1. Oh JY, Lim S, Kim DJ, Kim NH, Kim DJ, Moon SD, et al. The diagnosis of diabetes mellitus in Korea: a pooled analysis of four community-based cohort studies. Diabet Med. 2007; 24:217–8.

2. Oh JY, Lim S, Kim DJ, Kim NH, Kim DJ, Moon SD, et al. A report on the diagnosis of intermediate hyperglycemia in Korea: a pooled analysis of four community-based cohort studies. Diabetes Res Clin Pract. 2008; 80:463–8.

3. American Diabetes Association. Standards of medical care in diabetes: 2010. Diabetes Care. 2010; 33 Suppl 1:S11–61.
4. International Diabetes Federation Clinical Guidelines Task Force. Global guideline for type 2 diabetes. Brussels: International Diabetes Federation;2005.
5. World Health Organization Regional Office for the Western Pacific. Type 2 diabetes: practical targets and treatments. 4th ed. Melbourne: International Diabetes Institute;2005.
6. World Health Organization Department of Noncommunicable Disease Surveillance. Definition, diagnosis, and classification of diabetes mellitus and its complications: report of a WHO consultation. Geneva: World Health Organization;1999.
7. World Health Organization, Chronic Respiratory Diseases and Arthritis Team. Screening for type 2 diabetes: report of a World Health Organization and International Diabetes Federation meeting. Geneva: World Health Organization;2003.
8. Committee of the Japan Diabetes Society on the Diagnostic Criteria of Diabetes Mellitus, Seino Y, Nanjo K, Tajima N, Kadowaki T, Kashiwagi A, et al. Report of the committee on the classification and diagnostic criteria of diabetes mellitus. J Diabetes Investig. 2010; 1:212–28.

9. Ito C, Maeda R, Ishida S, Sasaki H, Harada H. Correlation among fasting plasma glucose, two-hour plasma glucose levels in OGTT and HbA1c. Diabetes Res Clin Pract. 2000; 50:225–30.
10. Ryu S, Shin H, Chang Y, Sung KC, Song J, Lee SJ. Should the lower limit of impaired fasting glucose be reduced from 110 mg/dL in Korea? Metabolism. 2006; 55:489–93.

11. Kim DL, Kim SD, Kim SK, Park S, Song KH. Is an oral glucose tolerance test still valid for diagnosing diabetes mellitus? Diabetes Metab J. 2016; 40:118–28.

12. Kim KS, Kim SK, Lee YK, Park SW, Cho YW. Diagnostic value of glycated haemoglobin HbA(1c) for the early detection of diabetes in high-risk subjects. Diabet Med. 2008; 25:997–1000.

13. Hong S, Kang JG, Kim CS, Lee SJ, Lee CB, Ihm SH. Fasting plasma glucose concentrations for specified HbA1c goals in Korean populations: data from the Fifth Korea National Health and Nutrition Examination Survey (KNHANES V-2, 2011). Diabetol Metab Syndr. 2016; 8:62.

14. Cowie CC, Rust KF, Byrd-Holt DD, Gregg EW, Ford ES, Geiss LS, et al. Prevalence of diabetes and high risk for diabetes using A1C criteria in the U.S. population in 1988-2006. Diabetes Care. 2010; 33:562–8.
15. Lee HC, Huh KB, Hong SK, Roh HJ, Choi BJ, An SH, et al. The prevalence of diabetes mellitus in chronic liver disease. Korean J Med. 1999; 57:281–8.
16. Kim HK, Kim JY, Park JY, Yoo B, Park YS, Lee KU, et al. Clinical typing and characterization of youth-onset diabetic patients in Korea. Korean Diabetes J. 1995; 19:202–7.
17. Lee KU, Ryu JS, Kim YT, Shong YK, Kim GS, Lee M, et al. Clinical characteristics of Korean diabetic patients classified by fasting plasma C-peptide level and degree of obesity. Korean J Med. 1992; 42:315–21.
18. Park M, Kang YI, Chon S, Oh SJ, Woo JT, Kim SW, et al. The clinical characteristics of young onset diabetes according to etiology based classification. Korean Diabetes J. 2006; 30:190–7.

19. Kim CS, Park J, Cho MH, Park JS, Nam JY, Kim DM, et al. Frequency of anti-GAD antibody in non-obese, adult-onset type 2 diabetes in Korea and clinical and biological characteristics according to anti-GAD antibody. Korean Diabetes J. 2004; 28:66–74.
20. Tuomi T, Groop LC, Zimmet PZ, Rowley MJ, Knowles W, Mackay IR. Antibodies to glutamic acid decarboxylase reveal latent autoimmune diabetes mellitus in adults with a non-insulin-dependent onset of disease. Diabetes. 1993; 42:359–62.

21. Lee HC, Kim DH, Nam JH, Ahn CW, Lim SK, Huh KB, et al. Follow-up study of clinical and immunogenetic characteristics and basal C-peptide in Korean young age onset diabetic patients. Korean Diabetes J. 1999; 23:288–98.
22. Schaefer UM, Songster G, Xiang A, Berkowitz K, Buchanan TA, Kjos SL. Congenital malformations in offspring of women with hyperglycemia first detected during pregnancy. Am J Obstet Gynecol. 1997; 177:1165–71.

23. Tennant PW, Glinianaia SV, Bilous RW, Rankin J, Bell R. Preexisting diabetes, maternal glycated haemoglobin, and the risks of fetal and infant death: a population-based study. Diabetologia. 2014; 57:285–94.

24. Tehrani FR, Naz MSG, Bidhendi-Yarandi R, Behboudi-Gandevani S. Effect of different types of diagnostic criteria for gestational diabetes mellitus on adverse neonatal outcomes: a systematic review, meta-analysis, and meta-regression. Diabetes Metab J. 2022; 46:605–19.
25. Yefet E, Jeda E, Tzur A, Nachum Z. Markers for undiagnosed type 2 diabetes mellitus during pregnancy: a population-based retrospective cohort study. J Diabetes. 2020; 12:205–14.
26. Murphy HR, Roland JM, Skinner TC, Simmons D, Gurnell E, Morrish NJ, et al. Effectiveness of a regional prepregnancy care program in women with type 1 and type 2 diabetes: benefits beyond glycemic control. Diabetes Care. 2010; 33:2514–20.

27. Wahabi HA, Fayed A, Esmaeil S, Elmorshedy H, Titi MA, Amer YS, et al. Systematic review and meta-analysis of the effectiveness of pre-pregnancy care for women with diabetes for improving maternal and perinatal outcomes. PLoS One. 2020; 15:e0237571.

28. Kim SY. Min Heon Ki’s clinical endocrinology. 3rd ed. Seoul: Korea Medicine;2006. Chapter 40. Gestational diabetes.
29. Jang HC, Cho YM, Park KS, Kim SY, Lee HK, Kim MY, et al. Pregnancy outcome in Korean women with gestational diabetes mellitus diagnosed by the Carpenter-Coustan criteria. J Korean Diabetes Assoc. 2004; 28:122–30.
30. HAPO Study Cooperative Research Group, Metzger BE, Lowe LP, Dyer AR, Trimble ER, Chaovarindr U, et al. Hyperglycemia and adverse pregnancy outcomes. N Engl J Med. 2008; 358:1991–2002.

31. International Association of Diabetes and Pregnancy Study Groups Consensus Panel, Metzger BE, Gabbe SG, Persson B, Buchanan TA, Catalano PA, et al. International association of diabetes and pregnancy study groups recommendations on the diagnosis and classification of hyperglycemia in pregnancy. Diabetes Care. 2010; 33:676–82.

32. Kim MH, Kwak SH, Kim SH, Hong JS, Chung HR, Choi SH, et al. Pregnancy outcomes of women additionally diagnosed as gestational diabetes by the International Association of the Diabetes and Pregnancy Study Groups Criteria. Diabetes Metab J. 2019; 43:766–75.

33. Hillier TA, Pedula KL, Ogasawara KK, Vesco KK, Oshiro CES, Lubarsky SL, et al. A pragmatic, randomized clinical trial of gestational diabetes screening. N Engl J Med. 2021; 384:895–904.

34. Crowther CA, Samuel D, McCowan LME, Edlin R, Tran T, McKinlay CJ, et al. Lower versus higher glycemic criteria for diagnosis of gestational diabetes. N Engl J Med. 2022; 387:587–98.
35. Bae JH, Han KD, Ko SH, Yang YS, Choi JH, Choi KM, et al. Diabetes fact sheet in Korea 2021. Diabetes Metab J. 2022; 46:417–26.

36. Yang YS, Han BD, Han K, Jung JH, Son JW; Taskforce Team of the Obesity Fact Sheet of the Korean Society for the Study of Obesity. Obesity fact sheet in Korea, 2021: trends in obesity prevalence and obesity-related comorbidity incidence stratified by age from 2009 to 2019. J Obes Metab Syndr. 2022; 31:169–77.

37. American Diabetes Association Professional Practice Committee. 2. Classification and diagnosis of diabetes: standards of medical care in diabetes-2022. Diabetes Care. 2022; 45(Suppl 1):S17–38.
38. Ha KH, Lee KA, Han KD, Moon MK, Kim DJ. Diabetes screening in South Korea: a new estimate of the number needed to screen to detect diabetes. Korean J Intern Med. 2023; 38:93–100.

39. Kim JH, Lim JS. Trends of diabetes and prediabetes prevalence among Korean adolescents from 2007 to 2018. J Korean Med Sci. 2021; 36:e112.

40. Gillett MJ. International Expert Committee report on the role of the A1c assay in the diagnosis of diabetes: Diabetes Care 2009; 32(7): 1327-1334. Clin Biochem Rev. 2009; 30:197–200.
41. Saudek CD, Herman WH, Sacks DB, Bergenstal RM, Edelman D, Davidson MB. A new look at screening and diagnosing diabetes mellitus. J Clin Endocrinol Metab. 2008; 93:2447–53.

42. Bennett CM, Guo M, Dharmage SC. HbA(1c) as a screening tool for detection of type 2 diabetes: a systematic review. Diabet Med. 2007; 24:333–43.
43. Kim JM, Hong JW, Won JC, Noh JH, Ko KS, Rhee BD, et al. Glycated hemoglobin value for fasting plasma glucose of 126 mg/dL in Korean: the 2011 Korea National Health and Nutrition Examination Survey. Diabetes Metab J. 2014; 38:480–3.

44. Lee H, Oh JY, Sung YA, Kim DJ, Kim SH, Kim SG, et al. Optimal hemoglobin A1C cutoff value for diagnosing type 2 diabetes mellitus in Korean adults. Diabetes Res Clin Pract. 2013; 99:231–6.

45. Lee JH, Chon S, Cha SA, Lim SY, Kim KR, Yun JS, et al. Impaired fasting glucose levels in overweight or obese subjects for screening of type 2 diabetes in Korea. Korean J Intern Med. 2021; 36:382–91.
46. Araki E, Goto A, Kondo T, Noda M, Noto H, Origasa H, et al. Japanese clinical practice guideline for diabetes 2019. J Diabetes Investig. 2020; 11:1020–76.

47. Diabetes Canada Clinical Practice Guidelines Expert Committee, Ekoe JM, Goldenberg R, Katz P. Screening for diabetes in adults. Can J Diabetes. 2018; 42 Suppl 1:S16–9.

48. American Diabetes Association. 13. Management of diabetes in pregnancy: standards of medical care in diabetes-2018. Diabetes Care. 2018; 41(Suppl 1):S137–43.
49. Jang HC. Gestational diabetes in Korea: incidence and risk factors of diabetes in women with previous gestational diabetes. Diabetes Metab J. 2011; 35:1–7.

50. Lee YH, Bang H, Kim HC, Kim HM, Park SW, Kim DJ. A simple screening score for diabetes for the Korean population: development, validation, and comparison with other scores. Diabetes Care. 2012; 35:1723–30.
51. Oh TJ, Moon JH, Choi SH, Cho YM, Park KS, Cho NH, et al. Development of a clinical risk score for incident diabetes: a 10-year prospective cohort study. J Diabetes Investig. 2021; 12:610–8.
52. Chan AY, Swaminathan R, Cockram CS. Effectiveness of sodium fluoride as a preservative of glucose in blood. Clin Chem. 1989; 35:315–7.

53. le Roux CW, Wilkinson SD, Pavitt DV, Muller BR, Alaghband-Zadeh J. A new antiglycolytic agent. Ann Clin Biochem. 2004; 41(Pt 1):43–6.

54. Pan XR, Li GW, Hu YH, Wang JX, Yang WY, An ZX, et al. Effects of diet and exercise in preventing NIDDM in people with impaired glucose tolerance. The Da Qing IGT and Diabetes Study. Diabetes Care. 1997; 20:537–44.

55. Tuomilehto J, Lindstrom J, Eriksson JG, Valle TT, Hamalainen H, Ilanne-Parikka P, et al. Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. N Engl J Med. 2001; 344:1343–50.

56. Knowler WC, Barrett-Connor E, Fowler SE, Hamman RF, Lachin JM, Walker EA, et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002; 346:393–403.
57. Kosaka K, Noda M, Kuzuya T. Prevention of type 2 diabetes by lifestyle intervention: a Japanese trial in IGT males. Diabetes Res Clin Pract. 2005; 67:152–62.

58. Ramachandran A, Snehalatha C, Mary S, Mukesh B, Bhaskar AD, Vijay V, et al. The Indian Diabetes Prevention Programme shows that lifestyle modification and metformin prevent type 2 diabetes in Asian Indian subjects with impaired glucose tolerance (IDPP-1). Diabetologia. 2006; 49:289–97.

59. Roumen C, Corpeleijn E, Feskens EJ, Mensink M, Saris WH, Blaak EE. Impact of 3-year lifestyle intervention on postprandial glucose metabolism: the SLIM study. Diabet Med. 2008; 25:597–605.

60. Lindahl B, Nilsson TK, Borch-Johnsen K, Roder ME, Soderberg S, Widman L, et al. A randomized lifestyle intervention with 5-year follow-up in subjects with impaired glucose tolerance: pronounced short-term impact but long-term adherence problems. Scand J Public Health. 2009; 37:434–42.

61. Penn L, White M, Oldroyd J, Walker M, Alberti KG, Mathers JC. Prevention of type 2 diabetes in adults with impaired glucose tolerance: the European Diabetes Prevention RCT in Newcastle upon Tyne, UK. BMC Public Health. 2009; 9:342.
62. Saito T, Watanabe M, Nishida J, Izumi T, Omura M, Takagi T, et al. Lifestyle modification and prevention of type 2 diabetes in overweight Japanese with impaired fasting glucose levels: a randomized controlled trial. Arch Intern Med. 2011; 171:1352–60.

63. Sakane N, Sato J, Tsushita K, Tsujii S, Kotani K, Tsuzaki K, et al. Prevention of type 2 diabetes in a primary healthcare setting: three-year results of lifestyle intervention in Japanese subjects with impaired glucose tolerance. BMC Public Health. 2011; 11:40.

64. Penn L, White M, Lindstrom J, den Boer AT, Blaak E, Eriksson JG, et al. Importance of weight loss maintenance and risk prediction in the prevention of type 2 diabetes: analysis of European Diabetes Prevention Study RCT. PLoS One. 2013; 8:e57143.

65. Li G, Zhang P, Wang J, Gregg EW, Yang W, Gong Q, et al. The long-term effect of lifestyle interventions to prevent diabetes in the China Da Qing Diabetes Prevention Study: a 20-year follow-up study. Lancet. 2008; 371:1783–9.

66. Gong Q, Gregg EW, Wang J, An Y, Zhang P, Yang W, et al. Long-term effects of a randomised trial of a 6-year lifestyle intervention in impaired glucose tolerance on diabetes-related microvascular complications: the China Da Qing Diabetes Prevention Outcome Study. Diabetologia. 2011; 54:300–7.
67. Li G, Zhang P, Wang J, An Y, Gong Q, Gregg EW, et al. Cardiovascular mortality, all-cause mortality, and diabetes incidence after lifestyle intervention for people with impaired glucose tolerance in the Da Qing Diabetes Prevention Study: a 23-year follow-up study. Lancet Diabetes Endocrinol. 2014; 2:474–80.

68. Gong Q, Zhang P, Wang J, Ma J, An Y, Chen Y, et al. Morbidity and mortality after lifestyle intervention for people with impaired glucose tolerance: 30-year results of the Da Qing Diabetes Prevention Outcome Study. Lancet Diabetes Endocrinol. 2019; 7:452–61.

69. Chen Y, Zhang P, Wang J, Gong Q, An Y, Qian X, et al. Associations of progression to diabetes and regression to normal glucose tolerance with development of cardiovascular and microvascular disease among people with impaired glucose tolerance: a secondary analysis of the 30 year Da Qing Diabetes Prevention Outcome Study. Diabetologia. 2021; 64:1279–87.

70. Lindstrom J, Ilanne-Parikka P, Peltonen M, Aunola S, Eriksson JG, Hemio K, et al. Sustained reduction in the incidence of type 2 diabetes by lifestyle intervention: follow-up of the Finnish Diabetes Prevention Study. Lancet. 2006; 368:1673–9.

71. Lindstrom J, Peltonen M, Eriksson JG, Ilanne-Parikka P, Aunola S, Keinanen-Kiukaanniemi S, et al. Improved lifestyle and decreased diabetes risk over 13 years: long-term follow-up of the randomised Finnish Diabetes Prevention Study (DPS). Diabetologia. 2013; 56:284–93.
72. Uusitupa M, Peltonen M, Lindstrom J, Aunola S, Ilanne-Parikka P, Keinanen-Kiukaanniemi S, et al. Ten-year mortality and cardiovascular morbidity in the Finnish Diabetes Prevention Study--secondary analysis of the randomized trial. PLoS One. 2009; 4:e5656.

73. Diabetes Prevention Program Research Group, Knowler WC, Fowler SE, Hamman RF, Christophi CA, Hoffman HJ, et al. 10-Year follow-up of diabetes incidence and weight loss in the Diabetes Prevention Program Outcomes Study. Lancet. 2009; 374:1677–86.

74. Fottrell E, Ahmed N, Morrison J, Kuddus A, Shaha SK, King C, et al. Community groups or mobile phone messaging to prevent and control type 2 diabetes and intermediate hyperglycaemia in Bangladesh (DMagic): a cluster-randomised controlled trial. Lancet Diabetes Endocrinol. 2019; 7:200–12.

75. McLeod M, Stanley J, Signal V, Stairmand J, Thompson D, Henderson K, et al. Impact of a comprehensive digital health programme on HbA1c and weight after 12 months for people with diabetes and prediabetes: a randomised controlled trial. Diabetologia. 2020; 63:2559–70.

76. Nanditha A, Thomson H, Susairaj P, Srivanichakorn W, Oliver N, Godsland IF, et al. A pragmatic and scalable strategy using mobile technology to promote sustained lifestyle changes to prevent type 2 diabetes in India and the UK: a randomised controlled trial. Diabetologia. 2020; 63:486–96.
77. Toro-Ramos T, Michaelides A, Anton M, Karim Z, Kang-Oh L, Argyrou C, et al. Mobile delivery of the diabetes prevention program in people with prediabetes: randomized controlled trial. JMIR Mhealth Uhealth. 2020; 8:e17842.

78. Lee JH, Lim SY, Cha SA, Han CJ, Jung AR, Kim KR, et al. Short-term effects of the internet-based Korea Diabetes Prevention Study: 6-month results of a community-based randomized controlled trial. Diabetes Metab J. 2021; 45:960–5.

79. Diabetes Prevention Program Research Group. Long-term effects of lifestyle intervention or metformin on diabetes development and microvascular complications over 15-year follow-up: the Diabetes Prevention Program Outcomes Study. Lancet Diabetes Endocrinol. 2015; 3:866–75.
80. Diabetes Prevention Program Research Group. Long-term effects of metformin on diabetes prevention: identification of subgroups that benefited most in the Diabetes Prevention Program and Diabetes Prevention Program Outcomes Study. Diabetes Care. 2019; 42:601–8.
81. Rhee SY, Chon S, Ahn KJ, Woo JT; Korean Diabetes Prevention Study Investigators. Hospital-based Korean diabetes prevention study: a prospective, multi-center, randomized, open-label controlled study. Diabetes Metab J. 2019; 43:49–58.

82. Chiasson JL, Josse RG, Gomis R, Hanefeld M, Karasik A, Laakso M, et al. Acarbose for prevention of type 2 diabetes mellitus: the STOP-NIDDM randomised trial. Lancet. 2002; 359:2072–7.

83. Torgerson JS, Hauptman J, Boldrin MN, Sjostrom L. XENical in the prevention of diabetes in obese subjects (XENDOS) study: a randomized study of orlistat as an adjunct to lifestyle changes for the prevention of type 2 diabetes in obese patients. Diabetes Care. 2004; 27:155–61.
84. Kawamori R, Tajima N, Iwamoto Y, Kashiwagi A, Shimamoto K, Kaku K, et al. Voglibose for prevention of type 2 diabetes mellitus: a randomised, double-blind trial in Japanese individuals with impaired glucose tolerance. Lancet. 2009; 373:1607–14.

85. NAVIGATOR Study Group, McMurray JJ, Holman RR, Haffner SM, Bethel MA, Holzhauer B, et al. Effect of valsartan on the incidence of diabetes and cardiovascular events. N Engl J Med. 2010; 362:1477–90.

86. DeFronzo RA, Tripathy D, Schwenke DC, Banerji M, Bray GA, Buchanan TA, et al. Pioglitazone for diabetes prevention in impaired glucose tolerance. N Engl J Med. 2011; 364:1104–15.

87. Garvey WT, Ryan DH, Henry R, Bohannon NJ, Toplak H, Schwiers M, et al. Prevention of type 2 diabetes in subjects with prediabetes and metabolic syndrome treated with phentermine and topiramate extended release. Diabetes Care. 2014; 37:912–21.

88. Kernan WN, Viscoli CM, Furie KL, Young LH, Inzucchi SE, Gorman M, et al. Pioglitazone after ischemic stroke or transient ischemic attack. N Engl J Med. 2016; 374:1321–31.
89. le Roux CW, Astrup A, Fujioka K, Greenway F, Lau DCW, Van Gaal L, et al. 3 Years of liraglutide versus placebo for type 2 diabetes risk reduction and weight management in individuals with prediabetes: a randomised, double-blind trial. Lancet. 2017; 389:1399–409.
90. Gerstein HC, Coleman RL, Scott CAB, Xu S, Tuomilehto J, Ryden L, et al. Impact of acarbose on incident diabetes and regression to normoglycemia in people with coronary heart disease and impaired glucose tolerance: insights from the ACE trial. Diabetes Care. 2020; 43:2242–7.

91. Diabetes Control and Complications Trial Research Group, Nathan DM, Genuth S, Lachin J, Cleary P, Crofford O, et al. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulindependent diabetes mellitus. N Engl J Med. 1993; 329:977–86.

92. Diabetes Control and Complications Trial (DCCT)/Epidemiology of Diabetes Interventions and Complications (EDIC) Research Group, Lachin JM, White NH, Hainsworth DP, Sun W, Cleary PA, et al. Effect of intensive diabetes therapy on the progression of diabetic retinopathy in patients with type 1 diabetes: 18 years of follow-up in the DCCT/EDIC. Diabetes. 2015; 64:631–42.

93. Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications Research Group, Lachin JM, Genuth S, Cleary P, Davis MD, Nathan DM. Retinopathy and nephropathy in patients with type 1 diabetes four years after a trial of intensive therapy. N Engl J Med. 2000; 342:381–9.
94. Ohkubo Y, Kishikawa H, Araki E, Miyata T, Isami S, Motoyoshi S, et al. Intensive insulin therapy prevents the progression of diabetic microvascular complications in Japanese patients with non-insulin-dependent diabetes mellitus: a randomized prospective 6-year study. Diabetes Res Clin Pract. 1995; 28:103–17.

95. UK Prospective Diabetes Study (UKPDS) Group. Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34). Lancet. 1998; 352:854–65.
96. UK Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet. 1998; 352:837–53.
97. Holman RR, Paul SK, Bethel MA, Matthews DR, Neil HA. 10-Year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med. 2008; 359:1577–89.

98. Stratton IM, Adler AI, Neil HA, Matthews DR, Manley SE, Cull CA, et al. Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study. BMJ. 2000; 321:405–12.

99. Ismail-Beigi F, Craven T, Banerji MA, Basile J, Calles J, Cohen RM, et al. Effect of intensive treatment of hyperglycaemia on microvascular outcomes in type 2 diabetes: an analysis of the ACCORD randomised trial. Lancet. 2010; 376:419–30.

100. ADVANCE Collaborative Group, Patel A, MacMahon S, Chalmers J, Neal B, Billot L, et al. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med. 2008; 358:2560–72.
101. Duckworth W, Abraira C, Moritz T, Reda D, Emanuele N, Reaven PD, et al. Glucose control and vascular complications in veterans with type 2 diabetes. N Engl J Med. 2009; 360:129–39.

102. Zoungas S, Chalmers J, Ninomiya T, Li Q, Cooper ME, Colagiuri S, et al. Association of HbA1c levels with vascular complications and death in patients with type 2 diabetes: evidence of glycaemic thresholds. Diabetologia. 2012; 55:636–43.

103. Nathan DM, Cleary PA, Backlund JY, Genuth SM, Lachin JM, Orchard TJ, et al. Intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes. N Engl J Med. 2005; 353:2643–53.

104. Writing Group for the DCCT/EDIC Research Group, Orchard TJ, Nathan DM, Zinman B, Cleary P, Brillon D, et al. Association between 7 years of intensive treatment of type 1 diabetes and long-term mortality. JAMA. 2015; 313:45–53.

105. Action to Control Cardiovascular Risk in Diabetes Study Group, Gerstein HC, Miller ME, Byington RP, Goff DC Jr, Bigger JT, et al. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med. 2008; 358:2545–59.
106. Lee AK, Warren B, Lee CJ, McEvoy JW, Matsushita K, Huang ES, et al. The association of severe hypoglycemia with incident cardiovascular events and mortality in adults with type 2 diabetes. Diabetes Care. 2018; 41:104–11.

107. Duckworth WC, Abraira C, Moritz TE, Davis SN, Emanuele N, Goldman S, et al. The duration of diabetes affects the response to intensive glucose control in type 2 subjects: the VA Diabetes Trial. J Diabetes Complications. 2011; 25:355–61.

108. Laiteerapong N, Ham SA, Gao Y, Moffet HH, Liu JY, Huang ES, et al. The legacy effect in type 2 diabetes: impact of early glycemic control on future complications (The Diabetes & Aging Study). Diabetes Care. 2019; 42:416–26.

109. Zoungas S, Chalmers J, Neal B, Billot L, Li Q, Hirakawa Y, et al. Follow-up of blood-pressure lowering and glucose control in type 2 diabetes. N Engl J Med. 2014; 371:1392–406.

110. Hayward RA, Reaven PD, Wiitala WL, Bahn GD, Reda DJ, Ge L, et al. Follow-up of glycemic control and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2015; 372:2197–206.
111. Matthews DR, Paldanius PM, Proot P, Chiang Y, Stumvoll M, Del Prato S, et al. Glycaemic durability of an early combination therapy with vildagliptin and metformin versus sequential metformin monotherapy in newly diagnosed type 2 diabetes (VERIFY): a 5-year, multicentre, randomised, double-blind trial. Lancet. 2019; 394:1519–29.

112. Beck RW, Bergenstal RM, Cheng P, Kollman C, Carlson AL, Johnson ML, et al. The relationships between time in range, hyperglycemia metrics, and HbA1c. J Diabetes Sci Technol. 2019; 13:614–26.

113. Writing Team for the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications Research Group. Effect of intensive therapy on the microvascular complications of type 1 diabetes mellitus. JAMA. 2002; 287:2563–9.
114. Jovanovic L, Savas H, Mehta M, Trujillo A, Pettitt DJ. Frequent monitoring of A1C during pregnancy as a treatment tool to guide therapy. Diabetes Care. 2011; 34:53–4.

115. Wei N, Zheng H, Nathan DM. Empirically establishing blood glucose targets to achieve HbA1c goals. Diabetes Care. 2014; 37:1048–51.

116. Nathan DM, Kuenen J, Borg R, Zheng H, Schoenfeld D, Heine RJ, et al. Translating the A1C assay into estimated average glucose values. Diabetes Care. 2008; 31:1473–8.
117. ElSayed NA, Aleppo G, Aroda VR, Bannuru RR, Brown FM, Bruemmer D, et al. 6. Glycemic targets: standards of care in diabetes-2023. Diabetes Care. 2023; 46(Suppl 1):S97–110.
118. Beck RW, Connor CG, Mullen DM, Wesley DM, Bergenstal RM. The fallacy of average: how using HbA1c alone to assess glycemic control can be misleading. Diabetes Care. 2017; 40:994–9.

119. Battelino T, Danne T, Bergenstal RM, Amiel SA, Beck R, Biester T, et al. Clinical targets for continuous glucose monitoring data interpretation: recommendations from the International Consensus on Time in Range. Diabetes Care. 2019; 42:1593–603.
120. ElSayed NA, Aleppo G, Aroda VR, Bannuru RR, Brown FM, Bruemmer D, et al. 7. Diabetes technology: standards of care in diabetes-2023. Diabetes Care. 2023; 46(Suppl 1):S111–27.
121. Norris SL, Engelgau MM, Narayan KM. Effectiveness of self-management training in type 2 diabetes: a systematic review of randomized controlled trials. Diabetes Care. 2001; 24:561–87.
122. Franciosi M, Pellegrini F, De Berardis G, Belfiglio M, Cavaliere D, Di Nardo B, et al. The impact of blood glucose self-monitoring on metabolic control and quality of life in type 2 diabetic patients: an urgent need for better educational strategies. Diabetes Care. 2001; 24:1870–7.
123. Polonsky WH, Fisher L, Schikman CH, Hinnen DA, Parkin CG, Jelsovsky Z, et al. Structured self-monitoring of blood glucose significantly reduces A1C levels in poorly controlled, noninsulin-treated type 2 diabetes: results from the Structured Testing Program study. Diabetes Care. 2011; 34:262–7.
124. Karter AJ, Ackerson LM, Darbinian JA, D’Agostino RB Jr, Ferrara A, Liu J, et al. Self-monitoring of blood glucose levels and glycemic control: the Northern California Kaiser Permanente Diabetes registry. Am J Med. 2001; 111:1–9.

126. Bergenstal R, Pearson J, Cembrowski GS, Bina D, Davidson J, List S. Identifying variables associated with inaccurate self-monitoring of blood glucose: proposed guidelines to improve accuracy. Diabetes Educ. 2000; 26:981–9.

127. Ziegler R, Heidtmann B, Hilgard D, Hofer S, Rosenbauer J, Holl R, et al. Frequency of SMBG correlates with HbA1c and acute complications in children and adolescents with type 1 diabetes. Pediatr Diabetes. 2011; 12:11–7.
128. Murata GH, Shah JH, Hoffman RM, Wendel CS, Adam KD, Solvas PA, et al. Intensified blood glucose monitoring improves glycemic control in stable, insulin-treated veterans with type 2 diabetes: the Diabetes Outcomes in Veterans Study (DOVES). Diabetes Care. 2003; 26:1759–63.
129. Rosenstock J, Davies M, Home PD, Larsen J, Koenen C, Schernthaner G. A randomised, 52-week, treat-to-target trial comparing insulin detemir with insulin glargine when administered as add-on to glucose-lowering drugs in insulin-naive people with type 2 diabetes. Diabetologia. 2008; 51:408–16.

130. Garber AJ. Treat-to-target trials: uses, interpretation and review of concepts. Diabetes Obes Metab. 2014; 16:193–205.

131. Farmer A, Wade A, Goyder E, Yudkin P, French D, Craven A, et al. Impact of self monitoring of blood glucose in the management of patients with non-insulin treated diabetes: open parallel group randomised trial. BMJ. 2007; 335:132.

132. O’Kane MJ, Bunting B, Copeland M, Coates VE; ESMON study group. Efficacy of self monitoring of blood glucose in patients with newly diagnosed type 2 diabetes (ESMON study): randomised controlled trial. BMJ. 2008; 336:1174–7.
133. Simon J, Gray A, Clarke P, Wade A, Neil A, Farmer A, et al. Cost effectiveness of self monitoring of blood glucose in patients with non-insulin treated type 2 diabetes: economic evaluation of data from the DiGEM trial. BMJ. 2008; 336:1177–80.

134. Young LA, Buse JB, Weaver MA, Vu MB, Mitchell CM, Blakeney T, et al. Glucose self-monitoring in non-insulin-treated patients with type 2 diabetes in primary care settings: a randomized trial. JAMA Intern Med. 2017; 177:920–9.

135. Malanda UL, Welschen LM, Riphagen II, Dekker JM, Nijpels G, Bot SD. Self-monitoring of blood glucose in patients with type 2 diabetes mellitus who are not using insulin. Cochrane Database Syst Rev. 2012; 1:CD005060.

136. Mannucci E, Antenore A, Giorgino F, Scavini M. Effects of structured versus unstructured self-monitoring of blood glucose on glucose control in patients with non-insulin-treated type 2 diabetes: a meta-analysis of randomized controlled trials. J Diabetes Sci Technol. 2018; 12:183–9.

137. Avignon A, Radauceanu A, Monnier L. Nonfasting plasma glucose is a better marker of diabetic control than fasting plasma glucose in type 2 diabetes. Diabetes Care. 1997; 20:1822–6.
138. Monnier L, Lapinski H, Colette C. Contributions of fasting and postprandial plasma glucose increments to the overall diurnal hyperglycemia of type 2 diabetic patients: variations with increasing levels of HbA(1c). Diabetes Care. 2003; 26:881–5.
139. Evert AB, Dennison M, Gardner CD, Garvey WT, Lau KHK, MacLeod J, et al. Nutrition therapy for adults with diabetes or prediabetes: a consensus report. Diabetes Care. 2019; 42:731–54.

140. Razaz JM, Rahmani J, Varkaneh HK, Thompson J, Clark C, Abdulazeem HM. The health effects of medical nutrition therapy by dietitians in patients with diabetes: a systematic review and meta-analysis: Nutrition therapy and diabetes. Prim Care Diabetes. 2019; 13:399–408.

141. Parker AR, Byham-Gray L, Denmark R, Winkle PJ. The effect of medical nutrition therapy by a registered dietitian nutritionist in patients with prediabetes participating in a randomized controlled clinical research trial. J Acad Nutr Diet. 2014; 114:1739–48.

142. Cho Y, Lee M, Jang H, Rha M, Kim J, Park Y, et al. The clinical and cost effectiveness of medical nutrition therapy for patients with type 2 diabetes mellitus. Korean J Nutr. 2008; 41:147–55.
143. Look AHEAD Research Group, Wing RR. Long-term effects of a lifestyle intervention on weight and cardiovascular risk factors in individuals with type 2 diabetes mellitus: four-year results of the Look AHEAD trial. Arch Intern Med. 2010; 170:1566–75.
144. Franz MJ, Boucher JL, Rutten-Ramos S, VanWormer JJ. Lifestyle weight-loss intervention outcomes in overweight and obese adults with type 2 diabetes: a systematic review and meta-analysis of randomized clinical trials. J Acad Nutr Diet. 2015; 115:1447–63.
145. Wing RR, Lang W, Wadden TA, Safford M, Knowler WC, Bertoni AG, et al. Benefits of modest weight loss in improving cardiovascular risk factors in overweight and obese individuals with type 2 diabetes. Diabetes Care. 2011; 34:1481–6.

146. Hamdy O, Mottalib A, Morsi A, El-Sayed N, Goebel-Fabbri A, Arathuzik G, et al. Long-term effect of intensive lifestyle intervention on cardiovascular risk factors in patients with diabetes in real-world clinical practice: a 5-year longitudinal study. BMJ Open Diabetes Res Care. 2017; 5:e000259.

147. Lean ME, Leslie WS, Barnes AC, Brosnahan N, Thom G, Mc-Combie L, et al. Primary care-led weight management for remission of type 2 diabetes (DiRECT): an open-label, cluster-randomised trial. Lancet. 2018; 391:541–51.
148. Blau JE, Tella SH, Taylor SI, Rother KI. Ketoacidosis associated with SGLT2 inhibitor treatment: analysis of FAERS data. Diabetes Metab Res Rev. 2017; 33:e2924.

149. Sellahewa L, Khan C, Lakkunarajah S, Idris I. A systematic review of evidence on the use of very low calorie diets in people with diabetes. Curr Diabetes Rev. 2017; 13:35–46.

150. Kirkpatrick CF, Bolick JP, Kris-Etherton PM, Sikand G, Aspry KE, Soffer DE, et al. Review of current evidence and clinical recommendations on the effects of low-carbohydrate and very-low-carbohydrate (including ketogenic) diets for the management of body weight and other cardiometabolic risk factors: a scientific statement from the National Lipid Association Nutrition and Lifestyle Task Force. J Clin Lipidol. 2019; 13:689–711.e1.

151. Schwingshackl L, Chaimani A, Hoffmann G, Schwedhelm C, Boeing H. A network meta-analysis on the comparative efficacy of different dietary approaches on glycaemic control in patients with type 2 diabetes mellitus. Eur J Epidemiol. 2018; 33:157–70.

152. Meng Y, Bai H, Wang S, Li Z, Wang Q, Chen L. Efficacy of low carbohydrate diet for type 2 diabetes mellitus management: a systematic review and meta-analysis of randomized controlled trials. Diabetes Res Clin Pract. 2017; 131:124–31.
153. Sainsbury E, Kizirian NV, Partridge SR, Gill T, Colagiuri S, Gibson AA. Effect of dietary carbohydrate restriction on glycemic control in adults with diabetes: a systematic review and meta-analysis. Diabetes Res Clin Pract. 2018; 139:239–52.

154. van Zuuren EJ, Fedorowicz Z, Kuijpers T, Pijl H. Effects of low-carbohydrate-compared with low-fat-diet interventions on metabolic control in people with type 2 diabetes: a systematic review including GRADE assessments. Am J Clin Nutr. 2018; 108:300–31.
155. Korsmo-Haugen HK, Brurberg KG, Mann J, Aas AM. Carbohydrate quantity in the dietary management of type 2 diabetes: a systematic review and meta-analysis. Diabetes Obes Metab. 2019; 21:15–27.

156. Jayedi A, Zeraattalab-Motlagh S, Jabbarzadeh B, Hosseini Y, Jibril AT, Shahinfar H, et al. Dose-dependent effect of carbohydrate restriction for type 2 diabetes management: a systematic review and dose-response meta-analysis of randomized controlled trials. Am J Clin Nutr. 2022; 116:40–56.

157. Goldenberg JZ, Day A, Brinkworth GD, Sato J, Yamada S, Jonsson T, et al. Efficacy and safety of low and very low carbohydrate diets for type 2 diabetes remission: systematic review and meta-analysis of published and unpublished randomized trial data. BMJ. 2021; 372:m4743.
158. Korean Diabetes Association. Diabetes fact sheet in Korea 2020. Seoul: Korean Diabetes Association;2020.
159. Ministry of Health and Welfare; The Korean Nutrition Society. Dietary reference intakes for Koreans 2020. Sejong: Ministry of Health and Welfare;2020.
160. Carter S, Clifton PM, Keogh JB. Effect of intermittent compared with continuous energy restricted diet on glycemic control in patients with type 2 diabetes: a randomized noninferiority trial. JAMA Netw Open. 2018; 1:e180756.
161. Hutchison AT, Regmi P, Manoogian ENC, Fleischer JG, Wittert GA, Panda S, et al. Time-restricted feeding improves glucose tolerance in men at risk for type 2 diabetes: a randomized crossover trial. Obesity (Silver Spring). 2019; 27:724–32.

162. Andriessen C, Fealy CE, Veelen A, van Beek SMM, Roumans KHM, Connell NJ, et al. Three weeks of time-restricted eating improves glucose homeostasis in adults with type 2 diabetes but does not improve insulin sensitivity: a randomised crossover trial. Diabetologia. 2022; 65:1710–20.

163. de Cabo R, Mattson MP. Effects of intermittent fasting on health, aging, and disease. N Engl J Med. 2019; 381:2541–51.

164. Vitale R, Kim Y. The effects of intermittent fasting on glycemic control and body composition in adults with obesity and type 2 diabetes: a systematic review. Metab Syndr Relat Disord. 2020; 18:450–61.
165. Corley BT, Carroll RW, Hall RM, Weatherall M, Parry-Strong A, Krebs JD. Intermittent fasting in type 2 diabetes mellitus and the risk of hypoglycaemia: a randomized controlled trial. Diabet Med. 2018; 35:588–94.

166. Uldal S, Clemmensen KKB, Persson F, Faerch K, Quist JS. Is time-restricted eating safe in the treatment of type 2 diabetes? A review of intervention studies. Nutrients. 2022; 14:2299.

167. ElSayed NA, Aleppo G, Aroda VR, Bannuru RR, Brown FM, Bruemmer D, et al. 5. Facilitating positive health behaviors and well-being to improve health outcomes: standards of care in diabetes-2023. Diabetes Care. 2023; 46(Supple 1):S68–96.
168. Yao B, Fang H, Xu W, Yan Y, Xu H, Liu Y, et al. Dietary fiber intake and risk of type 2 diabetes: a dose-response analysis of prospective studies. Eur J Epidemiol. 2014; 29:79–88.

169. Silva FM, Kramer CK, de Almeida JC, Steemburgo T, Gross JL, Azevedo MJ. Fiber intake and glycemic control in patients with type 2 diabetes mellitus: a systematic review with meta-analysis of randomized controlled trials. Nutr Rev. 2013; 71:790–801.
170. Threapleton DE, Greenwood DC, Evans CE, Cleghorn CL, Nykjaer C, Woodhead C, et al. Dietary fibre intake and risk of cardiovascular disease: systematic review and meta-analysis. BMJ. 2013; 347:f6879.

171. He M, van Dam RM, Rimm E, Hu FB, Qi L. Whole-grain, cereal fiber, bran, and germ intake and the risks of all-cause and cardiovascular disease-specific mortality among women with type 2 diabetes mellitus. Circulation. 2010; 121:2162–8.

172. Burger KN, Beulens JW, van der Schouw YT, Sluijs I, Spijkerman AM, Sluik D, et al. Dietary fiber, carbohydrate quality and quantity, and mortality risk of individuals with diabetes mellitus. PLoS One. 2012; 7:e43127.

173. Tseng TS, Lin WT, Gonzalez GV, Kao YH, Chen LS, Lin HY. Sugar intake from sweetened beverages and diabetes: a narrative review. World J Diabetes. 2021; 12:1530–8.

174. Neelakantan N, Park SH, Chen GC, van Dam RM. Sugar-sweetened beverage consumption, weight gain, and risk of type 2 diabetes and cardiovascular diseases in Asia: a systematic review. Nutr Rev. 2021; 80:50–67.
175. Partula V, Deschasaux M, Druesne-Pecollo N, Latino-Martel P, Desmetz E, Chazelas E, et al. Associations between consumption of dietary fibers and the risk of cardiovascular diseases, cancers, type 2 diabetes, and mortality in the prospective NutriNet-Santé cohort. Am J Clin Nutr. 2020; 112:195–207.

176. Reynolds A, Mann J, Cummings J, Winter N, Mete E, Te Morenga L. Carbohydrate quality and human health: a series of systematic reviews and meta-analyses. Lancet. 2019; 393:434–45.

177. MacLeod J, Franz MJ, Handu D, Gradwell E, Brown C, Evert A, et al. Academy of Nutrition and Dietetics nutrition practice guideline for type 1 and type 2 diabetes in adults: nutrition intervention evidence reviews and recommendations. J Acad Nutr Diet. 2017; 117:1637–58.

178. Miller PE, Perez V. Low-calorie sweeteners and body weight and composition: a meta-analysis of randomized controlled trials and prospective cohort studies. Am J Clin Nutr. 2014; 100:765–77.
179. Azad MB, Abou-Setta AM, Chauhan BF, Rabbani R, Lys J, Copstein L, et al. Nonnutritive sweeteners and cardiometabolic health: a systematic review and meta-analysis of randomized controlled trials and prospective cohort studies. CMAJ. 2017; 189:E929–39.
180. Lohner S, Kuellenberg de Gaudry D, Toews I, Ferenci T, Meerpohl JJ. Non-nutritive sweeteners for diabetes mellitus. Cochrane Database Syst Rev. 2020; 5:CD012885.

181. Johnson RK, Lichtenstein AH, Anderson CAM, Carson JA, Despres JP, Hu FB, et al. Low-calorie sweetened beverages and cardiometabolic health: a science advisory from the American Heart Association. Circulation. 2018; 138:e126–40.

182. Zhang R, Noronha JC, Khan TA, McGlynn N, Back S, Grant SM, et al. The effect of non-nutritive sweetened beverages on postprandial glycemic and endocrine responses: a systematic review and network meta-analysis. Nutrients. 2023; 15:1050.

183. Suez J, Cohen Y, Valdes-Mas R, Mor U, Dori-Bachash M, Federici S, et al. Personalized microbiome-driven effects of nonnutritive sweeteners on human glucose tolerance. Cell. 2022; 185:3307–28.e19.

184. Witkowski M, Nemet I, Alamri H, Wilcox J, Gupta N, Nimer N, et al. The artificial sweetener erythritol and cardiovascular event risk. Nat Med. 2023; 29:710–8.
185. Debras C, Chazelas E, Sellem L, Porcher R, Druesne-Pecollo N, Esseddik Y, et al. Artificial sweeteners and risk of cardiovascular diseases: results from the prospective NutriNet-Santé cohort. BMJ. 2022; 378:e071204.

186. Zafar MI, Mills KE, Zheng J, Regmi A, Hu SQ, Gou L, et al. Low-glycemic index diets as an intervention for diabetes: a systematic review and meta-analysis. Am J Clin Nutr. 2019; 110:891–902.

187. Moore LJ, Midgley AW, Thomas G, Thurlow S, McNaughton LR. The effects of low- and high-glycemic index meals on time trial performance. Int J Sports Physiol Perform. 2009; 4:331–44.

188. Wheeler ML, Dunbar SA, Jaacks LM, Karmally W, Mayer-Davis EJ, Wylie-Rosett J, et al. Macronutrients, food groups, and eating patterns in the management of diabetes: a systematic review of the literature, 2010. Diabetes Care. 2012; 35:434–45.
189. Zhu HG, Jiang ZS, Gong PY, Zhang DM, Zou ZW, et al. Efficacy of low-protein diet for diabetic nephropathy: a systematic review of randomized controlled trials. Lipids Health Dis. 2018; 17:141.
190. Pan Y, Guo LL, Jin HM. Low-protein diet for diabetic nephropathy: a meta-analysis of randomized controlled trials. Am J Clin Nutr. 2008; 88:660–6.

191. Robertson L, Waugh N, Robertson A. Protein restriction for diabetic renal disease. Cochrane Database Syst Rev. 2007; 2007:CD002181.

192. Layman DK, Clifton P, Gannon MC, Krauss RM, Nuttall FQ. Protein in optimal health: heart disease and type 2 diabetes. Am J Clin Nutr. 2008; 87:1571S–5S.

193. Vitale M, Masulli M, Rivellese AA, Babini AC, Boemi M, Bonora E, et al. Influence of dietary fat and carbohydrates proportions on plasma lipids, glucose control and low-grade inflammation in patients with type 2 diabetes: the TOSCA.IT Study. Eur J Nutr. 2016; 55:1645–51.

194. Estruch R, Ros E, Salas-Salvado J, Covas MI, Corella D, Aros F, et al. Primary prevention of cardiovascular disease with a Mediterranean diet. N Engl J Med. 2013; 368:1279–90.
195. Wang DD, Li Y, Chiuve SE, Stampfer MJ, Manson JE, Rimm EB, et al. Association of specific dietary fats with total and cause-specific mortality. JAMA Intern Med. 2016; 176:1134–45.

196. de Souza RJ, Mente A, Maroleanu A, Cozma AI, Ha V, Kishibe T, et al. Intake of saturated and trans unsaturated fatty acids and risk of all cause mortality, cardiovascular disease, and type 2 diabetes: systematic review and meta-analysis of observational studies. BMJ. 2015; 351:h3978.

197. Hooper L, Martin N, Abdelhamid A, Davey Smith G. Reduction in saturated fat intake for cardiovascular disease. Cochrane Database Syst Rev. 2015; 6:CD011737.

198. Julibert A, Bibiloni MDM, Tur JA. Dietary fat intake and metabolic syndrome in adults: a systematic review. Nutr Metab Cardiovasc Dis. 2019; 29:887–905.

199. Rizos EC, Ntzani EE, Bika E, Kostapanos MS, Elisaf MS. Association between omega-3 fatty acid supplementation and risk of major cardiovascular disease events: a systematic review and meta-analysis. JAMA. 2012; 308:1024–33.
200. Brown TJ, Brainard J, Song F, Wang X, Abdelhamid A, Hooper L, et al. Omega-3, omega-6, and total dietary polyunsaturated fat for prevention and treatment of type 2 diabetes mellitus: systematic review and meta-analysis of randomised controlled trials. BMJ. 2019; 366:l4697.

201. ASCEND Study Collaborative Group, Bowman L, Mafham M, Wallendszus K, Stevens W, Buck G, et al. Effects of n-3 fatty acid supplements in diabetes mellitus. N Engl J Med. 2018; 379:1540–50.
202. Bhatt DL, Steg PG, Miller M, Brinton EA, Jacobson TA, Ketchum SB, et al. Cardiovascular risk reduction with icosapent ethyl for hypertriglyceridemia. N Engl J Med. 2019; 380:11–22.

203. Intersalt: an international study of electrolyte excretion and blood pressure. Results for 24 hour urinary sodium and potassium excretion. BMJ. 1988; 297:319–28.
204. Sacks FM, Svetkey LP, Vollmer WM, Appel LJ, Bray GA, Harsha D, et al. Effects on blood pressure of reduced dietary sodium and the Dietary Approaches to Stop Hypertension (DASH) diet. DASH-Sodium Collaborative Research Group. N Engl J Med. 2001; 344:3–10.

205. Bibbins-Domingo K, Chertow GM, Coxson PG, Moran A, Lightwood JM, Pletcher MJ, et al. Projected effect of dietary salt reductions on future cardiovascular disease. N Engl J Med. 2010; 362:590–9.
206. Mozaffarian D, Fahimi S, Singh GM, Micha R, Khatibzadeh S, Engell RE, et al. Global sodium consumption and death from cardiovascular causes. N Engl J Med. 2014; 371:624–34.

207. Cook NR, Cutler JA, Obarzanek E, Buring JE, Rexrode KM, Kumanyika SK, et al. Long term effects of dietary sodium reduction on cardiovascular disease outcomes: observational follow-up of the trials of hypertension prevention (TOHP). BMJ. 2007; 334:885–8.

208. Suckling RJ, He FJ, Macgregor GA. Altered dietary salt intake for preventing and treating diabetic kidney disease. Cochrane Database Syst Rev. 2010; 12:CD006763.

209. Azadbakht L, Fard NR, Karimi M, Baghaei MH, Surkan PJ, Rahimi M, et al. Effects of the Dietary Approaches to Stop Hypertension (DASH) eating plan on cardiovascular risks among type 2 diabetic patients: a randomized crossover clinical trial. Diabetes Care. 2011; 34:55–7.
210. Thomas MC, Moran J, Forsblom C, Harjutsalo V, Thorn L, Ahola A, et al. The association between dietary sodium intake, ESRD, and all-cause mortality in patients with type 1 diabetes. Diabetes Care. 2011; 34:861–6.
211. Ekinci EI, Clarke S, Thomas MC, Moran JL, Cheong K, MacIsaac RJ, et al. Dietary salt intake and mortality in patients with type 2 diabetes. Diabetes Care. 2011; 34:703–9.

213. Barbarawi M, Zayed Y, Barbarawi O, Bala A, Alabdouh A, Gakhal I, et al. Effect of vitamin D supplementation on the incidence of diabetes mellitus. J Clin Endocrinol Metab. 2020; 105:dgaa335.

214. Zhang Y, Tan H, Tang J, Li J, Chong W, Hai Y, et al. Effects of vitamin D supplementation on prevention of type 2 diabetes in patients with prediabetes: a systematic review and meta-analysis. Diabetes Care. 2020; 43:1650–8.

215. Hu Z, Chen J, Sun X, Wang L, Wang A. Efficacy of vitamin D supplementation on glycemic control in type 2 diabetes patients: a meta-analysis of interventional studies. Medicine (Baltimore). 2019; 98:e14970.
216. Pittas AG, Dawson-Hughes B, Sheehan P, Ware JH, Knowler WC, Aroda VR, et al. Vitamin D supplementation and prevention of type 2 diabetes. N Engl J Med. 2019; 381:520–30.
217. US Preventive Services Task Force, Mangione CM, Barry MJ, Nicholson WK, Cabana M, Chelmow D, et al. Vitamin, mineral, and multivitamin supplementation to prevent cardiovascular disease and cancer: US Preventive Services Task Force Recommendation Statement. JAMA. 2022; 327:2326–33.

218. Aroda VR, Edelstein SL, Goldberg RB, Knowler WC, Marcovina SM, Orchard TJ, et al. Long-term metformin use and vitamin B12 deficiency in the diabetes prevention program outcomes study. J Clin Endocrinol Metab. 2016; 101:1754–61.

219. Han M. The dose-response relationship between alcohol consumption and the risk of type 2 diabetes among Asian men: a systematic review and meta-analysis of prospective cohort studies. J Diabetes Res. 2020; 2020:1032049.

220. Lee DY, Yoo MG, Kim HJ, Jang HB, Kim JH, Lee HJ, et al. Association between alcohol consumption pattern and the incidence risk of type 2 diabetes in Korean men: a 12-years follow-up study. Sci Rep. 2017; 7:7322.

221. Baliunas DO, Taylor BJ, Irving H, Roerecke M, Patra J, Mohapatra S, et al. Alcohol as a risk factor for type 2 diabetes: a systematic review and meta-analysis. Diabetes Care. 2009; 32:2123–32.
222. Richardson T, Weiss M, Thomas P, Kerr D. Day after the night before: influence of evening alcohol on risk of hypoglycemia in patients with type 1 diabetes. Diabetes Care. 2005; 28:1801–2.
224. Fowler-Brown A, Pignone M, Pletcher M, Tice JA, Sutton SF, Lohr KN, et al. Exercise tolerance testing to screen for coronary heart disease: a systematic review for the technical support for the U.S. Preventive Services Task Force. Ann Intern Med. 2004; 140:W9–24.

225. Bax JJ, Young LH, Frye RL, Bonow RO, Steinberg HO, Barrett EJ, et al. Screening for coronary artery disease in patients with diabetes. Diabetes Care. 2007; 30:2729–36.

226. Ruderman N, Devlin JT, Schneider SH, Kriska AM. Handbook of exercise in diabetes. 2nd ed. Alexandria: American Diabetes Association;2002. Chapter, Retinopathy. p. 401–13.
227. Colberg S. Exercise and diabetes: a clinician’s guide to prescribing physical activity. Alexandria: American Diabetes Association;2013.
228. Ruderman N, Devlin JT, Schneider SH, Kriska AM. Handbook of exercise in diabetes. 2nd ed. Alexandria: American Diabetes Association;2002. Chapter, Neuropathy. p. 463–96.
229. Ruderman N, Devlin JT, Schneider SH, Kriska AM. Handbook of exercise in diabetes. 2nd ed. Alexandria: American Diabetes Association;2002. Chapter, The diabetic foot. p. 385–99.
230. Valensi P, Sachs RN, Harfouche B, Lormeau B, Paries J, Cosson E, et al. Predictive value of cardiac autonomic neuropathy in diabetic patients with or without silent myocardial ischemia. Diabetes Care. 2001; 24:339–43.

231. Spallone V, Ziegler D, Freeman R, Bernardi L, Frontoni S, Pop-Busui R, et al. Cardiovascular autonomic neuropathy in diabetes: clinical impact, assessment, diagnosis, and management. Diabetes Metab Res Rev. 2011; 27:639–53.
232. Hasan S, Shaw SM, Gelling LH, Kerr CJ, Meads CA. Exercise modes and their association with hypoglycemia episodes in adults with type 1 diabetes mellitus: a systematic review. BMJ Open Diabetes Res Care. 2018; 6:e000578.

233. Colberg SR, Sigal RJ, Yardley JE, Riddell MC, Dunstan DW, Dempsey PC, et al. Physical activity/exercise and diabetes: a position statement of the American Diabetes Association. Diabetes Care. 2016; 39:2065–79.

234. Ruderman N, Devlin JT, Schneider SH, Kriska AM. Handbook of exercise in diabetes. 2nd ed. Alexandria: American Diabetes Association;2002. Chapter, Adjustment of insulin and oral agent therapy. p. 365–76.
235. Sigal RJ, Kenny GP, Wasserman DH, Castaneda-Sceppa C. Physical activity/exercise and type 2 diabetes. Diabetes Care. 2004; 27:2518–39.

236. Boule NG, Haddad E, Kenny GP, Wells GA, Sigal RJ. Effects of exercise on glycemic control and body mass in type 2 diabetes mellitus: a meta-analysis of controlled clinical trials. JAMA. 2001; 286:1218–27.
237. Boule NG, Kenny GP, Haddad E, Wells GA, Sigal RJ. Meta-analysis of the effect of structured exercise training on cardiorespiratory fitness in type 2 diabetes mellitus. Diabetologia. 2003; 46:1071–81.

238. Jang JE, Cho Y, Lee BW, Shin ES, Lee SH. Effectiveness of exercise intervention in reducing body weight and glycosylated hemoglobin levels in patients with type 2 diabetes mellitus in Korea: a systematic review and meta-analysis. Diabetes Metab J. 2019; 43:302–18.

239. Ostman C, Jewiss D, King N, Smart NA. Clinical outcomes to exercise training in type 1 diabetes: a systematic review and meta-analysis. Diabetes Res Clin Pract. 2018; 139:380–91.

240. Tikkanen-Dolenc H, Waden J, Forsblom C, Harjutsalo V, Thorn LM, Saraheimo M, et al. Physical activity reduces risk of premature mortality in patients with type 1 diabetes with and without kidney disease. Diabetes Care. 2017; 40:1727–32.

241. Colberg SR, Sigal RJ, Fernhall B, Regensteiner JG, Blissmer BJ, Rubin RR, et al. Exercise and type 2 diabetes: the American College of Sports Medicine and the American Diabetes Association: joint position statement. Diabetes Care. 2010; 33:e147–67.
243. de Mello MB, Righi NC, Schuch FB, Signori LU, da Silva AMV. Effect of high-intensity interval training protocols on VO2max and HbA1c level in people with type 2 diabetes: a systematic review and meta-analysis. Ann Phys Rehabil Med. 2022; 65:101586.

244. Dunstan DW, Daly RM, Owen N, Jolley D, De Courten M, Shaw J, et al. High-intensity resistance training improves glycemic control in older patients with type 2 diabetes. Diabetes Care. 2002; 25:1729–36.

245. Castaneda C, Layne JE, Munoz-Orians L, Gordon PL, Walsmith J, Foldvari M, et al. A randomized controlled trial of resistance exercise training to improve glycemic control in older adults with type 2 diabetes. Diabetes Care. 2002; 25:2335–41.

246. Church TS, Blair SN, Cocreham S, Johannsen N, Johnson W, Kramer K, et al. Effects of aerobic and resistance training on hemoglobin A1c levels in patients with type 2 diabetes: a randomized controlled trial. JAMA. 2010; 304:2253–62.

247. Sigal RJ, Kenny GP, Boule NG, Wells GA, Prud’homme D, Fortier M, et al. Effects of aerobic training, resistance training, or both on glycemic control in type 2 diabetes: a randomized trial. Ann Intern Med. 2007; 147:357–69.
248. Dempsey PC, Larsen RN, Sethi P, Sacre JW, Straznicky NE, Cohen ND, et al. Benefits for type 2 diabetes of interrupting prolonged sitting with brief bouts of light walking or simple resistance activities. Diabetes Care. 2016; 39:964–72.

249. Paing AC, McMillan KA, Kirk AF, Collier A, Hewitt A, Chastin SFM. Dose-response between frequency of interruption of sedentary time and fasting glucose, the dawn phenomenon and night-time glucose in type 2 diabetes. Diabet Med. 2019; 36:376–82.

250. Bell KJ, Barclay AW, Petocz P, Colagiuri S, Brand-Miller JC. Efficacy of carbohydrate counting in type 1 diabetes: a systematic review and meta-analysis. Lancet Diabetes Endocrinol. 2014; 2:133–40.

251. Rytter K, Schmidt S, Rasmussen LN, Pedersen-Bjergaard U, Norgaard K. Education programmes for persons with type 1 diabetes using an insulin pump: a systematic review. Diabetes Metab Res Rev. 2021; 37:e3412.

252. DAFNE Study Group. Training in flexible, intensive insulin management to enable dietary freedom in people with type 1 diabetes: dose adjustment for normal eating (DAFNE) randomised controlled trial. BMJ. 2002; 325:746.
253. Sanchez-Hernandez RM, Alvarado-Martel D, Lopez-Plasencia Y, Carrillo-Dominguez A, Jimenez-Rodriguez A, Rodriguez-Cordero J, et al. Assessment of Alimentación Normal con Ajuste de Insulina (ANAIS), a Spanish version of the DAFNE programme, in people with type 1 diabetes: a randomized controlled parallel trial. Diabet Med. 2019; 36:1037–45.
254. DeWitt DE, Hirsch IB. Outpatient insulin therapy in type 1 and type 2 diabetes mellitus: scientific review. JAMA. 2003; 289:2254–64.

255. Chiang JL, Kirkman MS, Laffel LM, Peters AL; Type 1 Diabetes Sourcebook Authors. Type 1 diabetes through the life span: a position statement of the American Diabetes Association. Diabetes Care. 2014; 37:2034–54.

256. Markowitz JT, Garvey KC, Laffel LM. Developmental changes in the roles of patients and families in type 1 diabetes management. Curr Diabetes Rev. 2015; 11:231–8.

257. ElSayed NA, Aleppo G, Aroda VR, Bannuru RR, Brown FM, Bruemmer D, et al. 14. Children and adolescents: standards of care in diabetes-2023. Diabetes Care. 2023; 46(Suppl 1):S230–53.
258. Siminerio LM, Albanese-O’Neill A, Chiang JL, Hathaway K, Jackson CC, Weissberg-Benchell J, et al. Care of young children with diabetes in the child care setting: a position statement of the American Diabetes Association. Diabetes Care. 2014; 37:2834–42.
259. Little SA, Leelarathna L, Walkinshaw E, Tan HK, Chapple O, Lubina-Solomon A, et al. Recovery of hypoglycemia awareness in long-standing type 1 diabetes: a multicenter 2 × 2 factorial randomized controlled trial comparing insulin pump with multiple daily injections and continuous with conventional glucose self-monitoring (HypoCOMPaSS). Diabetes Care. 2014; 37:2114–22.

260. Little SA, Speight J, Leelarathna L, Walkinshaw E, Tan HK, Bowes A, et al. Sustained reduction in severe hypoglycemia in adults with type 1 diabetes complicated by impaired awareness of hypoglycemia: two-year follow-up in the HypoCOMPaSS randomized clinical trial. Diabetes Care. 2018; 41:1600–7.

261. Chico A, Corcoy R. Intensive insulin therapy (Basal-Bolus). Am J Ther. 2020; 29:e64–73.

262. Bergenstal RM, Tamborlane WV, Ahmann A, Buse JB, Dailey G, Davis SN, et al. Effectiveness of sensor-augmented insulin-pump therapy in type 1 diabetes. N Engl J Med. 2010; 363:311–20.

263. Kim SK, Kwon SB, Yoon KH, Ahn KJ, Kang JG, Jung HS, et al. Assessment of glycemic lability and severity of hypoglycemia in Korean patients with type 1 diabetes. Endocr J. 2011; 58:433–40.
264. Yeh HC, Brown TT, Maruthur N, Ranasinghe P, Berger Z, Suh YD, et al. Comparative effectiveness and safety of methods of insulin delivery and glucose monitoring for diabetes mellitus: a systematic review and meta-analysis. Ann Intern Med. 2012; 157:336–47.

265. Tricco AC, Ashoor HM, Antony J, Beyene J, Veroniki AA, Isaranuwatchai W, et al. Safety, effectiveness, and cost effectiveness of long acting versus intermediate acting insulin for patients with type 1 diabetes: systematic review and network meta-analysis. BMJ. 2014; 349:g5459.

266. Laranjeira FO, de Andrade KRC, Figueiredo ACMG, Silva EN, Pereira MG. Long-acting insulin analogues for type 1 diabetes: an overview of systematic reviews and meta-analysis of randomized controlled trials. PLoS One. 2018; 13:e0194801.

267. Melo KFS, Bahia LR, Pasinato B, Porfirio GJM, Martimbianco AL, Riera R, et al. Short-acting insulin analogues versus regular human insulin on postprandial glucose and hypoglycemia in type 1 diabetes mellitus: a systematic review and meta-analysis. Diabetol Metab Syndr. 2019; 11:2.

268. Veroniki AA, Seitidis G, Stewart L, Clarke M, Tudur-Smith C, Mavridis D, et al. Comparative efficacy and complications of long-acting and intermediate-acting insulin regimens for adults with type 1 diabetes: an individual patient data network meta-analysis. BMJ Open. 2022; 12:e058034.
269. Saunders H, Pham B, Loong D, Mishra S, Ashoor HM, Antony J, et al. The cost-effectiveness of intermediate-acting, long-acting, ultralong-acting, and biosimilar insulins for type 1 diabetes mellitus: a systematic review. Value Health. 2022; 25:1235–52.

270. Tricco AC, Ashoor HM, Antony J, Bouck Z, Rodrigues M, Pham B, et al. Comparative efficacy and safety of ultra-long-acting, long-acting, intermediate-acting, and biosimilar insulins for type 1 diabetes mellitus: a systematic review and network meta-analysis. J Gen Intern Med. 2021; 36:2414–26.

271. Hemmingsen B, Metzendorf MI, Richter B. (Ultra-)long-acting insulin analogues for people with type 1 diabetes mellitus. Cochrane Database Syst Rev. 2021; 3:CD013498.

272. Bartley PC, Bogoev M, Larsen J, Philotheou A. Long-term efficacy and safety of insulin detemir compared to neutral protamine Hagedorn insulin in patients with type 1 diabetes using a treat-to-target basal-bolus regimen with insulin aspart at meals: a 2-year, randomized, controlled trial. Diabet Med. 2008; 25:442–9.

273. Agesen RM, Kristensen PL, Beck-Nielsen H, Norgaard K, Perrild H, Jensen T, et al. Effect of insulin analogs on frequency of non-severe hypoglycemia in patients with type 1 diabetes prone to severe hypoglycemia: much higher rates detected by continuous glucose monitoring than by self-monitoring of blood glucose: the HypoAna trial. Diabetes Technol Ther. 2018; 20:247–56.
274. Testa MA, Gill J, Su M, Turner RR, Blonde L, Simonson DC. Comparative effectiveness of basal-bolus versus premix analog insulin on glycemic variability and patient-centered outcomes during insulin intensification in type 1 and type 2 diabetes: a randomized, controlled, crossover trial. J Clin Endocrinol Metab. 2012; 97:3504–14.
275. Matsuhisa M, Koyama M, Cheng X, Takahashi Y, Riddle MC, Bolli GB, et al. New insulin glargine 300 U/ml versus glargine 100 U/ml in Japanese adults with type 1 diabetes using basal and mealtime insulin: glucose control and hypoglycaemia in a randomized controlled trial (EDITION JP 1). Diabetes Obes Metab. 2016; 18:375–83.
276. Home PD, Bergenstal RM, Bolli GB, Ziemen M, Rojeski M, Espinasse M, et al. Glycaemic control and hypoglycaemia during 12 months of randomized treatment with insulin glargine 300 U/mL versus glargine 100 U/mL in people with type 1 diabetes (EDITION 4). Diabetes Obes Metab. 2018; 20:121–8.
277. Lane W, Bailey TS, Gerety G, Gumprecht J, Philis-Tsimikas A, Hansen CT, et al. Effect of insulin degludec vs insulin glargine U100 on hypoglycemia in patients with type 1 diabetes: the SWITCH 1 randomized clinical trial. JAMA. 2017; 318:33–44.

278. Mathieu C, Hollander P, Miranda-Palma B, Cooper J, Franek E, Russell-Jones D, et al. Efficacy and safety of insulin degludec in a flexible dosing regimen vs insulin glargine in patients with type 1 diabetes (BEGIN: Flex T1): a 26-week randomized, treat-to-target trial with a 26-week extension. J Clin Endocrinol Metab. 2013; 98:1154–62.
279. Russell-Jones D, Bode BW, De Block C, Franek E, Heller SR, Mathieu C, et al. Fast-acting insulin aspart improves glycemic control in basal-bolus treatment for type 1 diabetes: results of a 26-week multicenter, active-controlled, treat-to-target, randomized, parallel-group trial (onset 1). Diabetes Care. 2017; 40:943–50.

280. Mathieu C, Bode BW, Franek E, Philis-Tsimikas A, Rose L, Graungaard T, et al. Efficacy and safety of fast-acting insulin aspart in comparison with insulin aspart in type 1 diabetes (onset 1): a 52-week, randomized, treat-to-target, phase III trial. Diabetes Obes Metab. 2018; 20:1148–55.

281. Klaff L, Cao D, Dellva MA, Tobian J, Miura J, Dahl D, et al. Ultra rapid lispro improves postprandial glucose control compared with lispro in patients with type 1 diabetes: results from the 26-week PRONTO-T1D study. Diabetes Obes Metab. 2020; 22:1799–807.
282. Ratner RE, Dickey R, Fineman M, Maggs DG, Shen L, Strobel SA, et al. Amylin replacement with pramlintide as an adjunct to insulin therapy improves long-term glycaemic and weight control in type 1 diabetes mellitus: a 1-year, randomized controlled trial. Diabet Med. 2004; 21:1204–12.
283. Edelman S, Garg S, Frias J, Maggs D, Wang Y, Zhang B, et al. A double-blind, placebo-controlled trial assessing pramlintide treatment in the setting of intensive insulin therapy in type 1 diabetes. Diabetes Care. 2006; 29:2189–95.
284. Meng H, Zhang A, Liang Y, Hao J, Zhang X, Lu J. Effect of metformin on glycaemic control in patients with type 1 diabetes: a meta-analysis of randomized controlled trials. Diabetes Metab Res Rev. 2018; 34:e2983.

285. Petrie JR, Chaturvedi N, Ford I, Brouwers MCGJ, Greenlaw N, Tillin T, et al. Cardiovascular and metabolic effects of metformin in patients with type 1 diabetes (REMOVAL): a double-blind, randomised, placebo-controlled trial. Lancet Diabetes Endocrinol. 2017; 5:597–609.
286. Wang W, Liu H, Xiao S, Liu S, Li X, Yu P. Effects of insulin plus glucagon-like peptide-1 receptor agonists (GLP-1RAs) in treating type 1 diabetes mellitus: a systematic review and meta-analysis. Diabetes Ther. 2017; 8:727–38.

287. Henry RR, Thakkar P, Tong C, Polidori D, Alba M. Efficacy and safety of canagliflozin, a sodium-glucose cotransporter 2 inhibitor, as add-on to insulin in patients with type 1 diabetes. Diabetes Care. 2015; 38:2258–65.

288. Dandona P, Mathieu C, Phillip M, Hansen L, Griffen SC, Tschope D, et al. Efficacy and safety of dapagliflozin in patients with inadequately controlled type 1 diabetes (DEPICT-1): 24 week results from a multicentre, double-blind, phase 3, randomised controlled trial. Lancet Diabetes Endocrinol. 2017; 5:864–76.
289. Rosenstock J, Marquard J, Laffel LM, Neubacher D, Kaspers S, Cherney DZ, et al. Empagliflozin as adjunctive to insulin therapy in type 1 diabetes: the EASE trials. Diabetes Care. 2018; 41:2560–9.

290. Buse JB, Garg SK, Rosenstock J, Bailey TS, Banks P, Bode BW, et al. Sotagliflozin in combination with optimized insulin therapy in adults with type 1 diabetes: the North American in-Tandem1 Study. Diabetes Care. 2018; 41:1970–80.

291. Zinman B, Wanner C, Lachin JM, Fitchett D, Bluhmki E, Hantel S, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015; 373:2117–28.

292. Wiviott SD, Raz I, Bonaca MP, Mosenzon O, Kato ET, Cahn A, et al. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2019; 380:347–57.

293. Marso SP, Daniels GH, Brown-Frandsen K, Kristensen P, Mann JF, Nauck MA, et al. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2016; 375:311–22.
294. Gerstein HC, Colhoun HM, Dagenais GR, Diaz R, Lakshmanan M, Pais P, et al. Dulaglutide and cardiovascular outcomes in type 2 diabetes (REWIND): a double-blind, randomised placebo-controlled trial. Lancet. 2019; 394:121–30.
295. Maloney A, Rosenstock J, Fonseca V. A model-based meta-analysis of 24 antihyperglycemic drugs for type 2 diabetes: comparison of treatment effects at therapeutic doses. Clin Pharmacol Ther. 2019; 105:1213–23.

296. Egede LE, Gebregziabher M, Echols C, Lynch CP. Longitudinal effects of medication nonadherence on glycemic control. Ann Pharmacother. 2014; 48:562–70.

297. Huber CA, Reich O. Medication adherence in patients with diabetes mellitus: does physician drug dispensing enhance quality of care? Evidence from a large health claims database in Switzerland. Patient Prefer Adherence. 2016; 10:1803–9.

298. Iglay K, Cartier SE, Rosen VM, Zarotsky V, Rajpathak SN, Radican L, et al. Meta-analysis of studies examining medication adherence, persistence, and discontinuation of oral antihyperglycemic agents in type 2 diabetes. Curr Med Res Opin. 2015; 31:1283–96.
299. McGovern A, Tippu Z, Hinton W, Munro N, Whyte M, de Lusignan S. Systematic review of adherence rates by medication class in type 2 diabetes: a study protocol. BMJ Open. 2016; 6:e010469.

300. Khunti K, Seidu S, Kunutsor S, Davies M. Association between adherence to pharmacotherapy and outcomes in type 2 diabetes: a meta-analysis. Diabetes Care. 2017; 40:1588–96.

301. Polonsky WH, Henry RR. Poor medication adherence in type 2 diabetes: recognizing the scope of the problem and its key contributors. Patient Prefer Adherence. 2016; 10:1299–307.

302. McGovern A, Tippu Z, Hinton W, Munro N, Whyte M, de Lusignan S. Comparison of medication adherence and persistence in type 2 diabetes: a systematic review and meta-analysis. Diabetes Obes Metab. 2018; 20:1040–3.

303. Lasalvia P, Barahona-Correa JE, Romero-Alvernia DM, Gil-Tamayo S, Castaneda-Cardona C, Bayona JG, et al. Pen devices for insulin self-administration compared with needle and vial: systematic review of the literature and meta-analysis. J Diabetes Sci Technol. 2016; 10:959–66.
304. American Diabetes Association. 3. Comprehensive medical evaluation and assessment of comorbidities: standards of medical care in diabetes-2018. Diabetes Care. 2018; 41(Suppl 1):S28–37.
305. Weng J, Li Y, Xu W, Shi L, Zhang Q, Zhu D, et al. Effect of intensive insulin therapy on beta-cell function and glycaemic control in patients with newly diagnosed type 2 diabetes: a multicentre randomised parallel-group trial. Lancet. 2008; 371:1753–60.
306. Kramer CK, Zinman B, Retnakaran R. Short-term intensive insulin therapy in type 2 diabetes mellitus: a systematic review and meta-analysis. Lancet Diabetes Endocrinol. 2013; 1:28–34.

307. Lee BW, Kim JH, Ko SH, Hur KY, Kim NH, Rhee SY, et al. Insulin therapy for adult patients with type 2 diabetes mellitus: a position statement of the Korean Diabetes Association, 2017. Diabetes Metab J. 2017; 41:367–73.

308. Wu T, Betty B, Downie M, Khanolkar M, Kilov G, Orr-Walker B, et al. Practical guidance on the use of premix insulin analogs in initiating, intensifying, or switching insulin regimens in type 2 diabetes. Diabetes Ther. 2015; 6:273–87.

309. Umpierrez GE, Smiley D, Zisman A, Prieto LM, Palacio A, Ceron M, et al. Randomized study of basal-bolus insulin therapy in the inpatient management of patients with type 2 diabetes (RABBIT 2 trial). Diabetes Care. 2007; 30:2181–6.
310. Moon SJ, Cho YM. Insulin treatment in hospitalized patients. J Korean Diabetes. 2018; 19:214–23.

311. Cai X, Gao X, Yang W, Han X, Ji L. Efficacy and safety of initial combination therapy in treatment-naïve type 2 diabetes patients: a systematic review and meta-analysis. Diabetes Ther. 2018; 9:1995–2014.

312. Phung OJ, Sobieraj DM, Engel SS, Rajpathak SN. Early combination therapy for the treatment of type 2 diabetes mellitus: systematic review and meta-analysis. Diabetes Obes Metab. 2014; 16:410–7.

313. Olansky L, Reasner C, Seck TL, Williams-Herman DE, Chen M, Terranella L, et al. A treatment strategy implementing combination therapy with sitagliptin and metformin results in superior glycaemic control versus metformin monotherapy due to a low rate of addition of antihyperglycaemic agents. Diabetes Obes Metab. 2011; 13:841–9.

314. Hadjadj S, Rosenstock J, Meinicke T, Woerle HJ, Broedl UC. Initial combination of empagliflozin and metformin in patients with type 2 diabetes. Diabetes Care. 2016; 39:1718–28.
315. Kim KJ, Choi J, Bae JH, Kim KJ, Yoo HJ, Seo JA, et al. Time to reach target glycosylated hemoglobin is associated with long-term durable glycemic control and risk of diabetic complications in patients with newly diagnosed type 2 diabetes mellitus: a 6-year observational study. Diabetes Metab J. 2021; 45:368–78.

316. Matthews DR, Paldanius PM, Stumvoll M, Han J, Bader G, Chiang Y, et al. A pre-specified statistical analysis plan for the VERIFY study: vildagliptin efficacy in combination with metformin for early treatment of T2DM. Diabetes Obes Metab. 2019; 21:2240–7.

317. Milder TY, Stocker SL, Abdel Shaheed C, McGrath-Cadell L, Samocha-Bonet D, Greenfield JR, et al. Combination therapy with an SGLT2 inhibitor as initial treatment for type 2 diabetes: a systematic review and meta-analysis. J Clin Med. 2019; 8:45.

318. Wu S, Gao L, Cipriani A, Huang Y, Yang Z, Yang J, et al. The effects of incretin-based therapies on β-cell function and insulin resistance in type 2 diabetes: a systematic review and network meta-analysis combining 360 trials. Diabetes Obes Metab. 2019; 21:975–83.

319. Chin KL, Ofori-Asenso R, Si S, Hird TR, Magliano DJ, Zoungas S, et al. Cost-effectiveness of first-line versus delayed use of combination dapagliflozin and metformin in patients with type 2 diabetes. Sci Rep. 2019; 9:3256.
320. Schectman JM, Nadkarni MM, Voss JD. The association between diabetes metabolic control and drug adherence in an indigent population. Diabetes Care. 2002; 25:1015–21.

321. Pladevall M, Williams LK, Potts LA, Divine G, Xi H, Lafata JE. Clinical outcomes and adherence to medications measured by claims data in patients with diabetes. Diabetes Care. 2004; 27:2800–5.

322. Capoccia K, Odegard PS, Letassy N. Medication adherence with diabetes medication: a systematic review of the literature. Diabetes Educ. 2016; 42:34–71.
323. Cheen MHH, Tan YZ, Oh LF, Wee HL, Thumboo J. Prevalence of and factors associated with primary medication nonadherence in chronic disease: a systematic review and meta-analysis. Int J Clin Pract. 2019; 73:e13350.

324. Choi YJ, Smaldone AM. Factors associated with medication engagement among older adults with diabetes: systematic review and meta-analysis. Diabetes Educ. 2018; 44:15–30.
325. Vignon Zomahoun HT, de Bruin M, Guillaumie L, Moisan J, Gregoire JP, Perez N, et al. Effectiveness and content analysis of interventions to enhance oral antidiabetic drug adherence in adults with type 2 diabetes: systematic review and meta-analysis. Value Health. 2015; 18:530–40.

326. Long H, Bartlett YK, Farmer AJ, French DP. Identifying brief message content for interventions delivered via mobile devices to improve medication adherence in people with type 2 diabetes mellitus: a rapid systematic review. J Med Internet Res. 2019; 21:e10421.

327. Farmer AJ, McSharry J, Rowbotham S, McGowan L, Ricci-Cabello I, French DP. Effects of interventions promoting monitoring of medication use and brief messaging on medication adherence for people with type 2 diabetes: a systematic review of randomized trials. Diabet Med. 2016; 33:565–79.

328. Bennett WL, Maruthur NM, Singh S, Segal JB, Wilson LM, Chatterjee R, et al. Comparative effectiveness and safety of medications for type 2 diabetes: an update including new drugs and 2-drug combinations. Ann Intern Med. 2011; 154:602–13.

329. Zaccardi F, Dhalwani NN, Dales J, Mani H, Khunti K, Davies MJ, et al. Comparison of glucose-lowering agents after dual therapy failure in type 2 diabetes: a systematic review and network meta-analysis of randomized controlled trials. Diabetes Obes Metab. 2018; 20:985–97.
330. Lee CM, Woodward M, Colagiuri S. Triple therapy combinations for the treatment of type 2 diabetes: a network meta-analysis. Diabetes Res Clin Pract. 2016; 116:149–58.
331. Lozano-Ortega G, Goring S, Bennett HA, Bergenheim K, Sternhufvud C, Mukherjee J. Network meta-analysis of treatments for type 2 diabetes mellitus following failure with metformin plus sulfonylurea. Curr Med Res Opin. 2016; 32:807–16.

332. Qian D, Zhang T, Zheng P, Liang Z, Wang S, Xie J, et al. Comparison of oral antidiabetic drugs as add-on treatments in patients with type 2 diabetes uncontrolled on metformin: a network meta-analysis. Diabetes Ther. 2018; 9:1945–58.

333. Qaseem A, Barry MJ, Humphrey LL, Forciea MA; Clinical Guidelines Committee of the American College of Physicians, Fitterman N, et al. Oral pharmacologic treatment of type 2 diabetes mellitus: a clinical practice guideline update from the American College of Physicians. Ann Intern Med. 2017; 166:279–90.

334. Kim MK, Suk JH, Kwon MJ, Chung HS, Yoon CS, Jun HJ, et al. Nateglinide and acarbose for postprandial glucose control after optimizing fasting glucose with insulin glargine in patients with type 2 diabetes. Diabetes Res Clin Pract. 2011; 92:322–8.
335. Palmer SC, Mavridis D, Nicolucci A, Johnson DW, Tonelli M, Craig JC, et al. Comparison of clinical outcomes and adverse events associated with glucose-lowering drugs in patients with type 2 diabetes: a meta-analysis. JAMA. 2016; 316:313–24.

336. ElSayed NA, Aleppo G, Aroda VR, Bannuru RR, Brown FM, Bruemmer D, et al. 9. Pharmacologic approaches to glycemic treatment: standards of care in diabetes-2023. Diabetes Care. 2023; 46(Suppl 1):S140–57.
337. Yoon KH, Shin JA, Kwon HS, Lee SH, Min KW, Ahn YB, et al. Comparison of the efficacy of glimepiride, metformin, and rosiglitazone monotherapy in Korean drug-naïve type 2 diabetic patients: the practical evidence of antidiabetic monotherapy study. Diabetes Metab J. 2011; 35:26–33.

338. Davies MJ, D’Alessio DA, Fradkin J, Kernan WN, Mathieu C, Mingrone G, et al. Management of hyperglycemia in type 2 diabetes, 2018. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care. 2018; 41:2669–701.

339. Zhang K, Yang W, Dai H, Deng Z. Cardiovascular risk following metformin treatment in patients with type 2 diabetes mellitus: results from meta-analysis. Diabetes Res Clin Pract. 2020; 160:108001.
340. Bailey CJ, Turner RC. Metformin. N Engl J Med. 1996; 334:574–9.

341. Yang W, Cai X, Wu H, Ji L. Associations between metformin use and vitamin B12 levels, anemia, and neuropathy in patients with diabetes: a meta-analysis. J Diabetes. 2019; 11:729–43.
342. Out M, Kooy A, Lehert P, Schalkwijk CA, Stehouwer CDA. Long-term treatment with metformin in type 2 diabetes and methylmalonic acid: post hoc analysis of a randomized controlled 4.3 year trial. J Diabetes Complications. 2018; 32:171–8.

344. Maruthur NM, Tseng E, Hutfless S, Wilson LM, Suarez-Cuervo C, Berger Z, et al. Diabetes medications as monotherapy or metformin-based combination therapy for type 2 diabetes: a systematic review and meta-analysis. Ann Intern Med. 2016; 164:740–51.

345. Hur KY, Moon MK, Park JS, Kim SK, Lee SH, Yun JS, et al. 2021 Clinical practice guidelines for diabetes mellitus of the Korean Diabetes Association. Diabetes Metab J. 2021; 45:461–81.
346. Tsapas A, Avgerinos I, Karagiannis T, Malandris K, Manolopoulos A, Andreadis P, et al. Comparative effectiveness of glucose-lowering drugs for type 2 diabetes: a systematic review and network meta-analysis. Ann Intern Med. 2020; 173:278–86.
347. Nauck MA, Mirna AEA, Quast DR. Meta-analysis of head-tohead clinical trials comparing incretin-based glucose-lowering medications and basal insulin: an update including recently developed glucagon-like peptide-1 (GLP-1) receptor agonists and the glucose-dependent insulinotropic polypeptide/GLP-1 receptor co-agonist tirzepatide. Diabetes Obes Metab. 2023; 25:1361–71.
348. DeVries JH, Bain SC, Rodbard HW, Seufert J, D’Alessio D, Thomsen AB, et al. Sequential intensification of metformin treatment in type 2 diabetes with liraglutide followed by randomized addition of basal insulin prompted by A1C targets. Diabetes Care. 2012; 35:1446–54.

349. Aroda VR, Bailey TS, Cariou B, Kumar S, Leiter LA, Raskin P, et al. Effect of adding insulin degludec to treatment in patients with type 2 diabetes inadequately controlled with metformin and liraglutide: a double-blind randomized controlled trial (BEGIN: ADD TO GLP-1 Study). Diabetes Obes Metab. 2016; 18:663–70.
350. Rosenstock J, Aronson R, Grunberger G, Hanefeld M, Piatti P, Serusclat P, et al. Benefits of Lixilan, a titratable fixed-ratio combination of insulin glargine plus lixisenatide, versus insulin glargine and lixisenatide monocomponents in type 2 diabetes inadequately controlled on oral agents: the Lixilan-O randomized trial. Diabetes Care. 2016; 39:2026–35.
351. Blonde L, Rosenstock J, Del Prato S, Henry R, Shehadeh N, Frias J, et al. Switching to iGlarLixi versus continuing daily or weekly GLP-1 RA in type 2 diabetes inadequately controlled by GLP-1 RA and oral antihyperglycemic therapy: the Lixi-Lan-G randomized clinical trial. Diabetes Care. 2019; 42:2108–16.

352. Gough SC, Bode B, Woo V, Rodbard HW, Linjawi S, Poulsen P, et al. Efficacy and safety of a fixed-ratio combination of insulin degludec and liraglutide (IDegLira) compared with its components given alone: results of a phase 3, open-label, randomised, 26-week, treat-to-target trial in insulin-naive patients with type 2 diabetes. Lancet Diabetes Endocrinol. 2014; 2:885–93.

353. Linjawi S, Bode BW, Chaykin LB, Courreges JP, Handelsman Y, Lehmann LM, et al. The efficacy of IDegLira (insulin degludec/liraglutide combination) in adults with type 2 diabetes inadequately controlled with a GLP-1 receptor agonist and oral therapy: DUAL III randomized clinical trial. Diabetes Ther. 2017; 8:101–14.

354. Buse JB, Bergenstal RM, Glass LC, Heilmann CR, Lewis MS, Kwan AY, et al. Use of twice-daily exenatide in basal insulin-treated patients with type 2 diabetes: a randomized, controlled trial. Ann Intern Med. 2011; 154:103–12.

355. Seino Y, Min KW, Niemoeller E, Takami A; EFC10887 GETGOAL-L Asia Study Investigators. Randomized, double-blind, placebo-controlled trial of the once-daily GLP-1 receptor agonist lixisenatide in Asian patients with type 2 diabetes insufficiently controlled on basal insulin with or without a sulfonylurea (GetGoal-L-Asia). Diabetes Obes Metab. 2012; 14:910–7.
356. Riddle MC, Aronson R, Home P, Marre M, Niemoeller E, Miossec P, et al. Adding once-daily lixisenatide for type 2 diabetes inadequately controlled by established basal insulin: a 24-week, randomized, placebo-controlled comparison (Get-Goal-L). Diabetes Care. 2013; 36:2489–96.
357. Riddle MC, Forst T, Aronson R, Sauque-Reyna L, Souhami E, Silvestre L, et al. Adding once-daily lixisenatide for type 2 diabetes inadequately controlled with newly initiated and continuously titrated basal insulin glargine: a 24-week, randomized, placebo-controlled study (GetGoal-Duo 1). Diabetes Care. 2013; 36:2497–503.
358. Yang W, Min K, Zhou Z, Li L, Xu X, Zhu D, et al. Efficacy and safety of lixisenatide in a predominantly Asian population with type 2 diabetes insufficiently controlled with basal insulin: the GetGoal-L-C randomized trial. Diabetes Obes Metab. 2018; 20:335–43.
359. Ahmann A, Rodbard HW, Rosenstock J, Lahtela JT, de Loredo L, Tornoe K, et al. Efficacy and safety of liraglutide versus placebo added to basal insulin analogues (with or without metformin) in patients with type 2 diabetes: a randomized, placebo-controlled trial. Diabetes Obes Metab. 2015; 17:1056–64.

360. Pozzilli P, Norwood P, Jodar E, Davies MJ, Ivanyi T, Jiang H, et al. Placebo-controlled, randomized trial of the addition of once-weekly glucagon-like peptide-1 receptor agonist dulaglutide to titrated daily insulin glargine in patients with type 2 diabetes (AWARD-9). Diabetes Obes Metab. 2017; 19:1024–31.
361. Rodbard HW, Lingvay I, Reed J, de la Rosa R, Rose L, Sugimoto D, et al. Semaglutide added to basal insulin in type 2 diabetes (SUSTAIN 5): a randomized, controlled trial. J Clin Endocrinol Metab. 2018; 103:2291–301.

362. Aroda VR, Rosenstock J, Wysham C, Unger J, Bellido D, Gonzalez-Galvez G, et al. Efficacy and safety of LixiLan, a titratable fixed-ratio combination of insulin glargine plus lixisenatide in type 2 diabetes inadequately controlled on basal insulin and metformin: the LixiLan-L randomized trial. Diabetes Care. 2016; 39:1972–80.

363. Rosenstock J, Diamant M, Aroda VR, Silvestre L, Souhami E, Zhou T, et al. Efficacy and safety of Lixilan, a titratable fixedratio combination of lixisenatide and insulin glargine, versus insulin glargine in type 2 diabetes inadequately controlled on metformin monotherapy: the LixiLan proof-of-concept randomized trial. Diabetes Care. 2016; 39:1579–86.

364. Buse JB, Vilsboll T, Thurman J, Blevins TC, Langbakke IH, Bottcher SG, et al. Contribution of liraglutide in the fixed-ratio combination of insulin degludec and liraglutide (IDegLira). Diabetes Care. 2014; 37:2926–33.

365. Lingvay I, Perez Manghi F, Garcia-Hernandez P, Norwood P, Lehmann L, Tarp-Johansen MJ, et al. Effect of insulin glargine up-titration vs insulin degludec/liraglutide on glycated hemoglobin levels in patients with uncontrolled type 2 diabetes: the DUAL V randomized clinical trial. JAMA. 2016; 315:898–907.
366. Owens DR, Luzio SD, Sert-Langeron C, Riddle MC. Effects of initiation and titration of a single pre-prandial dose of insulin glulisine while continuing titrated insulin glargine in type 2 diabetes: a 6-month ‘proof-of-concept’ study. Diabetes Obes Metab. 2011; 13:1020–7.
367. Lankisch MR, Ferlinz KC, Leahy JL, Scherbaum WA; Orals Plus Apidra and LANTUS (OPAL) study group. Introducing a simplified approach to insulin therapy in type 2 diabetes: a comparison of two single-dose regimens of insulin glulisine plus insulin glargine and oral antidiabetic drugs. Diabetes Obes Metab. 2008; 10:1178–85.
368. Leahy JL. Insulin therapy in type 2 diabetes mellitus. Endocrinol Metab Clin North Am. 2012; 41:119–44.
369. Giugliano D, Maiorino MI, Bellastella G, Chiodini P, Ceriello A, Esposito K. Efficacy of insulin analogs in achieving the hemoglobin A1c target of <7% in type 2 diabetes: meta-analysis of randomized controlled trials. Diabetes Care. 2011; 34:510–7.
370. Davidson MB, Raskin P, Tanenberg RJ, Vlajnic A, Hollander P. A stepwise approach to insulin therapy in patients with type 2 diabetes mellitus and basal insulin treatment failure. Endocr Pract. 2011; 17:395–403.
371. Castellana M, Cignarelli A, Brescia F, Laviola L, Giorgino F. GLP-1 receptor agonist added to insulin versus basal-plus or basal-bolus insulin therapy in type 2 diabetes: a systematic review and meta-analysis. Diabetes Metab Res Rev. 2019; 35:e3082.
372. Rosenstock J, Nino A, Soffer J, Erskine L, Acusta A, Dole J, et al. Impact of a weekly glucagon-like peptide 1 receptor agonist, albiglutide, on glycemic control and on reducing prandial insulin use in type 2 diabetes inadequately controlled on multiple insulin therapy: a randomized trial. Diabetes Care. 2020; 43:2509–18.

373. Holman RR, Thorne KI, Farmer AJ, Davies MJ, Keenan JF, Paul S, et al. Addition of biphasic, prandial, or basal insulin to oral therapy in type 2 diabetes. N Engl J Med. 2007; 357:1716–30.

374. Home P, Naggar NE, Khamseh M, Gonzalez-Galvez G, Shen C, Chakkarwar P, et al. An observational non-interventional study of people with diabetes beginning or changed to insulin analogue therapy in non-Western countries: the A1chieve study. Diabetes Res Clin Pract. 2011; 94:352–63.

375. Aschner P, Sethi B, Gomez-Peralta F, Landgraf W, Loizeau V, Dain MP, et al. Insulin glargine compared with premixed in sulin for management of insulin-naïve type 2 diabetes patients uncontrolled on oral antidiabetic drugs: the open-label, randomized GALAPAGOS study. J Diabetes Complications. 2015; 29:838–45.
376. Lee YH, Lee BW, Chun SW, Cha BS, Lee HC. Predictive characteristics of patients achieving glycaemic control with insulin after sulfonylurea failure. Int J Clin Pract. 2011; 65:1076–84.

377. Cannon CP, Pratley R, Dagogo-Jack S, Mancuso J, Huyck S, Masiukiewicz U, et al. Cardiovascular outcomes with ertugliflozin in type 2 diabetes. N Engl J Med. 2020; 383:1425–35.

378. Radholm K, Figtree G, Perkovic V, Solomon SD, Mahaffey KW, de Zeeuw D, et al. Canagliflozin and heart failure in type 2 diabetes mellitus: results from the CANVAS program. Circulation. 2018; 138:458–68.
379. Kato ET, Silverman MG, Mosenzon O, Zelniker TA, Cahn A, Furtado RHM, et al. Effect of dapagliflozin on heart failure and mortality in type 2 diabetes mellitus. Circulation. 2019; 139:2528–36.

380. Buse JB, Wexler DJ, Tsapas A, Rossing P, Mingrone G, Mathieu C, et al. 2019 Update to: management of hyperglycemia in type 2 diabetes, 2018. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care. 2020; 43:487–93.
381. McMurray JJV, Solomon SD, Inzucchi SE, Kober L, Kosiborod MN, Martinez FA, et al. Dapagliflozin in patients with heart failure and reduced ejection fraction. N Engl J Med. 2019; 381:1995–2008.
382. Zannad F, Ferreira JP, Pocock SJ, Anker SD, Butler J, Filippatos G, et al. SGLT2 inhibitors in patients with heart failure with reduced ejection fraction: a meta-analysis of the EMPERORReduced and DAPA-HF trials. Lancet. 2020; 396:819–29.

383. Anker SD, Butler J, Filippatos G, Ferreira JP, Bocchi E, Bohm M, et al. Empagliflozin in heart failure with a preserved ejection fraction. N Engl J Med. 2021; 385:1451–61.
384. Wanner Ch, Inzucchi SE, Zinman B. Empagliflozin and progression of kidney disease in type 2 diabetes. N Engl J Med. 2016; 375:1801–2.

385. Heerspink HJL, Stefansson BV, Correa-Rotter R, Chertow GM, Greene T, Hou FF, et al. Dapagliflozin in patients with chronic kidney disease. N Engl J Med. 2020; 383:1436–46.
386. McGuire DK, Shih WJ, Cosentino F, Charbonnel B, Cherney DZI, Dagogo-Jack S, et al. Association of SGLT2 inhibitors with cardiovascular and kidney outcomes in patients with type 2 diabetes: a meta-analysis. JAMA Cardiol. 2021; 6:148–58.

387. The EMPA-KIDNEY Collaborative Group, Herrington WG, Staplin N, Wanner C, Green JB, Hauske SJ, et al. Empagliflozin in patients with chronic kidney disease. N Engl J Med. 2023; 388:117–27.

388. Oh TJ, Moon JY, Hur KY, Ko SH, Kim HJ, Kim T, et al. Sodium-glucose cotransporter-2 inhibitor for renal function preservation in patients with type 2 diabetes mellitus: a Korean Diabetes Association and Korean Society of Nephrology consensus statement. Diabetes Metab J. 2020; 44:489–97.

389. Kadowaki T, Nangaku M, Hantel S, Okamura T, von Eynatten M, Wanner C, et al. Empagliflozin and kidney outcomes in Asian patients with type 2 diabetes and established cardiovascular disease: results from the EMPA-REG OUTCOME trial. J Diabetes Investig. 2019; 10:760–70.
390. Kaku K, Lee J, Mattheus M, Kaspers S, George J, Woerle HJ, et al. Empagliflozin and cardiovascular outcomes in Asian patients with type 2 diabetes and established cardiovascular disease: results from EMPA-REG OUTCOME. Circ J. 2017; 81:227–34.
391. Zhang XL, Zhu QQ, Chen YH, Li XL, Chen F, Huang JA, et al. Cardiovascular safety, long-term noncardiovascular safety, and efficacy of sodium-glucose cotransporter 2 inhibitors in patients with type 2 diabetes mellitus: a systemic review and meta-analysis with trial sequential analysis. J Am Heart Assoc. 2018; 7:e007165.

392. Arnott C, Li Q, Kang A, Neuen BL, Bompoint S, Lam CSP, et al. Sodium-glucose cotransporter 2 inhibition for the prevention of cardiovascular events in patients with type 2 diabetes mellitus: a systematic review and meta-analysis. J Am Heart Assoc. 2020; 9:e014908.

393. Lo KB, Gul F, Ram P, Kluger AY, Tecson KM, McCullough PA, et al. The effects of SGLT2 inhibitors on cardiovascular and renal outcomes in diabetic patients: a systematic review and meta-analysis. Cardiorenal Med. 2020; 10:1–10.

394. Zelniker TA, Wiviott SD, Raz I, Im K, Goodrich EL, Bonaca MP, et al. SGLT2 inhibitors for primary and secondary prevention of cardiovascular and renal outcomes in type 2 diabetes: a systematic review and meta-analysis of cardiovascular outcome trials. Lancet. 2019; 393:31–9.

395. Marso SP, Bain SC, Consoli A, Eliaschewitz FG, Jodar E, Leiter LA, et al. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med. 2016; 375:1834–44.
396. Dormandy JA, Charbonnel B, Eckland DJ, Erdmann E, Massi-Benedetti M, Moules IK, et al. Secondary prevention of macrovascular events in patients with type 2 diabetes in the PROactive Study (PROspective pioglitAzone Clinical Trial In macroVascular Events): a randomised controlled trial. Lancet. 2005; 366:1279–89.

397. Liu D, Jin B, Chen W, Yun P. Dipeptidyl peptidase 4 (DPP-4) inhibitors and cardiovascular outcomes in patients with type 2 diabetes mellitus (T2DM): a systematic review and meta-analysis. BMC Pharmacol Toxicol. 2019; 20:15.

398. Alfayez OM, Almutairi AR, Aldosari A, Al Yami MS. Update on cardiovascular safety of incretin-based therapy in adults with type 2 diabetes mellitus: a meta-analysis of cardiovascular outcome trials. Can J Diabetes. 2019; 43:538–45.e2.

399. Look AHEAD Research Group, Wing RR, Bolin P, Brancati FL, Bray GA, Clark JM, et al. Cardiovascular effects of intensive lifestyle intervention in type 2 diabetes. N Engl J Med. 2013; 369:145–54.

400. Look AHEAD Research Group, Gregg EW, Jakicic JM, Blackburn G, Bloomquist P, Bray GA, et al. Association of the magnitude of weight loss and changes in physical fitness with long-term cardiovascular disease outcomes in overweight or obese people with type 2 diabetes: a post-hoc analysis of the Look AHEAD randomised clinical trial. Lancet Diabetes Endocrinol. 2016; 4:913–21.
401. Lean MEJ, Leslie WS, Barnes AC, Brosnahan N, Thom G, Mc-Combie L, et al. Durability of a primary care-led weight-management intervention for remission of type 2 diabetes: 2-year results of the DiRECT open-label, cluster-randomised trial. Lancet Diabetes Endocrinol. 2019; 7:344–55.

402. Korean Society for the Study of Obesity. Clinical practice guidelines for obesity 2022. Seoul: Korean Society for the Study of Obesity;2022.
403. Look AHEAD Research Group. Eight-year weight losses with an intensive lifestyle intervention: the Look AHEAD study. Obesity (Silver Spring). 2014; 22:5–13.
404. Look AHEAD Research Group. Effects of intensive lifestyle intervention on all-cause mortality in older adults with type 2 diabetes and overweight/obesity: results from the Look AHEAD study. Diabetes Care. 2022; 45:1252–9.
405. Espeland MA, Glick HA, Bertoni A, Brancati FL, Bray GA, Clark JM, et al. Impact of an intensive lifestyle intervention on use and cost of medical services among overweight and obese adults with type 2 diabetes: the action for health in diabetes. Diabetes Care. 2014; 37:2548–56.

406. Tsapas A, Karagiannis T, Kakotrichi P, Avgerinos I, Mantsiou C, Tousinas G, et al. Comparative efficacy of glucose-lowering medications on body weight and blood pressure in patients with type 2 diabetes: a systematic review and network meta-analysis. Diabetes Obes Metab. 2021; 23:2116–24.
407. Miles JM, Leiter L, Hollander P, Wadden T, Anderson JW, Doyle M, et al. Effect of orlistat in overweight and obese patients with type 2 diabetes treated with metformin. Diabetes Care. 2002; 25:1123–8.

408. Hollander P, Gupta AK, Plodkowski R, Greenway F, Bays H, Burns C, et al. Effects of naltrexone sustained-release/bupropion sustained-release combination therapy on body weight and glycemic parameters in overweight and obese patients with type 2 diabetes. Diabetes Care. 2013; 36:4022–9.
409. Davies MJ, Bergenstal R, Bode B, Kushner RF, Lewin A, Skjoth TV, et al. Efficacy of liraglutide for weight loss among patients with type 2 diabetes: the SCALE diabetes randomized clinical trial. JAMA. 2015; 314:687–99.

410. Yanovski SZ, Yanovski JA. Long-term drug treatment for obesity: a systematic and clinical review. JAMA. 2014; 311:74–86.
411. Gadde KM, Allison DB, Ryan DH, Peterson CA, Troupin B, Schwiers ML, et al. Effects of low-dose, controlled-release, phentermine plus topiramate combination on weight and associated comorbidities in overweight and obese adults (CONQUER): a randomised, placebo-controlled, phase 3 trial. Lancet. 2011; 377:1341–52.

412. Davies M, Faerch L, Jeppesen OK, Pakseresht A, Pedersen SD, Perreault L, et al. Semaglutide 2.4 mg once a week in adults with overweight or obesity, and type 2 diabetes (STEP 2): a randomised, double-blind, double-dummy, placebo-controlled, phase 3 trial. Lancet. 2021; 397:971–84.

413. Kadowaki T, Isendahl J, Khalid U, Lee SY, Nishida T, Ogawa W, et al. Semaglutide once a week in adults with overweight or obesity, with or without type 2 diabetes in an east Asian population (STEP 6): a randomised, double-blind, double-dummy, placebo-controlled, phase 3a trial. Lancet Diabetes Endocrinol. 2022; 10:193–206.
414. Rosenstock J, Wysham C, Frias JP, Kaneko S, Lee CJ, Fernandez Lando L, et al. Efficacy and safety of a novel dual GIP and GLP-1 receptor agonist tirzepatide in patients with type 2 diabetes (SURPASS-1): a double-blind, randomised, phase 3 trial. Lancet. 2021; 398:143–55.

415. Frias JP, Davies MJ, Rosenstock J, Perez Manghi FC, Fernandez Lando L, Bergman BK, et al. Tirzepatide versus semaglutide once weekly in patients with type 2 diabetes. N Engl J Med. 2021; 385:503–15.

416. Ludvik B, Giorgino F, Jodar E, Frias JP, Fernandez Lando L, Brown K, et al. Once-weekly tirzepatide versus once-daily insulin degludec as add-on to metformin with or without SGLT2 inhibitors in patients with type 2 diabetes (SURPASS-3): a randomised, open-label, parallel-group, phase 3 trial. Lancet. 2021; 398:583–98.

417. Schauer PR, Bhatt DL, Kirwan JP, Wolski K, Aminian A, Brethauer SA, et al. Bariatric surgery versus intensive medical therapy for diabetes: 5-year outcomes. N Engl J Med. 2017; 376:641–51.

418. Mingrone G, Panunzi S, De Gaetano A, Guidone C, Iaconelli A, Capristo E, et al. Metabolic surgery versus conventional medical therapy in patients with type 2 diabetes: 10-year follow-up of an open-label, single-centre, randomised controlled trial. Lancet. 2021; 397:293–304.
419. Cohen R, Le Roux CW, Junqueira S, Ribeiro RA, Luque A. Roux-en-Y gastric bypass in type 2 diabetes patients with mild obesity: a systematic review and meta-analysis. Obes Surg. 2017; 27:2733–9.

420. Yeo D, Yeo C, Low TY, Ahmed S, Phua S, Oo AM, et al. Outcomes after metabolic surgery in Asians: a meta-analysis. Obes Surg. 2019; 29:114–26.

421. Kirwan JP, Courcoulas AP, Cummings DE, Goldfine AB, Kashyap SR, Simonson DC, et al. Diabetes remission in the alliance of randomized trials of medicine versus metabolic surgery in type 2 diabetes (ARMMS-T2D). Diabetes Care. 2022; 45:1574–83.

422. Syn NL, Cummings DE, Wang LZ, Lin DJ, Zhao JJ, Loh M, et al. Association of metabolic-bariatric surgery with long-term survival in adults with and without diabetes: a one-stage meta-analysis of matched cohort and prospective controlled studies with 174 772 participants. Lancet. 2021; 397:1830–41.
423. Bullen NL, Parmar J, Gilbert J, Clarke M, Cota A, Finlay IG. How effective is the multidisciplinary team approach in bariatric surgery? Obes Surg. 2019; 29:3232–8.
424. Aminian A, Zajichek A, Arterburn DE, Wolski KE, Brethauer SA, Schauer PR, et al. Association of metabolic surgery with major adverse cardiovascular outcomes in patients with type 2 diabetes and obesity. JAMA. 2019; 322:1271–82.

425. Billeter AT, Scheurlen KM, Probst P, Eichel S, Nickel F, Kopf S, et al. Meta-analysis of metabolic surgery versus medical treatment for microvascular complications in patients with type 2 diabetes mellitus. Br J Surg. 2018; 105:168–81.
426. Uhe I, Douissard J, Podetta M, Chevallay M, Toso C, Jung MK, et al. Roux-en-Y gastric bypass, sleeve gastrectomy, or one-anastomosis gastric bypass? A systematic review and meta-analysis of randomized-controlled trials. Obesity (Silver Spring). 2022; 30:614–27.

427. Sha Y, Huang X, Ke P, Wang B, Yuan H, Yuan W, et al. Laparoscopic Roux-en-Y gastric bypass versus sleeve gastrectomy for type 2 diabetes mellitus in nonseverely obese patients: a systematic review and meta-analysis of randomized controlled trials. Obes Surg. 2020; 30:1660–70.

428. Kasama K, Mui W, Lee WJ, Lakdawala M, Naitoh T, Seki Y, et al. IFSO-APC consensus statements 2011. Obes Surg. 2012; 22:677–84.
429. Rubino F, Nathan DM, Eckel RH, Schauer PR, Alberti KG, Zimmet PZ, et al. Metabolic surgery in the treatment algorithm for type 2 diabetes: a joint statement by International Diabetes Organizations. Diabetes Care. 2016; 39:861–77.
430. Aminian A, Chang J, Brethauer SA, Kim JJ; American Society for Metabolic and Bariatric Surgery Clinical Issues Committee. ASMBS updated position statement on bariatric surgery in class I obesity (BMI 30-35 kg/m2). Surg Obes Relat Dis. 2018; 14:1071–87.
431. Adler AI, Stratton IM, Neil HA, Yudkin JS, Matthews DR, Cull CA, et al. Association of systolic blood pressure with macrovascular and microvascular complications of type 2 diabetes (UKPDS 36): prospective observational study. BMJ. 2000; 321:412–9.

432. Korean Diabetes Association. Diabetes fact sheets in Korea 2022. Seoul: Korean Diabetes Association;2022.
433. Omboni S, Gazzola T, Carabelli G, Parati G. Clinical usefulness and cost effectiveness of home blood pressure telemonitoring: meta-analysis of randomized controlled studies. J Hypertens. 2013; 31:455–67.
434. Emdin CA, Rahimi K, Neal B, Callender T, Perkovic V, Patel A. Blood pressure lowering in type 2 diabetes: a systematic review and meta-analysis. JAMA. 2015; 313:603–15.
435. ACCORD Study Group, Cushman WC, Evans GW, Byington RP, Goff DC Jr, Grimm RH Jr, et al. Effects of intensive bloodpressure control in type 2 diabetes mellitus. N Engl J Med. 2010; 362:1575–85.

436. SPRINT Research Group, Wright JT Jr, Williamson JD, Whelton PK, Snyder JK, Sink KM, et al. A randomized trial of intensive versus standard blood-pressure control. N Engl J Med. 2015; 373:2103–16.

437. Buckley LF, Dixon DL, Wohlford GF 4th, Wijesinghe DS, Baker WL, Van Tassell BW. Intensive versus standard blood pressure control in SPRINT-eligible participants of ACCORD-BP. Diabetes Care. 2017; 40:1733–8.

438. Whelton PK, Carey RM, Aronow WS, Casey DE Jr, Collins KJ, Dennison Himmelfarb C, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension. 2018; 71:e13–115.
439. Williams B, Mancia G, Spiering W, Agabiti Rosei E, Azizi M, Burnier M, et al. 2018 ESC/ESH guidelines for the management of arterial hypertension: the task force for the management of arterial hypertension of the European Society of Cardiology and the European Society of Hypertension. J Hypertens. 2018; 36:1953–2041.
440. Zhang W, Zhang S, Deng Y, Wu S, Ren J, Sun G, et al. Trial of intensive blood-pressure control in older patients with hypertension. N Engl J Med. 2021; 385:1268–79.

441. American Society of Hypertension Guideline Committee. 2022 Hypertension guidelines. Seoul: Korean Society of Hypertension;2022.
442. UK Prospective Diabetes Study Group. Tight blood pressure control and risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 38. BMJ. 1998; 317:703–13.
443. Hansson L, Zanchetti A, Carruthers SG, Dahlof B, Elmfeldt D, Julius S, et al. Effects of intensive blood-pressure lowering and low-dose aspirin in patients with hypertension: principal results of the Hypertension Optimal Treatment (HOT) randomised trial. HOT Study Group. Lancet. 1998; 351:1755–62.
444. Beddhu S, Greene T, Boucher R, Cushman WC, Wei G, Stoddard G, et al. Intensive systolic blood pressure control and incident chronic kidney disease in people with and without diabetes mellitus: secondary analyses of two randomised controlled trials. Lancet Diabetes Endocrinol. 2018; 6:555–63.

445. Sink KM, Evans GW, Shorr RI, Bates JT, Berlowitz D, Conroy MB, et al. Syncope, hypotension, and falls in the treatment of hypertension: results from the randomized clinical systolic blood pressure intervention trial. J Am Geriatr Soc. 2018; 66:679–86.

446. Bakris GL, Gaxiola E, Messerli FH, Mancia G, Erdine S, Cooper-DeHoff R, et al. Clinical outcomes in the diabetes cohort of the INternational VErapamil SR-Trandolapril study. Hypertension. 2004; 44:637–42.
447. Elmer PJ, Obarzanek E, Vollmer WM, Simons-Morton D, Stevens VJ, Young DR, et al. Effects of comprehensive lifestyle modification on diet, weight, physical fitness, and blood pressure control: 18-month results of a randomized trial. Ann Intern Med. 2006; 144:485–95.

448. Neter JE, Stam BE, Kok FJ, Grobbee DE, Geleijnse JM. Influence of weight reduction on blood pressure: a meta-analysis of randomized controlled trials. Hypertension. 2003; 42:878–84.
449. Graudal NA, Hubeck-Graudal T, Jurgens G. Effects of low-sodium diet vs. high-sodium diet on blood pressure, renin, aldosterone, catecholamines, cholesterol, and triglyceride (Cochrane Review). Am J Hypertens. 2012; 25:1–15.

450. He FJ, MacGregor GA. Salt reduction lowers cardiovascular risk: meta-analysis of outcome trials. Lancet. 2011; 378:380–2.

451. Xin X, He J, Frontini MG, Ogden LG, Motsamai OI, Whelton PK. Effects of alcohol reduction on blood pressure: a meta-analysis of randomized controlled trials. Hypertension. 2001; 38:1112–7.
452. Turnbull F, Neal B, Algert C, Chalmers J, Chapman N, Cutler J, et al. Effects of different blood pressure-lowering regimens on major cardiovascular events in individuals with and without diabetes mellitus: results of prospectively designed overviews of randomized trials. Arch Intern Med. 2005; 165:1410–9.

453. Bangalore S, Fakheri R, Toklu B, Messerli FH. Diabetes mellitus as a compelling indication for use of renin angiotensin system blockers: systematic review and meta-analysis of randomized trials. BMJ. 2016; 352:i438.

454. Lindholm LH, Ibsen H, Dahlof B, Devereux RB, Beevers G, de Faire U, et al. Cardiovascular morbidity and mortality in patients with diabetes in the Losartan Intervention For Endpoint reduction in hypertension study (LIFE): a randomised trial against atenolol. Lancet. 2002; 359:1004–10.

455. Berl T, Hunsicker LG, Lewis JB, Pfeffer MA, Porush JG, Rouleau JL, et al. Cardiovascular outcomes in the Irbesartan Diabetic Nephropathy Trial of patients with type 2 diabetes and overt nephropathy. Ann Intern Med. 2003; 138:542–9.

456. Palmer SC, Mavridis D, Navarese E, Craig JC, Tonelli M, Salanti G, et al. Comparative efficacy and safety of blood pressure-lowering agents in adults with diabetes and kidney disease: a network meta-analysis. Lancet. 2015; 385:2047–56.
457. Heart Outcomes Prevention Evaluation Study Investigators. Effects of ramipril on cardiovascular and microvascular outcomes in people with diabetes mellitus: results of the HOPE study and MICRO-HOPE substudy. Lancet. 2000; 355:253–9.
458. Arnold SV, Bhatt DL, Barsness GW, Beatty AL, Deedwania PC, Inzucchi SE, et al. Clinical management of stable coronary artery disease in patients with type 2 diabetes mellitus: a scientific statement from the American Heart Association. Circulation. 2020; 141:e779–806.

459. Telmisartan Randomised AssessmeNt Study in ACE iNtolerant subjects with cardiovascular Disease (TRANSCEND) Investigators, Yusuf S, Teo K, Anderson C, Pogue J, Dyal L, et al. Effects of the angiotensin-receptor blocker telmisartan on cardiovascular events in high-risk patients intolerant to angiotensin-converting enzyme inhibitors: a randomised controlled trial. Lancet. 2008; 372:1174–83.

460. Qiao Y, Shin JI, Chen TK, Inker LA, Coresh J, Alexander GC, et al. Association between renin-angiotensin system blockade discontinuation and all-cause mortality among persons with low estimated glomerular filtration rate. JAMA Intern Med. 2020; 180:718–26.

461. Wald DS, Law M, Morris JK, Bestwick JP, Wald NJ. Combination therapy versus monotherapy in reducing blood pressure: meta-analysis on 11,000 participants from 42 trials. Am J Med. 2009; 122:290–300.

462. Weber MA, Bakris GL, Jamerson K, Weir M, Kjeldsen SE, Devereux RB, et al. Cardiovascular events during differing hypertension therapies in patients with diabetes. J Am Coll Cardiol. 2010; 56:77–85.
463. ONTARGET Investigators, Yusuf S, Teo KK, Pogue J, Dyal L, Copland I, et al. Telmisartan, ramipril, or both in patients at high risk for vascular events. N Engl J Med. 2008; 358:1547–59.
464. Fried LF, Emanuele N, Zhang JH, Brophy M, Conner TA, Duckworth W, et al. Combined angiotensin inhibition for the treatment of diabetic nephropathy. N Engl J Med. 2013; 369:1892–903.
465. Makani H, Bangalore S, Desouza KA, Shah A, Messerli FH. Efficacy and safety of dual blockade of the renin-angiotensin system: meta-analysis of randomised trials. BMJ. 2013; 346:f360.
466. Williams B, MacDonald TM, Morant S, Webb DJ, Sever P, McInnes G, et al. Spironolactone versus placebo, bisoprolol, and doxazosin to determine the optimal treatment for drugresistant hypertension (PATHWAY-2): a randomised, double-blind, crossover trial. Lancet. 2015; 386:2059–68.
467. Bakris GL, Weir MR; Study of Hypertension and the Efficacy of Lotrel in Diabetes (SHIELD) Investigators. Achieving goal blood pressure in patients with type 2 diabetes: conventional versus fixed-dose combination approaches. J Clin Hypertens (Greenwich). 2003; 5:202–9.
468. Feldman RD, Zou GY, Vandervoort MK, Wong CJ, Nelson SA, Feagan BG. A simplified approach to the treatment of uncomplicated hypertension: a cluster randomized, controlled trial. Hypertension. 2009; 53:646–53.

469. Webster R, Salam A, de Silva HA, Selak V, Stepien S, Rajapakse S, et al. Fixed low-dose triple combination antihypertensive medication vs usual care for blood pressure control in patients with mild to moderate hypertension in Sri Lanka: a randomized clinical trial. JAMA. 2018; 320:566–79.

470. Mottillo S, Filion KB, Genest J, Joseph L, Pilote L, Poirier P, et al. The metabolic syndrome and cardiovascular risk a systematic review and meta-analysis. J Am Coll Cardiol. 2010; 56:1113–32.
471. Haffner SM, Lehto S, Ronnemaa T, Pyorala K, Laakso M. Mortality from coronary heart disease in subjects with type 2 diabetes and in nondiabetic subjects with and without prior myocardial infarction. N Engl J Med. 1998; 339:229–34.

472. ElSayed NA, Aleppo G, Aroda VR, Bannuru RR, Brown FM, Bruemmer D, et al. 10. Cardiovascular disease and risk management: standards of care in diabetes-2023. Diabetes Care. 2023; 46(Suppl 1):S158–90.
473. Rhee EJ, Kim HC, Kim JH, Lee EY, Kim BJ, Kim EM, et al. 2018 Guidelines for the management of dyslipidemia in Korea. Korean J Intern Med. 2019; 34:1171.

474. Boekholdt SM, Arsenault BJ, Mora S, Pedersen TR, LaRosa JC, Nestel PJ, et al. Association of LDL cholesterol, non-HDL cholesterol, and apolipoprotein B levels with risk of cardiovascular events among patients treated with statins: a meta-analysis. JAMA. 2012; 307:1302–9.
475. Brugts JJ, Yetgin T, Hoeks SE, Gotto AM, Shepherd J, Westendorp RG, et al. The benefits of statins in people without established cardiovascular disease but with cardiovascular risk factors: meta-analysis of randomised controlled trials. BMJ. 2009; 338:b2376.

476. Cholesterol Treatment Trialists’ (CTT) Collaboration, Baigent C, Blackwell L, Emberson J, Holland LE, Reith C, et al. Efficacy and safety of more intensive lowering of LDL cholesterol: a meta-analysis of data from 170,000 participants in 26 randomised trials. Lancet. 2010; 376:1670–81.

477. Ahmed S, Cannon CP, Murphy SA, Braunwald E. Acute coronary syndromes and diabetes: is intensive lipid lowering beneficial? Results of the PROVE IT-TIMI 22 trial. Eur Heart J. 2006; 27:2323–9.
478. Nissen SE, Tuzcu EM, Schoenhagen P, Brown BG, Ganz P, Vogel RA, et al. Effect of intensive compared with moderate lipid-lowering therapy on progression of coronary atherosclerosis: a randomized controlled trial. JAMA. 2004; 291:1071–80.
479. Shepherd J, Barter P, Carmena R, Deedwania P, Fruchart JC, Haffner S, et al. Effect of lowering LDL cholesterol substantial ly below currently recommended levels in patients with coronary heart disease and diabetes: the Treating to New Targets (TNT) study. Diabetes Care. 2006; 29:1220–6.
480. Moon MK, Noh J, Rhee EJ, Park SH, Kim HC, Kim BJ, et al. Cardiovascular outcomes according to comorbidities and low-density lipoprotein cholesterol in Korean people with type 2 diabetes mellitus. Diabetes Metab J. 2023; 47:45–58.
481. Stevens RJ, Kothari V, Adler AI, Stratton IM; United Kingdom Prospective Diabetes Study (UKPDS) Group. The UKPDS risk engine: a model for the risk of coronary heart disease in type II diabetes (UKPDS 56). Clin Sci (Lond). 2001; 101:671–9.
482. Heart Protection Study Collaborative Group. MRC/BHF Heart Protection Study of cholesterol lowering with simvastatin in 20,536 high-risk individuals: a randomised placebo-controlled trial. Lancet. 2002; 360:7–22.
483. Cholesterol Treatment Trialists’ (CTT) Collaborators, Kearney PM, Blackwell L, Collins R, Keech A, Simes J, et al. Efficacy of cholesterol-lowering therapy in 18,686 people with diabetes in 14 randomised trials of statins: a meta-analysis. Lancet. 2008; 371:117–25.
484. Cannon CP, Blazing MA, Giugliano RP, McCagg A, White JA, Theroux P, et al. Ezetimibe added to statin therapy after acute coronary syndromes. N Engl J Med. 2015; 372:2387–97.

485. Sabatine MS, Giugliano RP, Keech AC, Honarpour N, Wiviott SD, Murphy SA, et al. Evolocumab and clinical outcomes in patients with cardiovascular disease. N Engl J Med. 2017; 376:1713–22.

486. Schwartz GG, Steg PG, Szarek M, Bhatt DL, Bittner VA, Diaz R, et al. Alirocumab and cardiovascular outcomes after acute coronary syndrome. N Engl J Med. 2018; 379:2097–107.

487. Hong N, Lee YH, Tsujita K, Gonzalez JA, Kramer CM, Kovarnik T, et al. Comparison of the effects of ezetimibe-statin combination therapy on major adverse cardiovascular events in patients with and without diabetes: a meta-analysis. Endocrinol Metab (Seoul). 2018; 33:219–27.

488. Sabatine MS, Leiter LA, Wiviott SD, Giugliano RP, Deedwania P, De Ferrari GM, et al. Cardiovascular safety and efficacy of the PCSK9 inhibitor evolocumab in patients with and without diabetes and the effect of evolocumab on glycaemia and risk of new-onset diabetes: a prespecified analysis of the FOURIER randomised controlled trial. Lancet Diabetes Endocrinol. 2017; 5:941–50.
489. Ray KK, Colhoun HM, Szarek M, Baccara-Dinet M, Bhatt DL, Bittner VA, et al. Effects of alirocumab on cardiovascular and metabolic outcomes after acute coronary syndrome in patients with or without diabetes: a prespecified analysis of the ODYSSEY OUTCOMES randomised controlled trial. Lancet Diabetes Endocrinol. 2019; 7:618–28.
490. Grundy SM, Stone NJ, Bailey AL, Beam C, Birtcher KK, Blumenthal RS, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol: a report of the American College of Cardiology/American Heart Association task force on clinical practice guidelines. Circulation. 2019; 139:e1082–143.
491. Mach F, Baigent C, Catapano AL, Koskinas KC, Casula M, Badimon L, et al. 2019 ESC/EAS guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk. Eur Heart J. 2020; 41:111–88.
492. Gordon DJ, Probstfield JL, Garrison RJ, Neaton JD, Castelli WP, Knoke JD, et al. High-density lipoprotein cholesterol and cardiovascular disease. Four prospective American studies. Circulation. 1989; 79:8–15.

493. Grundy SM, Cleeman JI, Merz CN, Brewer HB Jr, Clark LT, Hunninghake DB, et al. Implications of recent clinical trials for the National Cholesterol Education Program Adult Treatment Panel III guidelines. Circulation. 2004; 110:227–39.
494. Charlton-Menys V, Betteridge DJ, Colhoun H, Fuller J, France M, Hitman GA, et al. Targets of statin therapy: LDL cholesterol, non-HDL cholesterol, and apolipoprotein B in type 2 diabetes in the Collaborative Atorvastatin Diabetes Study (CARDS). Clin Chem. 2009; 55:473–80.
495. Estruch R, Ros E, Salas-Salvado J, Covas MI, Corella D, Aros F, et al. Primary prevention of cardiovascular disease with a Mediterranean diet supplemented with extra-virgin olive oil or nuts. N Engl J Med. 2018; 378:e34.
496. Colhoun HM, Betteridge DJ, Durrington PN, Hitman GA, Neil HA, Livingstone SJ, et al. Primary prevention of cardiovascular disease with atorvastatin in type 2 diabetes in the Collaborative Atorvastatin Diabetes Study (CARDS): multicentre randomised placebo-controlled trial. Lancet. 2004; 364:685–96.
497. Dale KM, White CM, Henyan NN, Kluger J, Coleman CI. Impact of statin dosing intensity on transaminase and creatine kinase. Am J Med. 2007; 120:706–12.
498. Dongiovanni P, Petta S, Mannisto V, Mancina RM, Pipitone R, Karja V, et al. Statin use and non-alcoholic steatohepatitis in at risk individuals. J Hepatol. 2015; 63:705–12.
499. Bruckert E, Hayem G, Dejager S, Yau C, Begaud B. Mild to moderate muscular symptoms with high-dosage statin therapy in hyperlipidemic patients: the PRIMO study. Cardiovasc Drugs Ther. 2005; 19:403–14.
500. Ridker PM, Danielson E, Fonseca FA, Genest J, Gotto AM Jr, Kastelein JJ, et al. Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N Engl J Med. 2008; 359:2195–207.

501. Law M, Rudnicka AR. Statin safety: a systematic review. Am J Cardiol. 2006; 97(8A):52C–60C.
502. Waters DD, Ho JE, Boekholdt SM, DeMicco DA, Kastelein JJ, Messig M, et al. Cardiovascular event reduction versus newonset diabetes during atorvastatin therapy: effect of baseline risk factors for diabetes. J Am Coll Cardiol. 2013; 61:148–52.
503. Karalis DG, Hill AN, Clifton S, Wild RA. The risks of statin use in pregnancy: a systematic review. J Clin Lipidol. 2016; 10:1081–90.
504. Giugliano RP, Cannon CP, Blazing MA, Nicolau JC, Corbalan R, Spinar J, et al. Benefit of adding ezetimibe to statin therapy on cardiovascular outcomes and safety in patients with versus without diabetes mellitus: results from IMPROVE-IT (Improved Reduction of Outcomes: Vytorin Efficacy International Trial). Circulation. 2018; 137:1571–82.

505. Ben-Yehuda O, Wenger NK, Constance C, Zieve F, Hanson ME, Lin JX, et al. The comparative efficacy of ezetimibe added to atorvastatin 10 mg versus uptitration to atorvastatin 40 mg in subgroups of patients aged 65 to 74 years or greater than or equal to 75 years. J Geriatr Cardiol. 2011; 8:1–11.

506. Bays HE, Conard SE, Leiter LA, Bird SR, Lowe RS, Tershakovec AM. Influence of age, gender, and race on the efficacy of adding ezetimibe to atorvastatin vs. atorvastatin up-titration in patients at moderately high or high risk for coronary heart disease. Int J Cardiol. 2011; 153:141–7.

507. Nicholls SJ, Lincoff AM, Garcia M, Bash D, Ballantyne CM, Barter PJ, et al. Effect of high-dose omega-3 fatty acids vs corn oil on major adverse cardiovascular events in patients at high cardiovascular risk: the STRENGTH randomized clinical trial. JAMA. 2020; 324:2268–80.

508. ACCORD Study Group, Ginsberg HN, Elam MB, Lovato LC, Crouse JR 3rd, Leiter LA, et al. Effects of combination lipid therapy in type 2 diabetes mellitus. N Engl J Med. 2010; 362:1563–74.
509. Scott R, O’Brien R, Fulcher G, Pardy C, D’Emden M, Tse D, et al. Effects of fenofibrate treatment on cardiovascular disease risk in 9,795 individuals with type 2 diabetes and various components of the metabolic syndrome: the Fenofibrate Intervention and Event Lowering in Diabetes (FIELD) study. Diabetes Care. 2009; 32:493–8.
510. Hermans MP. Impact of fenofibrate on type 2 diabetes patients with features of the metabolic syndrome: subgroup analysis from FIELD. Curr Cardiol Rev. 2010; 6:112–8.

511. Guedeney P, Giustino G, Sorrentino S, Claessen BE, Camaj A, Kalkman DN, et al. Efficacy and safety of alirocumab and evolocumab: a systematic review and meta-analysis of randomized controlled trials. Eur Heart J. 2022; 43:e17–25.

512. Moriarty PM, Jacobson TA, Bruckert E, Thompson PD, Guyton JR, Baccara-Dinet MT, et al. Efficacy and safety of alirocumab, a monoclonal antibody to PCSK9, in statin-intolerant patients: design and rationale of ODYSSEY ALTERNATIVE, a randomized phase 3 trial. J Clin Lipidol. 2014; 8:554–61.

513. Zhang XL, Zhu QQ, Zhu L, Chen JZ, Chen QH, Li GN, et al. Safety and efficacy of anti-PCSK9 antibodies: a meta-analysis of 25 randomized, controlled trials. BMC Med. 2015; 13:123.
514. Rosenson RS, Daviglus ML, Handelsman Y, Pozzilli P, Bays H, Monsalvo ML, et al. Efficacy and safety of evolocumab in individuals with type 2 diabetes mellitus: primary results of the randomised controlled BANTING study. Diabetologia. 2019; 62:948–58.

515. Triglyceride Coronary Disease Genetics Consortium and Emerging Risk Factors Collaboration, Sarwar N, Sandhu MS, Ricketts SL, Butterworth AS, Di Angelantonio E, et al. Triglyceride-mediated pathways and coronary disease: collaborative analysis of 101 studies. Lancet. 2010; 375:1634–9.

516. Berglund L, Brunzell JD, Goldberg AC, Goldberg IJ, Sacks F, Murad MH, et al. Evaluation and treatment of hypertriglyceridemia: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2012; 97:2969–89.

517. Antithrombotic Trialists’ (ATT) Collaboration, Baigent C, Blackwell L, Collins R, Emberson J, Godwin J, et al. Aspirin in the primary and secondary prevention of vascular disease: collaborative meta-analysis of individual participant data from randomised trials. Lancet. 2009; 373:1849–60.

518. Steg PG, Bhatt DL, Simon T, Fox K, Mehta SR, Harrington RA, et al. Ticagrelor in patients with stable coronary disease and diabetes. N Engl J Med. 2019; 381:1309–20.
519. Leiter LA, Bhatt DL, McGuire DK, Teoh H, Fox K, Simon T, et al. Diabetes-related factors and the effects of ticagrelor plus aspirin in the THEMIS and THEMIS-PCI trials. J Am Coll Cardiol. 2021; 77:2366–77.
520. Bhatt DL, Steg PG, Mehta SR, Leiter LA, Simon T, Fox K, et al. Ticagrelor in patients with diabetes and stable coronary artery disease with a history of previous percutaneous coronary intervention (THEMIS-PCI): a phase 3, placebo-controlled, randomised trial. Lancet. 2019; 394:1169–80.
521. Eikelboom JW, Connolly SJ, Bosch J, Dagenais GR, Hart RG, Shestakovska O, et al. Rivaroxaban with or without aspirin in stable cardiovascular disease. N Engl J Med. 2017; 377:1319–30.

522. Bhatt DL, Eikelboom JW, Connolly SJ, Steg PG, Anand SS, Verma S, et al. Role of combination antiplatelet and anticoagulation therapy in diabetes mellitus and cardiovascular disease: insights from the COMPASS trial. Circulation. 2020; 141:1841–54.

523. Bonaca MP, Bauersachs RM, Anand SS, Debus ES, Nehler MR, Patel MR, et al. Rivaroxaban in peripheral artery disease after revascularization. N Engl J Med. 2020; 382:1994–2004.
524. Xie C, Hang Y, Zhu J, Li C, Jiang B, Zhang Y, et al. Benefit and risk of adding rivaroxaban in patients with coronary artery disease: a systematic review and meta-analysis. Clin Cardiol. 2021; 44:20–6.

525. CAPRIE Steering Committee. A randomised, blinded, trial of clopidogrel versus aspirin in patients at risk of ischaemic events (CAPRIE). CAPRIE Steering Committee. Lancet. 1996; 348:1329–39.
526. Qin ZY, Yang XF, Lian CY, Yan XJ, Lin MS, Bundhun PK, et al. Aspirin versus clopidogrel monotherapy for the secondary prevention of recurrent cerebrovascular attack following previous ischemic stroke in patients with type 2 diabetes mellitus: a systematic review and meta-analysis. Diabetes Ther. 2020; 11:1091–101.

527. Bhatt DL, Marso SP, Hirsch AT, Ringleb PA, Hacke W, Topol EJ. Amplified benefit of clopidogrel versus aspirin in patients with diabetes mellitus. Am J Cardiol. 2002; 90:625–8.

528. Angiolillo DJ, Suryadevara S. Aspirin and clopidogrel: efficacy and resistance in diabetes mellitus. Best Pract Res Clin Endocrinol Metab. 2009; 23:375–88.

529. Kim JD, Park CY, Ahn KJ, Cho JH, Choi KM, Kang JG, et al. Non-HDL cholesterol is an independent risk factor for aspirin resistance in obese patients with type 2 diabetes. Atherosclerosis. 2014; 234:146–51.
530. Ogawa H, Nakayama M, Morimoto T, Uemura S, Kanauchi M, Doi N, et al. Low-dose aspirin for primary prevention of atherosclerotic events in patients with type 2 diabetes: a randomized controlled trial. JAMA. 2008; 300:2134–41.

531. ASCEND Study Collaborative Group, Bowman L, Mafham M, Wallendszus K, Stevens W, Buck G, et al. Effects of aspirin for primary prevention in persons with diabetes mellitus. N Engl J Med. 2018; 379:1529–39.

532. Gaziano JM, Brotons C, Coppolecchia R, Cricelli C, Darius H, Gorelick PB, et al. Use of aspirin to reduce risk of initial vascular events in patients at moderate risk of cardiovascular disease (ARRIVE): a randomised, double-blind, placebo-controlled trial. Lancet. 2018; 392:1036–46.

533. McNeil JJ, Nelson MR, Woods RL, Lockery JE, Wolfe R, Reid CM, et al. Effect of aspirin on all-cause mortality in the healthy elderly. N Engl J Med. 2018; 379:1519–28.

534. Zheng SL, Roddick AJ. Association of aspirin use for primary prevention with cardiovascular events and bleeding events: a systematic review and meta-analysis. JAMA. 2019; 321:277–87.
535. Khan SU, Ul Abideen Asad Z, Khan MU, Talluri S, Ali F, Shahzeb Khan M, et al. Aspirin for primary prevention of cardiovascular outcomes in diabetes mellitus: an updated systematic review and meta-analysis. Eur J Prev Cardiol. 2020; 27:2034–41.

536. Masson W, Barbagelata L, Lavalle-Cobo A, Lobo M, Masson G, Nogueira JP, et al. Low-doses aspirin in the primary prevention of cardiovascular disease in patients with diabetes: meta-analysis stratified by baseline cardiovascular risk. Diabetes Metab Syndr. 2022; 16:102391.

537. Saito Y, Okada S, Ogawa H, Soejima H, Sakuma M, Nakayama M, et al. Low-dose aspirin for primary prevention of cardiovascular events in patients with type 2 diabetes mellitus: 10-year follow-up of a randomized controlled trial. Circulation. 2017; 135:659–70.

538. Kim YJ, Choi NK, Kim MS, Lee J, Chang Y, Seong JM, et al. Evaluation of low-dose aspirin for primary prevention of ischemic stroke among patients with diabetes: a retrospective cohort study. Diabetol Metab Syndr. 2015; 7:8.

539. Cryer PE. Mechanisms of hypoglycemia-associated autonomic failure in diabetes. N Engl J Med. 2013; 369:362–72.
540. Cryer PE. Individualized glycemic goals and an expanded classification of severe hypoglycemia in diabetes. Diabetes Care. 2017; 40:1641–3.

541. Bonds DE, Miller ME, Bergenstal RM, Buse JB, Byington RP, Cutler JA, et al. The association between symptomatic, severe hypoglycaemia and mortality in type 2 diabetes: retrospective epidemiological analysis of the ACCORD study. BMJ. 2010; 340:b4909.

542. ORIGIN Trial Investigators, Mellbin LG, Ryden L, Riddle MC, Probstfield J, Rosenstock J, et al. Does hypoglycaemia increase the risk of cardiovascular events? A report from the ORIGIN trial. Eur Heart J. 2013; 34:3137–44.

543. Griesdale DE, de Souza RJ, van Dam RM, Heyland DK, Cook DJ, Malhotra A, et al. Intensive insulin therapy and mortality among critically ill patients: a meta-analysis including NICESUGAR study data. CMAJ. 2009; 180:821–7.

544. Yeoh E, Choudhary P, Nwokolo M, Ayis S, Amiel SA. Interventions that restore awareness of hypoglycemia in adults with type 1 diabetes: a systematic review and meta-analysis. Diabetes Care. 2015; 38:1592–609.
545. Hermanns N, Kulzer B, Ehrmann D, Bergis-Jurgan N, Haak T. The effect of a diabetes education programme (PRIMAS) for people with type 1 diabetes: results of a randomized trial. Diabetes Res Clin Pract. 2013; 102:149–57.

546. Amiel SA, Potts L, Goldsmith K, Jacob P, Smith EL, Gonder-Frederick L, et al. A parallel randomised controlled trial of the Hypoglycaemia Awareness Restoration Programme for adults with type 1 diabetes and problematic hypoglycaemia despite optimised self-care (HARPdoc). Nat Commun. 2022; 13:2229.

547. Stanton-Fay SH, Hamilton K, Chadwick PM, Lorencatto F, Gianfrancesco C, de Zoysa N, et al. The DAFNEplus programme for sustained type 1 diabetes self management: intervention development using the behaviour change wheel. Diabet Med. 2021; 38:e14548.

548. Hopkins D, Lawrence I, Mansell P, Thompson G, Amiel S, Campbell M, et al. Improved biomedical and psychological outcomes 1 year after structured education in flexible insulin therapy for people with type 1 diabetes: the U.K. DAFNE experience. Diabetes Care. 2012; 35:1638–42.
549. Cryer PE. Diverse causes of hypoglycemia-associated autonomic failure in diabetes. N Engl J Med. 2004; 350:2272–9.
550. Beck RW, Riddlesworth T, Ruedy K, Ahmann A, Bergenstal R, Haller S, et al. Effect of continuous glucose monitoring on glycemic control in adults with type 1 diabetes using insulin injections: the DIAMOND randomized clinical trial. JAMA. 2017; 317:371–8.

551. Ruedy KJ, Parkin CG, Riddlesworth TD, Graham C; DIAMOND Study Group. Continuous glucose monitoring in older adults with type 1 and type 2 diabetes using multiple daily injections of insulin: results from the DIAMOND trial. J Diabetes Sci Technol. 2017; 11:1138–46.

552. Dicembrini I, Mannucci E, Monami M, Pala L. Impact of technology on glycaemic control in type 2 diabetes: a meta-analysis of randomized trials on continuous glucose monitoring and continuous subcutaneous insulin infusion. Diabetes Obes Metab. 2019; 21:2619–25.

553. Heinemann L, Freckmann G, Ehrmann D, Faber-Heinemann G, Guerra S, Waldenmaier D, et al. Real-time continuous glucose monitoring in adults with type 1 diabetes and impaired hypoglycaemia awareness or severe hypoglycaemia treated with multiple daily insulin injections (HypoDE): a multicentre, randomised controlled trial. Lancet. 2018; 391:1367–77.

554. He J, Ryder AG, Li S, Liu W, Zhu X. Glycemic extremes are related to cognitive dysfunction in children with type 1 diabetes: a meta-analysis. J Diabetes Investig. 2018; 9:1342–53.
555. Rama Chandran S, Jacob P, Choudhary P. A systematic review of the effect of prior hypoglycaemia on cognitive function in type 1 diabetes. Ther Adv Endocrinol Metab. 2020; 11:204201–8820906017.

556. de Galan BE, Zoungas S, Chalmers J, Anderson C, Dufouil C, Pillai A, et al. Cognitive function and risks of cardiovascular disease and hypoglycaemia in patients with type 2 diabetes: the Action in Diabetes and Vascular Disease: Preterax and Diamicron Modified Release Controlled Evaluation (ADVANCE) trial. Diabetologia. 2009; 52:2328–36.

557. Punthakee Z, Miller ME, Launer LJ, Williamson JD, Lazar RM, Cukierman-Yaffee T, et al. Poor cognitive function and risk of severe hypoglycemia in type 2 diabetes: post hoc epidemiologic analysis of the ACCORD trial. Diabetes Care. 2012; 35:787–93.
558. Feinkohl I, Aung PP, Keller M, Robertson CM, Morling JR, McLachlan S, et al. Severe hypoglycemia and cognitive decline in older people with type 2 diabetes: the Edinburgh type 2 diabetes study. Diabetes Care. 2014; 37:507–15.

559. Kim YG, Park DG, Moon SY, Jeon JY, Kim HJ, Kim DJ, et al. Hypoglycemia and dementia risk in older patients with type 2 diabetes mellitus: a propensity-score matched analysis of a population-based cohort study. Diabetes Metab J. 2020; 44:125–33.
560. Lee AK, Rawlings AM, Lee CJ, Gross AL, Huang ES, Sharrett AR, et al. Severe hypoglycaemia, mild cognitive impairment, dementia and brain volumes in older adults with type 2 diabetes: the Atherosclerosis Risk in Communities (ARIC) cohort study. Diabetologia. 2018; 61:1956–65.

561. Mehta HB, Mehta V, Goodwin JS. Association of hypoglycemia with subsequent dementia in older patients with type 2 diabetes mellitus. J Gerontol A Biol Sci Med Sci. 2017; 72:1110–6.

562. Whitmer RA, Karter AJ, Yaffe K, Quesenberry CP Jr, Selby JV. Hypoglycemic episodes and risk of dementia in older patients with type 2 diabetes mellitus. JAMA. 2009; 301:1565–72.

563. Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications Study Research Group, Jacobson AM, Musen G, Ryan CM, Silvers N, Cleary P, et al. Long-term effect of diabetes and its treatment on cognitive function. N Engl J Med. 2007; 356:1842–52.

564. Yun JS, Ko SH, Ko SH, Song KH, Yoo KD, Yoon KH, et al. Cardiovascular disease predicts severe hypoglycemia in patients with type 2 diabetes. Diabetes Metab J. 2015; 39:498–506.
565. Hermanns N, Kulzer B, Krichbaum M, Kubiak T, Haak T. Long-term effect of an education program (HyPOS) on the incidence of severe hypoglycemia in patients with type 1 diabetes. Diabetes Care. 2010; 33:e36.

566. LaManna J, Litchman ML, Dickinson JK, Todd A, Julius MM, Whitehouse CR, et al. diabetes education impact on hypoglycemia outcomes: a systematic review of evidence and gaps in the literature. Diabetes Educ. 2019; 45:349–69.

567. Yong YM, Shin KM, Lee KM, Cho JY, Ko SH, Yoon MH, et al. Intensive individualized reinforcement education is important for the prevention of hypoglycemia in patients with type 2 diabetes. Diabetes Metab J. 2015; 39:154–63.

568. Ghandi K, Pieri B, Dornhorst A, Hussain S. A comparison of validated methods used to assess impaired awareness of hypoglycaemia in type 1 diabetes: an observational study. Diabetes Ther. 2021; 12:441–51.

569. Rubin NT, Seaquist ER, Eberly L, Kumar A, Mangia S, Oz G, et al. Relationship between hypoglycemia awareness status on clarke/gold methods and counterregulatory response to hypoglycemia. J Endocr Soc. 2022; 6:bvac107.
570. Gold AE, MacLeod KM, Frier BM. Frequency of severe hypoglycemia in patients with type I diabetes with impaired awareness of hypoglycemia. Diabetes Care. 1994; 17:697–703.

571. Clarke WL, Cox DJ, Gonder-Frederick LA, Julian D, Schlundt D, Polonsky W. Reduced awareness of hypoglycemia in adults with IDDM. A prospective study of hypoglycemic frequency and associated symptoms. Diabetes Care. 1995; 18:517–22.

572. Korean Diabetes Association. Diabetic neuropathy manual. Seoul: Korean Diabetes Association;2020.
573. Pop-Busui R, Ang L, Boulton AJM, Feldman EL, Marcus RL, Mizokami-Stout K, et al. Diagnosis and treatment of painful diabetic peripheral neuropathy. ADA Clin Compend. 2022; 2022:1–32.

574. ElSayed NA, Aleppo G, Aroda VR, Bannuru RR, Brown FM, Bruemmer D, et al. 12. Retinopathy, neuropathy, and foot care: standards of care in diabetes-2023. Diabetes Care. 2023; 46(Suppl 1):S203–15.
575. Kim SS, Won JC, Kwon HS, Kim CH, Lee JH, Park TS, et al. Prevalence and clinical implications of painful diabetic peripheral neuropathy in type 2 diabetes: results from a nationwide hospital-based study of diabetic neuropathy in Korea. Diabetes Res Clin Pract. 2014; 103:522–9.
576. Won JC, Kwon HS, Kim CH, Lee JH, Park TS, Ko KS, et al. Prevalence and clinical characteristics of diabetic peripheral neuropathy in hospital patients with type 2 diabetes in Korea. Diabet Med. 2012; 29:e290–6.
577. Moon SS, Kim CH, Kang SM, Kim ES, Oh TJ, Yun JS, et al. Status of diabetic neuropathy in Korea: a National Health Insurance Service-National Sample Cohort Analysis (2006 to 2015). Diabetes Metab J. 2021; 45:115–9.

578. Pop-Busui R, Boulton AJ, Feldman EL, Bril V, Freeman R, Malik RA, et al. Diabetic neuropathy: a position statement by the American Diabetes Association. Diabetes Care. 2017; 40:136–54.

579. Feldman EL, Stevens MJ, Thomas PK, Brown MB, Canal N, Greene DA. A practical two-step quantitative clinical and electrophysiological assessment for the diagnosis and staging of diabetic neuropathy. Diabetes Care. 1994; 17:1281–9.

580. Greco C, Di Gennaro F, D’Amato C, Morganti R, Corradini D, Sun A, et al. Validation of the Composite Autonomic Symptom Score 31 (COMPASS 31) for the assessment of symptoms of autonomic neuropathy in people with diabetes. Diabet Med. 2017; 34:834–8.
581. Maser RE, Mitchell BD, Vinik AI, Freeman R. The association between cardiovascular autonomic neuropathy and mortality in individuals with diabetes: a meta-analysis. Diabetes Care. 2003; 26:1895–901.
582. Lykke JA, Tarnow L, Parving HH, Hilsted J. A combined abnormality in heart rate variation and QT corrected interval is a strong predictor of cardiovascular death in type 1 diabetes. Scand J Clin Lab Invest. 2008; 68:654–9.

583. Lonn EM, Rambihar S, Gao P, Custodis FF, Sliwa K, Teo KK, et al. Heart rate is associated with increased risk of major cardiovascular events, cardiovascular and all-cause death in patients with stable chronic cardiovascular disease: an analysis of ONTARGET/TRANSCEND. Clin Res Cardiol. 2014; 103:149–59.

584. Pop-Busui R, Evans GW, Gerstein HC, Fonseca V, Fleg JL, Hoogwerf BJ, et al. Effects of cardiac autonomic dysfunction on mortality risk in the Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial. Diabetes Care. 2010; 33:1578–84.

585. Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Heart rate variability: standards of measurement, physiological interpretation and clinical use. Circulation. 1996; 93:1043–65.
586. Pop-Busui R. Cardiac autonomic neuropathy in diabetes: a clinical perspective. Diabetes Care. 2010; 33:434–41.
587. Spallone V. Update on the impact, diagnosis and management of cardiovascular autonomic neuropathy in diabetes: what is defined, what is new, and what is unmet. Diabetes Metab J. 2019; 43:3–30.

588. Boulton AJ, Kempler P, Ametov A, Ziegler D. Whither pathogenetic treatments for diabetic polyneuropathy? Diabetes Metab Res Rev. 2013; 29:327–33.

589. The Diabetes Control and Complications Trial Research Group. The effect of intensive diabetes therapy on measures of autonomic nervous system function in the Diabetes Control and Complications Trial (DCCT). Diabetologia. 1998; 41:416–23.
590. Albers JW, Herman WH, Pop-Busui R, Feldman EL, Martin CL, Cleary PA, et al. Effect of prior intensive insulin treatment during the Diabetes Control and Complications Trial (DCCT) on peripheral neuropathy in type 1 diabetes during the Epidemiology of Diabetes Interventions and Complications (EDIC) study. Diabetes Care. 2010; 33:1090–6.

591. Pop-Busui R, Low PA, Waberski BH, Martin CL, Albers JW, Feldman EL, et al. Effects of prior intensive insulin therapy on cardiac autonomic nervous system function in type 1 diabetes mellitus: the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications study (DCCT/EDIC). Circulation. 2009; 119:2886–93.

592. Callaghan BC, Little AA, Feldman EL, Hughes RA. Enhanced glucose control for preventing and treating diabetic neuropathy. Cochrane Database Syst Rev. 2012; 6:CD007543.
593. Pop-Busui R, Lu J, Brooks MM, Albert S, Althouse AD, Escobedo J, et al. Impact of glycemic control strategies on the progression of diabetic peripheral neuropathy in the Bypass Angioplasty Revascularization Investigation 2 Diabetes (BARI 2D) Cohort. Diabetes Care. 2013; 36:3208–15.

594. Tang Y, Shah H, Bueno Junior CR, Sun X, Mitri J, Sambataro M, et al. Intensive risk factor management and cardiovascular autonomic neuropathy in type 2 diabetes: the ACCORD trial. Diabetes Care. 2021; 44:164–73.
595. Waldfogel JM, Nesbit SA, Dy SM, Sharma R, Zhang A, Wilson LM, et al. Pharmacotherapy for diabetic peripheral neuropathy pain and quality of life: a systematic review. Neurology. 2017; 88:1958–67.
596. Finnerup NB, Attal N, Haroutounian S, McNicol E, Baron R, Dworkin RH, et al. Pharmacotherapy for neuropathic pain in adults: a systematic review and meta-analysis. Lancet Neurol. 2015; 14:162–73.
597. Griebeler ML, Morey-Vargas OL, Brito JP, Tsapas A, Wang Z, Carranza Leon BG, et al. Pharmacologic interventions for painful diabetic neuropathy: an umbrella systematic review and comparative effectiveness network meta-analysis. Ann Intern Med. 2014; 161:639–49.
598. Ziegler D, Fonseca V. From guideline to patient: a review of recent recommendations for pharmacotherapy of painful diabetic neuropathy. J Diabetes Complications. 2015; 29:146–56.

599. Freeman R, Durso-Decruz E, Emir B. Efficacy, safety, and tolerability of pregabalin treatment for painful diabetic peripheral neuropathy: findings from seven randomized, controlled trials across a range of doses. Diabetes Care. 2008; 31:1448–54.
600. Raskin P, Huffman C, Toth C, Asmus MJ, Messig M, Sanchez RJ, et al. Pregabalin in patients with inadequately treated painful diabetic peripheral neuropathy: a randomized withdrawal trial. Clin J Pain. 2014; 30:379–90.
601. Tesfaye S, Wilhelm S, Lledo A, Schacht A, Tolle T, Bouhassira D, et al. Duloxetine and pregabalin: high-dose monotherapy or their combination? The “COMBO-DN study”: a multinational, randomized, double-blind, parallel-group study in patients with diabetic peripheral neuropathic pain. Pain. 2013; 154:2616–25.
602. Quilici S, Chancellor J, Lothgren M, Simon D, Said G, Le TK, et al. Meta-analysis of duloxetine vs. pregabalin and gabapentin in the treatment of diabetic peripheral neuropathic pain. BMC Neurol. 2009; 9:6.
603. Dworkin RH, Jensen MP, Gammaitoni AR, Olaleye DO, Galer BS. Symptom profiles differ in patients with neuropathic versus non-neuropathic pain. J Pain. 2007; 8:118–26.

604. Wiffen PJ, Derry S, Bell RF, Rice AS, Tolle TR, Phillips T, et al. Gabapentin for chronic neuropathic pain in adults. Cochrane Database Syst Rev. 2017; 6:CD007938.

605. Wernicke JF, Pritchett YL, D’Souza DN, Waninger A, Tran P, Iyengar S, et al. A randomized controlled trial of duloxetine in diabetic peripheral neuropathic pain. Neurology. 2006; 67:1411–20.

606. Schwartz S, Etropolski M, Shapiro DY, Okamoto A, Lange R, Haeussler J, et al. Safety and efficacy of tapentadol ER in patients with painful diabetic peripheral neuropathy: results of a randomized-withdrawal, placebo-controlled trial. Curr Med Res Opin. 2011; 27:151–62.

607. Vinik AI, Shapiro DY, Rauschkolb C, Lange B, Karcher K, Pennett D, et al. A randomized withdrawal, placebo-controlled study evaluating the efficacy and tolerability of tapentadol extended release in patients with chronic painful diabetic peripheral neuropathy. Diabetes Care. 2014; 37:2302–9.
608. Won JC, Kwon HS, Moon SS, Chun SW, Kim CH, Park IB, et al. γ-Linolenic acid versus α-lipoic acid for treating painful diabetic neuropathy in adults: a 12-week, double-placebo, randomized, noninferiority trial. Diabetes Metab J. 2020; 44:542–54.

609. Tesfaye S, Sloan G, Petrie J, White D, Bradburn M, Julious S, et al. Comparison of amitriptyline supplemented with pregabalin, pregabalin supplemented with amitriptyline, and duloxetine supplemented with pregabalin for the treatment of diabetic peripheral neuropathic pain (OPTION-DM): a multicentre, double-blind, randomised crossover trial. Lancet. 2022; 400:680–90.

610. Lee CW. Diagnosis and management of diabetic foot. J Korean Diabetes. 2018; 19:168–74.

611. Bakker K, Apelqvist J, Schaper NC; International Working Group on Diabetic Foot Editorial Board. Practical guidelines on the management and prevention of the diabetic foot 2011. Diabetes Metab Res Rev. 2012; 28 Suppl 1:225–31.

612. Boulton AJ, Armstrong DG, Albert SF, Frykberg RG, Hellman R, Kirkman MS, et al. Comprehensive foot examination and risk assessment: a report of the task force of the foot care interest group of the American Diabetes Association, with endorsement by the American Association of Clinical Endocrinologists. Diabetes Care. 2008; 31:1679–85.
613. Bus SA, Lavery LA, Monteiro-Soares M, Rasmussen A, Raspovic A, Sacco ICN, et al. Guidelines on the prevention of foot ulcers in persons with diabetes (IWGDF 2019 update). Diabetes Metab Res Rev. 2020; 36 Suppl 1:e3269.

614. Bonner T, Foster M, Spears-Lanoix E. Type 2 diabetes-related foot care knowledge and foot self-care practice interventions in the United States: a systematic review of the literature. Diabet Foot Ankle. 2016; 7:29758.

615. Lipsky BA, Berendt AR, Cornia PB, Pile JC, Peters EJ, Armstrong DG, et al. 2012 Infectious Diseases Society of America clinical practice guideline for the diagnosis and treatment of diabetic foot infections. Clin Infect Dis. 2012; 54:e132–73.
616. Elraiyah T, Tsapas A, Prutsky G, Domecq JP, Hasan R, Firwana B, et al. A systematic review and meta-analysis of adjunctive therapies in diabetic foot ulcers. J Vasc Surg. 2016; 63(2 Suppl):46S–58S.e1.

617. Game FL, Apelqvist J, Attinger C, Hartemann A, Hinchliffe RJ, Londahl M, et al. Effectiveness of interventions to enhance healing of chronic ulcers of the foot in diabetes: a systematic review. Diabetes Metab Res Rev. 2016; 32 Suppl 1:154–68.
618. Heng ML, Kwan YH, Ilya N, Ishak IA, Jin PH, Hogan D, et al. A collaborative approach in patient education for diabetes foot and wound care: a pragmatic randomised controlled trial. Int Wound J. 2020; 17:1678–86.

619. Yau JW, Rogers SL, Kawasaki R, Lamoureux EL, Kowalski JW, Bek T, et al. Global prevalence and major risk factors of diabetic retinopathy. Diabetes Care. 2012; 35:556–64.

620. Jee D, Lee WK, Kang S. Prevalence and risk factors for diabetic retinopathy: the Korea National Health and Nutrition Examination Survey 2008-2011. Invest Ophthalmol Vis Sci. 2013; 54:6827–33.

621. The Diabetes Control and Complications Trial. The effect of intensive diabetes treatment on the progression of diabetic retinopathy in insulin-dependent diabetes mellitus. Arch Ophthalmol. 1995; 113:36–51.
622. ACCORD Study Group; ACCORD Eye Study Group, Chew EY, Ambrosius WT, Davis MD, Danis RP, et al. Effects of medical therapies on retinopathy progression in type 2 diabetes. N Engl J Med. 2010; 363:233–44.

623. Keech AC, Mitchell P, Summanen PA, O’Day J, Davis TM, Moffitt MS, et al. Effect of fenofibrate on the need for laser treatment for diabetic retinopathy (FIELD study): a randomised controlled trial. Lancet. 2007; 370:1687–97.
624. Wright AD, Dodson PM. Medical management of diabetic retinopathy: fenofibrate and ACCORD Eye studies. Eye (Lond). 2011; 25:843–9.

625. DCCT/EDIC Research Group, Nathan DM, Bebu I, Hainsworth D, Klein R, Tamborlane W, et al. Frequency of evidence-based screening for retinopathy in type 1 diabetes. N Engl J Med. 2017; 376:1507–16.

626. Taylor-Phillips S, Mistry H, Leslie R, Todkill D, Tsertsvadze A, Connock M, et al. Extending the diabetic retinopathy screening interval beyond 1 year: systematic review. Br J Ophthalmol. 2016; 100:105–14.

627. American Diabetes Association. 10. Microvascular complications and foot care: standards of medical care in diabetes-2018. Diabetes Care. 2018; 41(Suppl 1):S105–18.
628. Wong TY, Sun J, Kawasaki R, Ruamviboonsuk P, Gupta N, Lansingh VC, et al. Guidelines on diabetic eye care: the International Council of Ophthalmology recommendations for screening, follow-up, referral, and treatment based on resource settings. Ophthalmology. 2018; 125:1608–22.
629. Kim SW, Kang GW. Cost-utility analysis of screening strategies for diabetic retinopathy in Korea. J Korean Med Sci. 2015; 30:1723–32.
630. Diabetes Control and Complications Trial Research Group. Effect of pregnancy on microvascular complications in the diabetes control and complications trial. Diabetes Care. 2000; 23:1084–91.
631. Gunderson EP, Lewis CE, Tsai AL, Chiang V, Carnethon M, Quesenberry CP Jr, et al. A 20-year prospective study of childbearing and incidence of diabetes in young women, controlling for glycemia before conception: the Coronary Artery Risk Development in Young Adults (CARDIA) Study. Diabetes. 2007; 56:2990–6.
632. Chew EY, Klein ML, Murphy RP, Remaley NA, Ferris FL 3rd. Effects of aspirin on vitreous/preretinal hemorrhage in patients with diabetes mellitus. Early Treatment Diabetic Retinopathy Study report no. 20. Arch Ophthalmol. 1995; 113:52–5.

633. The Diabetic Retinopathy Study Research Group. Preliminary report on effects of photocoagulation therapy. Am J Ophthalmol. 1976; 81:383–96.
634. Early Treatment Diabetic Retinopathy Study research group. Photocoagulation for diabetic macular edema. Early Treatment Diabetic Retinopathy Study report number 1. Arch Ophthalmol. 1985; 103:1796–806.
635. Vergmann AS, Grauslund J. Changes of visual fields in treatment of proliferative diabetic retinopathy: a systematic review. Acta Ophthalmol. 2020; 98:763–73.

636. Writing Committee for the Diabetic Retinopathy Clinical Research Network, Gross JG, Glassman AR, Jampol LM, Inusah S, Aiello LP, et al. Panretinal photocoagulation vs intravitreous ranibizumab for proliferative diabetic retinopathy: a randomized clinical trial. JAMA. 2015; 314:2137–46.

637. Sivaprasad S, Prevost AT, Vasconcelos JC, Riddell A, Murphy C, Kelly J, et al. Clinical efficacy of intravitreal aflibercept versus panretinal photocoagulation for best corrected visual acuity in patients with proliferative diabetic retinopathy at 52 weeks (CLARITY): a multicentre, single-blinded, randomised, controlled, phase 2b, non-inferiority trial. Lancet. 2017; 389:2193–203.
638. Hutton DW, Stein JD, Glassman AR, Bressler NM, Jampol LM, Sun JK, et al. Five-year cost-effectiveness of intravitreous ranibizumab therapy vs panretinal photocoagulation for treating proliferative diabetic retinopathy: a secondary analysis of a randomized clinical trial. JAMA Ophthalmol. 2019; 137:1424–32.

639. Wells JA, Glassman AR, Ayala AR, Jampol LM, Bressler NM, Bressler SB, et al. Aflibercept, bevacizumab, or ranibizumab for diabetic macular edema: two-year results from a comparative effectiveness randomized clinical trial. Ophthalmology. 2016; 123:1351–9.

640. He Y, Ren XJ, Hu BJ, Lam WC, Li XR. A meta-analysis of the effect of a dexamethasone intravitreal implant versus intravitreal anti-vascular endothelial growth factor treatment for diabetic macular edema. BMC Ophthalmol. 2018; 18:121.

641. Lau PE, Jenkins KS, Layton CJ. Current evidence for the prevention of endophthalmitis in anti-VEGF intravitreal injections. J Ophthalmol. 2018; 2018:8567912.

642. Porta M, Striglia E. Intravitreal anti-VEGF agents and cardiovascular risk. Intern Emerg Med. 2020; 15:199–210.
643. Ludvigsson JF, Neovius M, Soderling J, Gudbjornsdottir S, Svensson AM, Franzen S, et al. Maternal glycemic control in type 1 diabetes and the risk for preterm birth: a populationbased cohort study. Ann Intern Med. 2019; 170:691–701.

644. Moon JH, Jang HC. Gestational diabetes mellitus: diagnostic approaches and maternal-offspring complications. Diabetes Metab J. 2022; 46:3–14.

645. Chung HR, Moon JH, Lim JS, Lee YA, Shin CH, Hong JS, et al. Maternal hyperglycemia during pregnancy increases adiposity of offspring. Diabetes Metab J. 2021; 45:730–8.

646. de Veciana M, Major CA, Morgan MA, Asrat T, Toohey JS, Lien JM, et al. Postprandial versus preprandial blood glucose monitoring in women with gestational diabetes mellitus requiring insulin therapy. N Engl J Med. 1995; 333:1237–41.

647. Jovanovic-Peterson L, Peterson CM, Reed GF, Metzger BE, Mills JL, Knopp RH, et al. Maternal postprandial glucose levels and infant birth weight: the Diabetes in Early Pregnancy Study. The National Institute of Child Health and Human Development: Diabetes in Early Pregnancy Study. Am J Obstet Gynecol. 1991; 164(1 Pt 1):103–11.
648. Inkster ME, Fahey TP, Donnan PT, Leese GP, Mires GJ, Murphy DJ. Poor glycated haemoglobin control and adverse pregnancy outcomes in type 1 and type 2 diabetes mellitus: systematic review of observational studies. BMC Pregnancy Childbirth. 2006; 6:30.

649. Hernandez TL, Friedman JE, Van Pelt RE, Barbour LA. Patterns of glycemia in normal pregnancy: should the current therapeutic targets be challenged? Diabetes Care. 2011; 34:1660–8.
650. Oh TJ, Jang HC. Gestational diabetes mellitus: diagnosis and glycemic control. J Korean Diabetes. 2020; 21:69–74.

651. Viana LV, Gross JL, Azevedo MJ. Dietary intervention in patients with gestational diabetes mellitus: a systematic review and meta-analysis of randomized clinical trials on maternal and newborn outcomes. Diabetes Care. 2014; 37:3345–55.

652. Zhang R, Han S, Chen GC, Li ZN, Silva-Zolezzi I, Pares GV, et al. Effects of low-glycemic-index diets in pregnancy on maternal and newborn outcomes in pregnant women: a meta-analysis of randomized controlled trials. Eur J Nutr. 2018; 57:167–77.
653. Huang X, Huang J, Wu J, Li M, Yang Z, Liu L, et al. Different exercises for pregnant women with gestational diabetes: a meta-analysis of randomized controlled trials. J Sports Med Phys Fitness. 2020; 60:464–71.

654. Padayachee C, Coombes JS. Exercise guidelines for gestational diabetes mellitus. World J Diabetes. 2015; 6:1033–44.

655. Hernandez TL, Mande A, Barbour LA. Nutrition therapy within and beyond gestational diabetes. Diabetes Res Clin Pract. 2018; 145:39–50.

656. American Diabetes Association Professional Practice Committee. 15. Management of diabetes in pregnancy: standards of medical care in diabetes-2022. Diabetes Care. 2022; 45:S232–43.
657. Rowan JA, Hague WM, Gao W, Battin MR, Moore MP; MiG Trial Investigators. Metformin versus insulin for the treatment of gestational diabetes. N Engl J Med. 2008; 358:2003–15.

658. Balsells M, Garcia-Patterson A, Sola I, Roque M, Gich I, Corcoy R. Glibenclamide, metformin, and insulin for the treatment of gestational diabetes: a systematic review and meta-analysis. BMJ. 2015; 350:h102.
659. Brown J, Grzeskowiak L, Williamson K, Downie MR, Crowther CA. Insulin for the treatment of women with gestational diabetes. Cochrane Database Syst Rev. 2017; 11:CD012037.

660. Brown J, Martis R, Hughes B, Rowan J, Crowther CA. Oral anti-diabetic pharmacological therapies for the treatment of women with gestational diabetes. Cochrane Database Syst Rev. 2017; 1:CD011967.

661. ACOG Practice Bulletin No. 190: Gestational diabetes mellitus. Obstet Gynecol. 2018; 131:e49–64.
662. Rowan JA, Rush EC, Plank LD, Lu J, Obolonkin V, Coat S, et al. Metformin in gestational diabetes: the offspring follow-up (MiG TOFU): body composition and metabolic outcomes at 7-9 years of age. BMJ Open Diabetes Res Care. 2018; 6:e000456.

663. O’Neill SM, Kenny LC, Khashan AS, West HM, Smyth RM, Kearney PM. Different insulin types and regimens for pregnant women with pre-existing diabetes. Cochrane Database Syst Rev. 2017; 2:CD011880.
664. Roberge S, Bujold E, Nicolaides KH. Aspirin for the prevention of preterm and term preeclampsia: systematic review and meta-analysis. Am J Obstet Gynecol. 2018; 218:287–93.e1.
665. Hauspurg A, Sutton EF, Catov JM, Caritis SN. Aspirin effect on adverse pregnancy outcomes associated with stage 1 hypertension in a high-risk cohort. Hypertension. 2018; 72:202–7.

666. Tolcher MC, Sangi-Haghpeykar H, Mendez-Figueroa H, Aagaard KM. Low-dose aspirin for preeclampsia prevention: efficacy by ethnicity and race. Am J Obstet Gynecol MFM. 2020; 2:100184.

667. Adkins K, Allshouse AA, Metz TD, Heyborne KD. Impact of aspirin on fetal growth in diabetic pregnancies according to White classification. Am J Obstet Gynecol. 2017; 217:465.e1–5.

668. Vounzoulaki E, Khunti K, Abner SC, Tan BK, Davies MJ, Gillies CL. Progression to type 2 diabetes in women with a known history of gestational diabetes: systematic review and meta-analysis. BMJ. 2020; 369:m1361.

669. Kwak SH, Choi SH, Jung HS, Cho YM, Lim S, Cho NH, et al. Clinical and genetic risk factors for type 2 diabetes at early or late post partum after gestational diabetes mellitus. J Clin Endocrinol Metab. 2013; 98:E744–52.
670. Oh TJ, Kim YG, Kang S, Moon JH, Kwak SH, Choi SH, et al. Oral glucose tolerance testing allows better prediction of diabetes in women with a history of gestational diabetes mellitus. Diabetes Metab J. 2019; 43:342–9.

671. Moon JH, Kwak SH, Jang HC. Prevention of type 2 diabetes mellitus in women with previous gestational diabetes mellitus. Korean J Intern Med. 2017; 32:26–41.

672. Kramer CK, Campbell S, Retnakaran R. Gestational diabetes and the risk of cardiovascular disease in women: a systematic review and meta-analysis. Diabetologia. 2019; 62:905–14.

673. Grandi SM, Filion KB, Yoon S, Ayele HT, Doyle CM, Hutcheon JA, et al. Cardiovascular disease-related morbidity and mortality in women with a history of pregnancy complications. Circulation. 2019; 139:1069–79.

674. Moon JH, Kwak SH, Jung HS, Choi SH, Lim S, Cho YM, et al. Weight gain and progression to type 2 diabetes in women with a history of gestational diabetes mellitus. J Clin Endocrinol Metab. 2015; 100:3548–55.
675. Moon JH, Kim H, Kim H, Park J, Choi W, Choi W, et al. Lactation improves pancreatic β cell mass and function through serotonin production. Sci Transl Med. 2020; 12:eaay0455.

676. Pinho-Gomes AC, Morelli G, Jones A, Woodward M. Association of lactation with maternal risk of type 2 diabetes: a systematic review and meta-analysis of observational studies. Diabetes Obes Metab. 2021; 23:1902–16.

677. Guo J, Chen JL, Whittemore R, Whitaker E. Postpartum lifestyle interventions to prevent type 2 diabetes among women with history of gestational diabetes: a systematic review of randomized clinical trials. J Womens Health (Larchmt). 2016; 25:38–49.

678. Won CW. Evaluation and management of frailty. J Korean Med Assoc. 2017; 60:314–20.

679. Fried LP, Ferrucci L, Darer J, Williamson JD, Anderson G. Untangling the concepts of disability, frailty, and comorbidity: implications for improved targeting and care. J Gerontol A Biol Sci Med Sci. 2004; 59:255–63.
680. Kirkman MS, Briscoe VJ, Clark N, Florez H, Haas LB, Halter JB, et al. Diabetes in older adults. Diabetes Care. 2012; 35:2650–64.

681. LeRoith D, Biessels GJ, Braithwaite SS, Casanueva FF, Draznin B, Halter JB, et al. Treatment of diabetes in older adults: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2019; 104:1520–74.

682. Committee Report: glycemic targets for elderly patients with diabetes: Japan Diabetes Society (JDS)/Japan Geriatrics Society (JGS) Joint Committee on Improving Care for Elderly Patients with Diabetes. J Diabetes Investig. 2017; 8:126–8.
683. Abellan van Kan G, Rolland YM, Morley JE, Vellas B. Frailty: toward a clinical definition. J Am Med Dir Assoc. 2008; 9:71–2.

684. Rockwood K, Song X, MacKnight C, Bergman H, Hogan DB, McDowell I, et al. A global clinical measure of fitness and frailty in elderly people. CMAJ. 2005; 173:489–95.

685. Thorp AA, Healy GN, Owen N, Salmon J, Ball K, Shaw JE, et al. Deleterious associations of sitting time and television viewing time with cardiometabolic risk biomarkers: Australian Diabetes, Obesity and Lifestyle (AusDiab) study 2004-2005. Diabetes Care. 2010; 33:327–34.
686. American Diabetes Association. 12. Older adults: standards of medical care in diabetes-2021. Diabetes care. 2021; 44(Suppl 1):S168–79.
687. American Geriatrics Society Expert Panel on Care of Older Adults with Diabetes Mellitus, Moreno G, Mangione CM, Kimbro L, Vaisberg E. Guidelines abstracted from the American Geriatrics Society guidelines for improving the care of older adults with diabetes mellitus: 2013 update. J Am Geriatr Soc. 2013; 61:2020–6.

688. Villareal DT, Aguirre L, Gurney AB, Waters DL, Sinacore DR, Colombo E, et al. Aerobic or resistance exercise, or both, in dieting obese older adults. N Engl J Med. 2017; 376:1943–55.

689. Lee J, Kim D, Kim C. Resistance training for glycemic control, muscular strength, and lean body mass in old type 2 diabetic patients: a meta-analysis. Diabetes Ther. 2017; 8:459–73.

690. Thorpe CT, Gellad WF, Good CB, Zhang S, Zhao X, Mor M, et al. Tight glycemic control and use of hypoglycemic medications in older veterans with type 2 diabetes and comorbid dementia. Diabetes Care. 2015; 38:588–95.

691. Gilbert MP, Bain SC, Franek E, Jodar-Gimeno E, Nauck MA, Pratley R, et al. Effect of liraglutide on cardiovascular outcomes in elderly patients: a post hoc analysis of a randomized controlled trial. Ann Intern Med. 2019; 170:423–6.
692. Beckett NS, Peters R, Fletcher AE, Staessen JA, Liu L, Dumitrascu D, et al. Treatment of hypertension in patients 80 years of age or older. N Engl J Med. 2008; 358:1887–98.

693. Massing MW, Sueta CA, Chowdhury M, Biggs DP, Simpson RJ Jr. Lipid management among coronary artery disease patients with diabetes mellitus or advanced age. Am J Cardiol. 2001; 87:646–9. A10.

694. Ko RE, Moon SM, Kang D, Cho J, Chung CR, Lee Y, et al. Translation and validation of the Korean version of the clinical frailty scale in older patients. BMC Geriatr. 2021; 21:47.

695. Jung HW, Yoo HJ, Park SY, Kim SW, Choi JY, Yoon SJ, et al. The Korean version of the FRAIL scale: clinical feasibility and validity of assessing the frailty status of Korean elderly. Korean J Intern Med. 2016; 31:594–600.

696. Lloyd SM, Stott DJ, de Craen AJ, Kearney PM, Sattar N, Perry I, et al. Long-term effects of statin treatment in elderly people: extended follow-up of the PROspective Study of Pravastatin in the Elderly at Risk (PROSPER). PLoS One. 2013; 8:e72642.
697. Pratley RE, Kanapka LG, Rickels MR, Ahmann A, Aleppo G, Beck R, et al. Effect of continuous glucose monitoring on hypoglycemia in older adults with type 1 diabetes: a randomized clinical trial. JAMA. 2020; 323:2397–406.

698. Wagenknecht LE, Lawrence JM, Isom S, Jensen ET, Dabelea D, Liese AD, et al. Trends in incidence of youth-onset type 1 and type 2 diabetes in the USA, 2002-18: results from the population-based SEARCH for Diabetes in Youth study. Lancet Diabetes Endocrinol. 2023; 11:242–50.

699. Hong YH, Chung IH, Han K, Chung S; Taskforce Team of the Obesity Fact Sheet of the Korean Society for the Study of Obesity. Prevalence of type 2 diabetes mellitus among Korean children, adolescents, and adults younger than 30 years: changes from 2002 to 2016. Diabetes Metab J. 2022; 46:297–306.

700. Shah AS, Zeitler PS, Wong J, Pena AS, Wicklow B, Arslanian S, et al. ISPAD clinical practice consensus guidelines 2022: type 2 diabetes in children and adolescents. Pediatr Diabetes. 2022; 23:872–902.

701. Arslanian S, Bacha F, Grey M, Marcus MD, White NH, Zeitler P. Evaluation and management of youth-onset type 2 diabetes: a position statement by the American Diabetes Association. Diabetes Care. 2018; 41:2648–68.
702. Jonas DE, Vander Schaaf EB, Riley S, Allison BA, Middleton JC, Baker C, et al. Screening for prediabetes and type 2 diabetes in children and adolescents: evidence report and systematic review for the US preventive services task force. JAMA. 2022; 328:968–79.

703. Urakami T. Pediatric type 2 diabetes in Japan: similarities and differences from type 2 diabetes in other pediatric populations. Curr Diab Rep. 2018; 18:29.

704. Wei JN, Chuang LM, Lin CC, Chiang CC, Lin RS, Sung FC. Childhood diabetes identified in mass urine screening program in Taiwan, 1993-1999. Diabetes Res Clin Pract. 2003; 59:201–6.

705. Kim MS, Lee DY. Urinary glucose screening for early detection of asymptomatic type 2 diabetes in Jeonbuk province Korean schoolchildren. J Korean Med Sci. 2017; 32:985–91.

706. Marcus MD, Wilfley DE, El Ghormli L, Zeitler P, Linder B, Hirst K, et al. Weight change in the management of youth-onset type 2 diabetes: the TODAY clinical trial experience. Pediatr Obes. 2017; 12:337–45.
707. Berkowitz RI, Marcus MD, Anderson BJ, Delahanty L, Grover N, Kriska A, et al. Adherence to a lifestyle program for youth with type 2 diabetes and its association with treatment outcome in the TODAY clinical trial. Pediatr Diabetes. 2018; 19:191–8.

708. TODAY Study Group, Bjornstad P, Drews KL, Caprio S, Gubitosi-Klug R, Nathan DM, et al. Long-term complications in youth-onset type 2 diabetes. N Engl J Med. 2021; 385:416–26.

709. Lee YJ, Yoo S, Yi S, Kim S, Lee C, Cho J, et al. Trajectories in glycated hemoglobin and body mass index in children and adolescents with diabetes using the common data model. Sci Rep. 2021; 11:14614.

710. Savoye M, Nowicka P, Shaw M, Yu S, Dziura J, Chavent G, et al. Long-term results of an obesity program in an ethnically diverse pediatric population. Pediatrics. 2011; 127:402–10.

711. TODAY Study Group. Safety and tolerability of the treatment of youth-onset type 2 diabetes: the TODAY experience. Diabetes Care. 2013; 36:1765–71.
712. Zeitler P, Hirst K, Copeland KC, El Ghormli L, Levitt Katz L, Levitsky LL, et al. HbA1c after a short period of monotherapy with metformin identifies durable glycemic control among adolescents with type 2 diabetes. Diabetes Care. 2015; 38:2285–92.
713. TODAY Study Group, Zeitler P, Hirst K, Pyle L, Linder B, Copeland K, et al. A clinical trial to maintain glycemic control in youth with type 2 diabetes. N Engl J Med. 2012; 366:2247–56.

714. Tamborlane WV, Barrientos-Perez M, Fainberg U, Frimer-Larsen H, Hafez M, Hale PM, et al. Liraglutide in children and adolescents with type 2 diabetes. N Engl J Med. 2019; 381:637–46.

715. Arslanian SA, Hannon T, Zeitler P, Chao LC, Boucher-Berry C, Barrientos-Perez M, et al. Once-weekly dulaglutide for the treatment of youths with type 2 diabetes. N Engl J Med. 2022; 387:433–43.

716. Kelly AS, Auerbach P, Barrientos-Perez M, Gies I, Hale PM, Marcus C, et al. A randomized, controlled trial of liraglutide for adolescents with obesity. N Engl J Med. 2020; 382:2117–28.

717. Inge TH, Jenkins TM, Xanthakos SA, Dixon JB, Daniels SR, Zeller MH, et al. Long-term outcomes of bariatric surgery in adolescents with severe obesity (FABS-5+): a prospective follow-up analysis. Lancet Diabetes Endocrinol. 2017; 5:165–73.
718. Inge TH, Laffel LM, Jenkins TM, Marcus MD, Leibel NI, Brandt ML, et al. Comparison of surgical and medical therapy for type 2 diabetes in severely obese adolescents. JAMA Pediatr. 2018; 172:452–60.

719. Bjornstad P, Hughan K, Kelsey MM, Shah AS, Lynch J, Nehus E, et al. Effect of surgical versus medical therapy on diabetic kidney disease over 5 years in severely obese adolescents with type 2 diabetes. Diabetes Care. 2020; 43:187–95.

720. Alqahtani AR, Elahmedi M, Abdurabu HY, Alqahtani S. Tenyear outcomes of children and adolescents who underwent sleeve gastrectomy: weight loss, comorbidity resolution, adverse events, and growth velocity. J Am Coll Surg. 2021; 233:657–64.

721. Peters A, Laffel L; American Diabetes Association Transitions Working Group. Diabetes care for emerging adults: recommendations for transition from pediatric to adult diabetes care systems: a position statement of the American Diabetes Association, with representation by the American College of Osteopathic Family Physicians, the American Academy of Pediatrics, the American Association of Clinical Endocrinologists, the American Osteopathic Association, the Centers for Disease Control and Prevention, Children with Diabetes, The Endocrine Society, the International Society for Pediatric and Adolescent Diabetes, Juvenile Diabetes Research Foundation International, the National Diabetes Education Program, and the Pediatric Endocrine Society (formerly Lawson Wilkins Pediatric Endocrine Society). Diabetes Care. 2011; 34:2477–85.
722. Jin SM, Kim TH, Bae JC, Hur KY, Lee MS, Lee MK, et al. Clinical factors associated with absolute and relative measures of glycemic variability determined by continuous glucose monitoring: an analysis of 480 subjects. Diabetes Res Clin Pract. 2014; 104:266–72.
723. Jun JE, Lee SE, Lee YB, Ahn JY, Kim G, Hur KY, et al. Continuous glucose monitoring defined glucose variability is associated with cardiovascular autonomic neuropathy in type 1 diabetes. Diabetes Metab Res Rev. 2019; 35:e3092.

724. Yoo JH, Choi MS, Ahn J, Park SW, Kim Y, Hur KY, et al. Association between continuous glucose monitoring-derived time in range, other core metrics, and albuminuria in type 2 diabetes. Diabetes Technol Ther. 2020; 22:768–76.

725. Foster NC, Beck RW, Miller KM, Clements MA, Rickels MR, DiMeglio LA, et al. State of type 1 diabetes management and outcomes from the T1D exchange in 2016-2018. Diabetes Technol Ther. 2019; 21:66–72.

726. Lind M, Polonsky W, Hirsch IB, Heise T, Bolinder J, Dahlqvist S, et al. Continuous glucose monitoring vs conventional therapy for glycemic control in adults with type 1 diabetes treated with multiple daily insulin injections: the GOLD randomized clinical trial. JAMA. 2017; 317:379–87.

727. Tumminia A, Crimi S, Sciacca L, Buscema M, Frittitta L, Squatrito S, et al. Efficacy of real-time continuous glucose monitoring on glycaemic control and glucose variability in type 1 diabetic patients treated with either insulin pumps or multiple insulin injection therapy: a randomized controlled crossover trial. Diabetes Metab Res Rev. 2015; 31:61–8.
728. Sequeira PA, Montoya L, Ruelas V, Xing D, Chen V, Beck R, et al. Continuous glucose monitoring pilot in low-income type 1 diabetes patients. Diabetes Technol Ther. 2013; 15:855–8.

729. Juvenile Diabetes Research Foundation Continuous Glucose Monitoring Study Group, Tamborlane WV, Beck RW, Bode BW, Buckingham B, Chase HP, et al. Continuous glucose monitoring and intensive treatment of type 1 diabetes. N Engl J Med. 2008; 359:1464–76.

730. Battelino T, Conget I, Olsen B, Schutz-Fuhrmann I, Hommel E, Hoogma R, et al. The use and efficacy of continuous glucose monitoring in type 1 diabetes treated with insulin pump therapy: a randomised controlled trial. Diabetologia. 2012; 55:3155–62.

731. Deiss D, Bolinder J, Riveline JP, Battelino T, Bosi E, Tubiana-Rufi N, et al. Improved glycemic control in poorly controlled patients with type 1 diabetes using real-time continuous glucose monitoring. Diabetes Care. 2006; 29:2730–2.

732. O’Connell MA, Donath S, O’Neal DN, Colman PG, Ambler GR, Jones TW, et al. Glycaemic impact of patient-led use of sensor-guided pump therapy in type 1 diabetes: a randomised controlled trial. Diabetologia. 2009; 52:1250–7.
733. Bolinder J, Antuna R, Geelhoed-Duijvestijn P, Kroger J, Weitgasser R. Novel glucose-sensing technology and hypoglycaemia in type 1 diabetes: a multicentre, non-masked, randomised controlled trial. Lancet. 2016; 388:2254–63.

734. Hermanns N, Schumann B, Kulzer B, Haak T. The impact of continuous glucose monitoring on low interstitial glucose values and low blood glucose values assessed by point-of-care blood glucose meters: results of a crossover trial. J Diabetes Sci Technol. 2014; 8:516–22.

735. van Beers CA, DeVries JH, Kleijer SJ, Smits MM, Geelhoed-Duijvestijn PH, Kramer MH, et al. Continuous glucose monitoring for patients with type 1 diabetes and impaired awareness of hypoglycaemia (IN CONTROL): a randomised, open-label, crossover trial. Lancet Diabetes Endocrinol. 2016; 4:893–902.

736. Juvenile Diabetes Research Foundation Continuous Glucose Monitoring Study Group, Beck RW, Hirsch IB, Laffel L, Tamborlane WV, Bode BW, et al. The effect of continuous glucose monitoring in well-controlled type 1 diabetes. Diabetes Care. 2009; 32:1378–83.

737. Battelino T, Phillip M, Bratina N, Nimri R, Oskarsson P, Bolinder J. Effect of continuous glucose monitoring on hypoglycemia in type 1 diabetes. Diabetes Care. 2011; 34:795–800.
738. Seyed Ahmadi S, Westman K, Pivodic A, Olafsdottir AF, Dahlqvist S, Hirsch IB, et al. The association between HbA1c and time in hypoglycemia during CGM and self-monitoring of blood glucose in people with type 1 diabetes and multiple daily insulin injections: a randomized clinical trial (GOLD-4). Diabetes Care. 2020; 43:2017–24.

739. Haskova A, Radovnicka L, Petruzelkova L, Parkin CG, Grunberger G, Horova E, et al. Real-time CGM is superior to flash glucose monitoring for glucose control in type 1 diabetes: the CORRIDA randomized controlled trial. Diabetes Care. 2020; 43:2744–50.

740. Beck RW, Riddlesworth TD, Ruedy K, Ahmann A, Haller S, Kruger D, et al. Continuous glucose monitoring versus usual care in patients with type 2 diabetes receiving multiple daily insulin injections: a randomized trial. Ann Intern Med. 2017; 167:365–74.

741. Haak T, Hanaire H, Ajjan R, Hermanns N, Riveline JP, Rayman G. Flash glucose-sensing technology as a replacement for blood glucose monitoring for the management of insulin-treated type 2 diabetes: a multicenter, open-label randomized controlled trial. Diabetes Ther. 2017; 8:55–73.

742. Yaron M, Roitman E, Aharon-Hananel G, Landau Z, Ganz T, Yanuv I, et al. Effect of flash glucose monitoring technology on glycemic control and treatment satisfaction in patients with type 2 diabetes. Diabetes Care. 2019; 42:1178–84.
743. Martens T, Beck RW, Bailey R, Ruedy KJ, Calhoun P, Peters AL, et al. Effect of continuous glucose monitoring on glycemic control in patients with type 2 diabetes treated with basal insulin: a randomized clinical trial. JAMA. 2021; 325:2262–72.
744. Aleppo G, Beck RW, Bailey R, Ruedy KJ, Calhoun P, Peters AL, et al. The effect of discontinuing continuous glucose monitoring in adults with type 2 diabetes treated with basal insulin. Diabetes Care. 2021; 44:2729–37.
745. Choe HJ, Rhee EJ, Won JC, Park KS, Lee WY, Cho YM. Effects of patient-driven lifestyle modification using intermittently scanned continuous glucose monitoring in patients with type 2 diabetes: results from the randomized open-label PDF study. Diabetes Care. 2022; 45:2224–30.

746. Vigersky RA, Fonda SJ, Chellappa M, Walker MS, Ehrhardt NM. Short- and long-term effects of real-time continuous glucose monitoring in patients with type 2 diabetes. Diabetes Care. 2012; 35:32–8.

747. Ehrhardt NM, Chellappa M, Walker MS, Fonda SJ, Vigersky RA. The effect of real-time continuous glucose monitoring on glycemic control in patients with type 2 diabetes mellitus. J Diabetes Sci Technol. 2011; 5:668–75.
748. Yoo HJ, An HG, Park SY, Ryu OH, Kim HY, Seo JA, et al. Use of a real time continuous glucose monitoring system as a motivational device for poorly controlled type 2 diabetes. Diabetes Res Clin Pract. 2008; 82:73–9.

749. Moon SJ, Kim KS, Lee WJ, Lee MY, Vigersky R, Park CY. Efficacy of intermittent short-term use of a real-time continuous glucose monitoring system in non-insulin-treated patients with type 2 diabetes: a randomized controlled trial. Diabetes Obes Metab. 2023; 25:110–20.

750. Feig DS, Donovan LE, Corcoy R, Murphy KE, Amiel SA, Hunt KF, et al. Continuous glucose monitoring in pregnant women with type 1 diabetes (CONCEPTT): a multicentre international randomised controlled trial. Lancet. 2017; 390:2347–59.
751. Secher AL, Ringholm L, Andersen HU, Damm P, Mathiesen ER. The effect of real-time continuous glucose monitoring in pregnant women with diabetes: a randomized controlled trial. Diabetes Care. 2013; 36:1877–83.
752. Wei Q, Sun Z, Yang Y, Yu H, Ding H, Wang S. Effect of a CGMS and SMBG on maternal and neonatal outcomes in gestational diabetes mellitus: a randomized controlled trial. Sci Rep. 2016; 6:19920.
753. Brown SA, Kovatchev BP, Raghinaru D, Lum JW, Buckingham BA, Kudva YC, et al. Six-month randomized, multicenter trial of closed-loop control in type 1 diabetes. N Engl J Med. 2019; 381:1707–17.

754. Tauschmann M, Thabit H, Bally L, Allen JM, Hartnell S, Wilinska ME, et al. Closed-loop insulin delivery in suboptimally controlled type 1 diabetes: a multicentre, 12-week randomised trial. Lancet. 2018; 392:1321–9.

755. Ekhlaspour L, Raghinaru D, Forlenza GP, Isganaitis E, Kudva YC, Lam DW, et al. Outcomes in pump- and CGM-baseline use subgroups in the International Diabetes Closed-Loop (iDCL) trial. J Diabetes Sci Technol. 2023; 17:935–42.

756. Ekhlaspour L, Town M, Raghinaru D, Lum JW, Brown SA, Buckingham BA. Glycemic outcomes in baseline hemoglobin A1C subgroups in the International Diabetes Closed-Loop trial. Diabetes Technol Ther. 2022; 24:588–91.

757. Schoelwer MJ, Kanapka LG, Wadwa RP, Breton MD, Ruedy KJ, Ekhlaspour L, et al. Predictors of time-in-range (70-180 mg/dL) achieved using a closed-loop control system. Diabetes Technol Ther. 2021; 23:475–81.
758. Lal RA, Basina M, Maahs DM, Hood K, Buckingham B, Wilson DM. One year clinical experience of the first commercial hybrid closed-loop system. Diabetes Care. 2019; 42:2190–6.

759. Bergenstal RM, Nimri R, Beck RW, Criego A, Laffel L, Schatz D, et al. A comparison of two hybrid closed-loop systems in adolescents and young adults with type 1 diabetes (FLAIR): a multicentre, randomised, crossover trial. Lancet. 2021; 397:208–19.

760. Weinzimer SA, Bailey RJ, Bergenstal RM, Nimri R, Beck RW, Schatz D, et al. A comparison of postprandial glucose control in the medtronic advanced hybrid closed-loop system versus 670G. Diabetes Technol Ther. 2022; 24:573–82.
761. Matejko B, Juza A, Kiec-Wilk B, Cyranka K, Krzyzowska S, Chen X, et al. Transitioning of people with type 1 diabetes from multiple daily injections and self-monitoring of blood glucose directly to MiniMed 780G advanced hybrid closed-loop system: a two-center, randomized, controlled study. Diabetes Care. 2022; 45:2628–35.

762. McAuley SA, Trawley S, Vogrin S, Ward GM, Fourlanos S, Grills CA, et al. Closed-loop insulin delivery versus sensor-augmented pump therapy in older adults with type 1 diabetes (ORACL): a randomized, crossover trial. Diabetes Care. 2022; 45:381–90.
763. 1Soupal J, Petruzelkova L, Grunberger G, Haskova A, Flekac M, Matoulek M, et al. Glycemic outcomes in adults with T1D are impacted more by continuous glucose monitoring than by insulin delivery method: 3 years of follow-up from the COMISAIR study. Diabetes Care. 2020; 43:37–43.

764. Bergenstal RM, Klonoff DC, Garg SK, Bode BW, Meredith M, Slover RH, et al. Threshold-based insulin-pump interruption for reduction of hypoglycemia. N Engl J Med. 2013; 369:224–32.

765. Forlenza GP, Li Z, Buckingham BA, Pinsker JE, Cengiz E, Wadwa RP, et al. Predictive low-glucose suspend reduces hypoglycemia in adults, adolescents, and children with type 1 diabetes in an at-home randomized crossover study: results of the PROLOG trial. Diabetes Care. 2018; 41:2155–61.

766. Calhoun PM, Buckingham BA, Maahs DM, Hramiak I, Wilson DM, Aye T, et al. Efficacy of an overnight predictive low-glucose suspend system in relation to hypoglycemia risk factors in youth and adults with type 1 diabetes. J Diabetes Sci Technol. 2016; 10:1216–21.

767. Derosa G, Maffioli P, D’Angelo A, Salvadeo SA, Ferrari I, Fogari E, et al. Effects of insulin therapy with continuous subcutaneous insulin infusion (CSII) in diabetic patients: comparison with multi-daily insulin injections therapy (MDI). Endocr J. 2009; 56:571–8.
768. Herman WH, Ilag LL, Johnson SL, Martin CL, Sinding J, Al Harthi A, et al. A clinical trial of continuous subcutaneous insulin infusion versus multiple daily injections in older adults with type 2 diabetes. Diabetes Care. 2005; 28:1568–73.

769. Raskin P, Bode BW, Marks JB, Hirsch IB, Weinstein RL, Mc-Gill JB, et al. Continuous subcutaneous insulin infusion and multiple daily injection therapy are equally effective in type 2 diabetes: a randomized, parallel-group, 24-week study. Diabetes Care. 2003; 26:2598–603.
770. Wainstein J, Metzger M, Boaz M, Minuchin O, Cohen Y, Yaffe A, et al. Insulin pump therapy vs. multiple daily injections in obese type 2 diabetic patients. Diabet Med. 2005; 22:1037–46.
771. Reznik Y, Cohen O, Aronson R, Conget I, Runzis S, Castaneda J, et al. Insulin pump treatment compared with multiple daily injections for treatment of type 2 diabetes (OpT2mise): a randomised open-label controlled trial. Lancet. 2014; 384:1265–72.

772. DeVries JH, Snoek FJ, Kostense PJ, Masurel N, Heine RJ; Dutch Insulin Pump Study Group. A randomized trial of continuous subcutaneous insulin infusion and intensive injection therapy in type 1 diabetes for patients with long-standing poor glycemic control. Diabetes Care. 2002; 25:2074–80.
773. Thomas RM, Aldibbiat A, Griffin W, Cox MA, Leech NJ, Shaw JA. A randomized pilot study in type 1 diabetes complicated by severe hypoglycaemia, comparing rigorous hypoglycaemia avoidance with insulin analogue therapy, CSII or education alone. Diabet Med. 2007; 24:778–83.
774. Tsui E, Barnie A, Ross S, Parkes R, Zinman B. Intensive insulin therapy with insulin lispro: a randomized trial of continuous subcutaneous insulin infusion versus multiple daily insulin injection. Diabetes Care. 2001; 24:1722–7.
775. Bolli GB, Kerr D, Thomas R, Torlone E, Sola-Gazagnes A, Vitacolonna E, et al. Comparison of a multiple daily insulin injection regimen (basal once-daily glargine plus mealtime lispro) and continuous subcutaneous insulin infusion (lispro) in type 1 diabetes: a randomized open parallel multicenter study. Diabetes Care. 2009; 32:1170–6.
776. REPOSE Study Group. Relative effectiveness of insulin pump treatment over multiple daily injections and structured education during flexible intensive insulin treatment for type 1 diabetes: cluster randomised trial (REPOSE). BMJ. 2017; 356:j1285.
777. Pickup JC, Sutton AJ. Severe hypoglycaemia and glycaemic control in type 1 diabetes: meta-analysis of multiple daily insulin injections compared with continuous subcutaneous insulin infusion. Diabet Med. 2008; 25:765–74.
778. Choudhary P, Rickels MR, Senior PA, Vantyghem MC, Maffi P, Kay TW, et al. Evidence-informed clinical practice recommendations for treatment of type 1 diabetes complicated by problematic hypoglycemia. Diabetes Care. 2015; 38:1016–29.




 PDF
PDF Citation
Citation Print
Print



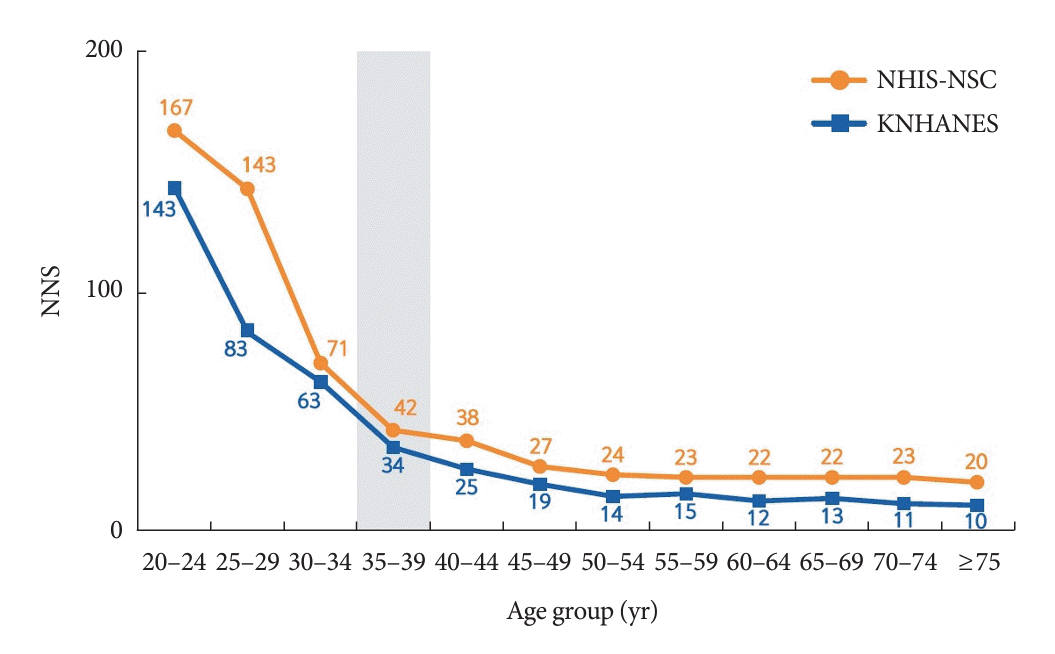
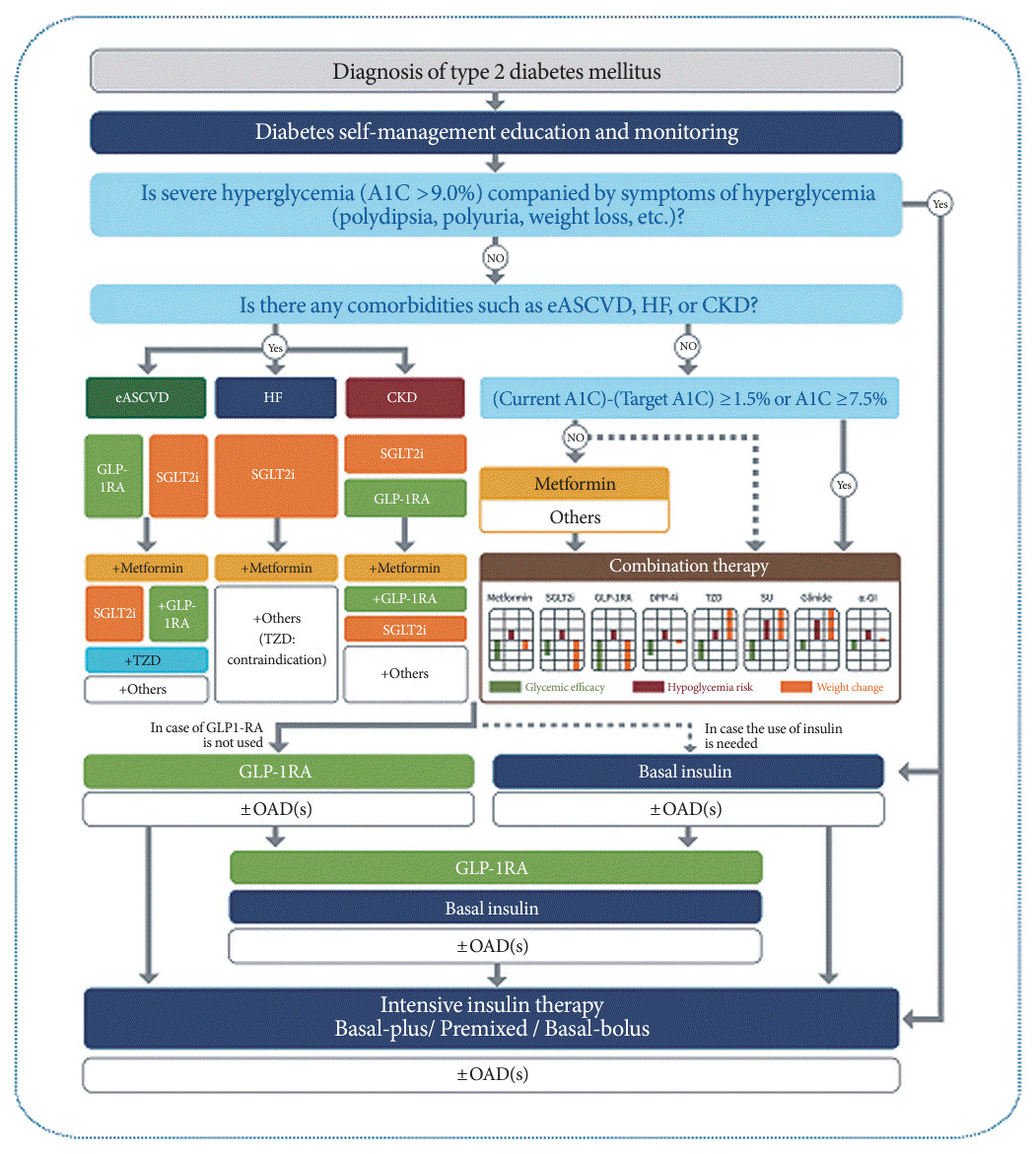
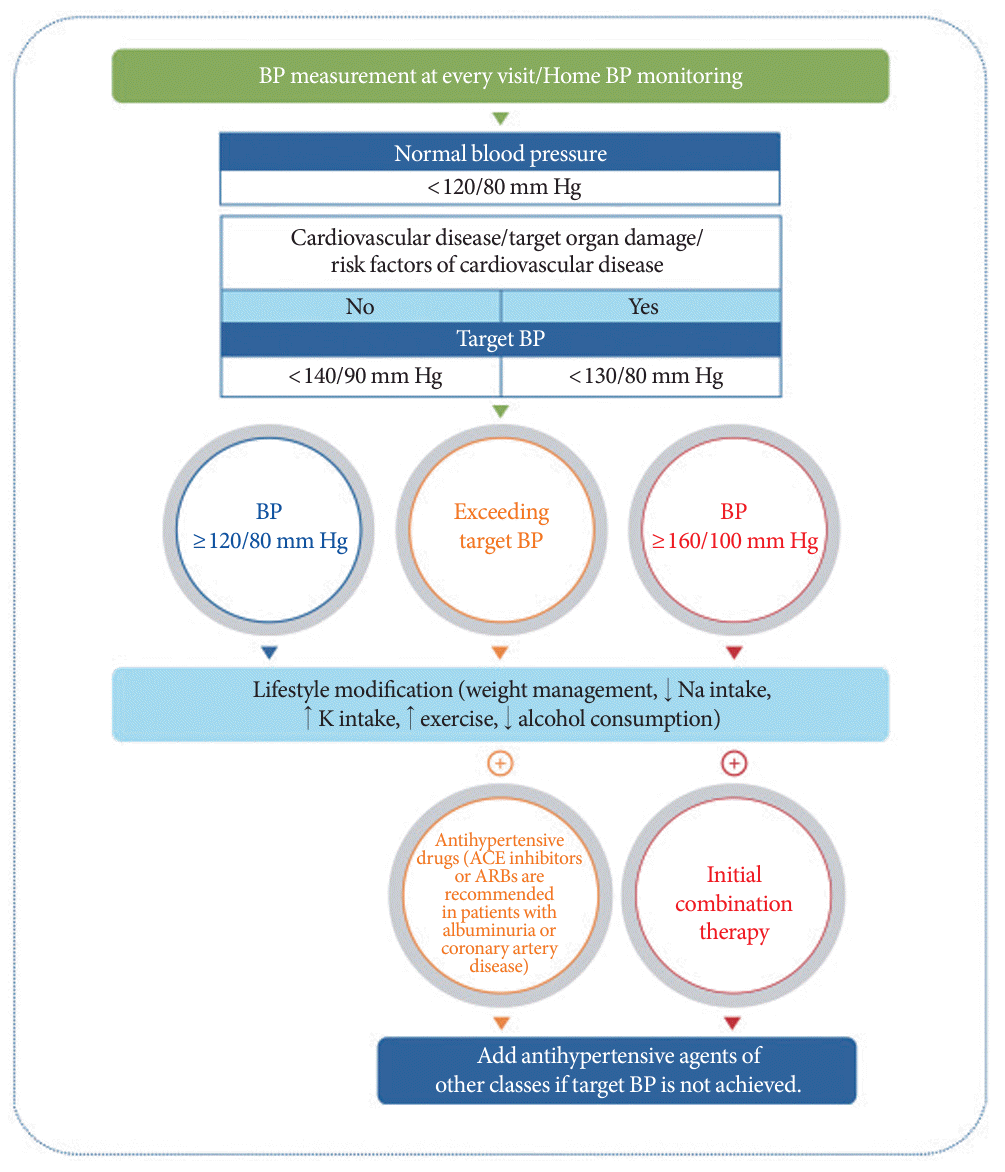
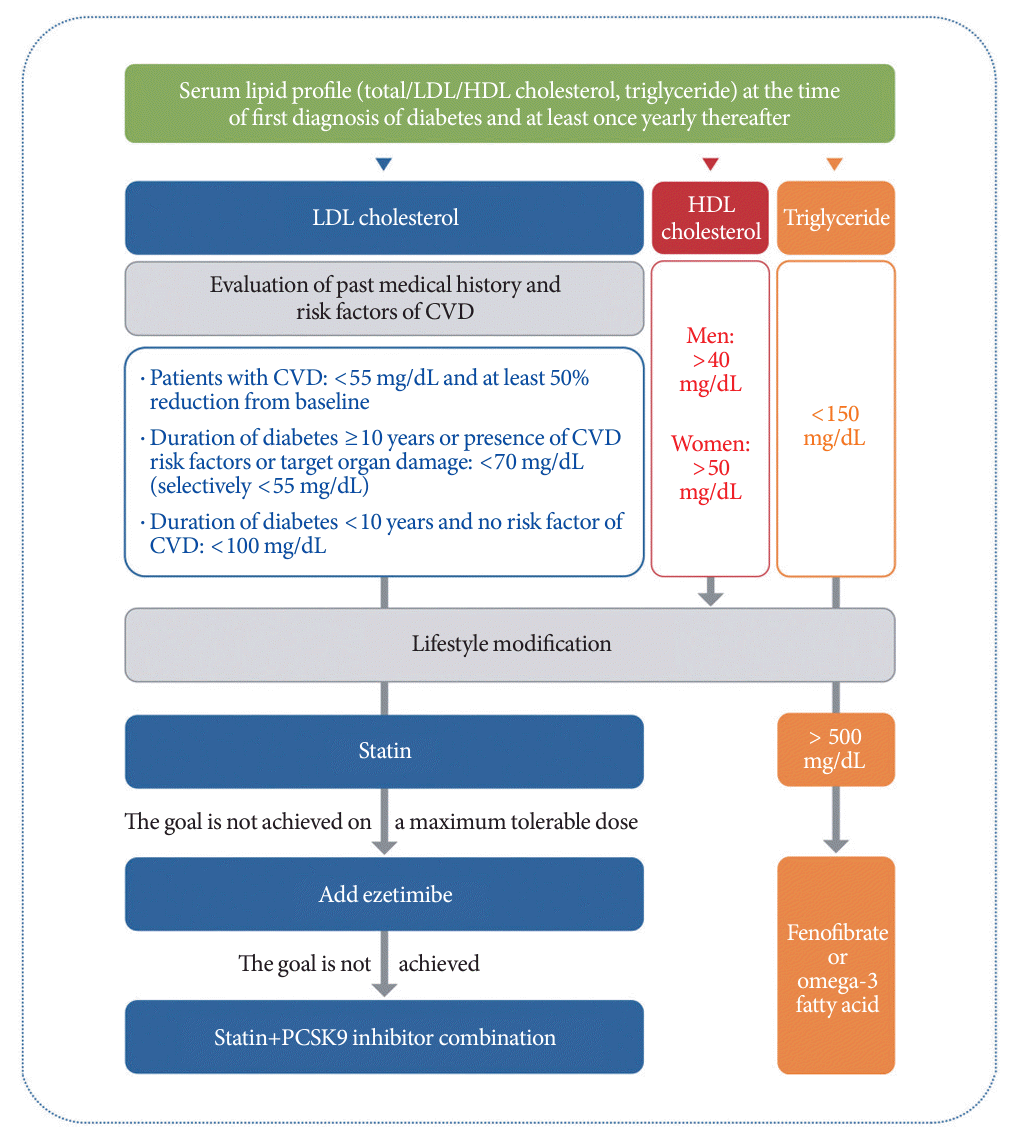
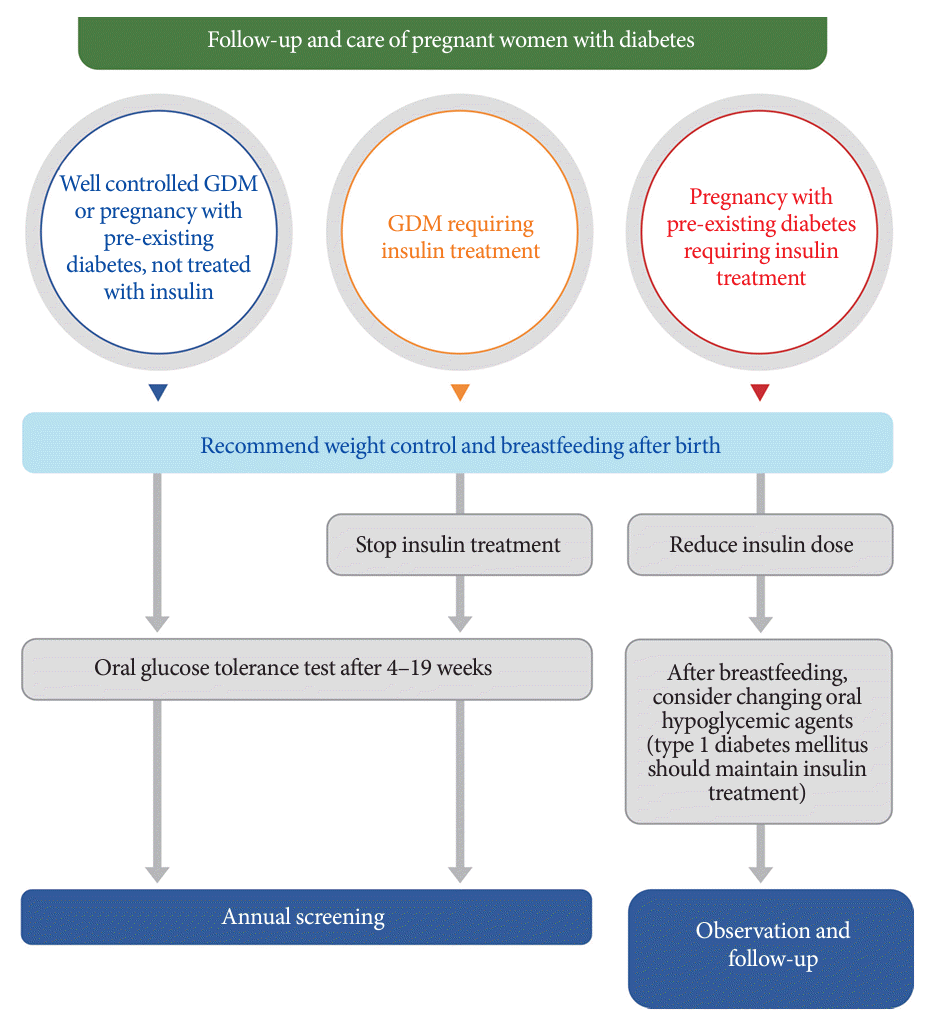
 XML Download
XML Download