDS [54] | |||
| Category I | Intact cognitive function | Low risk of hypoglycemia | |
| No impairment of ADL | HbA1c <7.0% | ||
| High risk of hypoglycemia | |||
| Age 65–74 years: HbA1c <7.5% | |||
| Age ≥75 years: HbA1c <8.0% | |||
| Category II | Mild cognitive impairment to mild dementia | Low risk of hypoglycemia | High-risk of hypoglycemia: use of drugs potentially associated with severe hypoglycemia, e.g., insulin formulation, sulfonylurea, glinides. |
| Impairment of instrumental ADL/no impairment of basic ADL | HbA1c <7.0% | ||
| High risk of hypoglycemia | In end-of-life care, priority is to be given to preventing significant hyperglycemia and subsequent dehydration and acute complications through appropriate therapeutic measures. | ||
| HbA1c 7.0%–8.0% | |||
| Category III | Moderate or severe dementia | Low risk of hypoglycemia | For patients categorized as type I, targets can be individualized: <6.0% for those managing well with diet/exercise or medications without side effects, and up to 8.0% if intensifying therapy is challenging. |
| Impairment of basic ADL presence of multiple comorbidities or functional impairments | HbA1c <8.0% | ||
| High risk of hypoglycemia | For patients categorized as type III, especially those with serious comorbidities, those with poor social support, and those at risk of developing adverse reactions to multi-drug combination therapy, a glycemic target of <8.5% may be allowed. | ||
| HbA1c 7.5%–8.5% | |||
| 2019 Canadian Diabetes Association [55] | |||
| Functionally independent | Clinical frailty index 1–3 | HbA1c ≤7.0% | |
| Functionally dependent | Clinical frailty index 4–5 | Low risk of hypoglycemia | |
| HbA1c <8.0% | |||
| High risk of hypoglycemia | |||
| HbA1c 7.1%–8.0% | |||
| Frail and/or with dementia | Clinical frailty index 6–8 | Low risk of hypoglycemia | |
| HbA1c <8.5% | |||
| High risk of hypoglycemia | |||
| HbA1c 7.1%–8.5% | |||
| End of life | Clinical frailty index 9 | HbA1c measurement not recommended. |
T2DM, type 2 diabetes mellitus; KDA, Korean Diabetes Association; HbA1c, glycosylated hemoglobin; ADA, American Diabetes Association; ADL, activity of daily living; EuGMS-EDWPOP, European Geriatric Medicine Society-European diabetes working party for older people; LTC, long-term care; IADL, instrumental activity of daily living; JDS, Japan Diabetes Society.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download