Abstract
Objective
This study aimed to investigate the levels of chitinase-3-like protein-1 (CHI3L1), matrix metalloproteinase-9 (MMP-9), and monocyte chemoattractant protein-1 (MCP-1) in adenomyosis, as compared to normal myometrial tissue. These biomarkers may be useful for determining potential treatment targets.
Methods
This was a correlative, analytical, and observational study with a cross-sectional design. Participants with a diagnosis of moderate-to-severe adenomyosis, as determined through transvaginal ultrasonography and histological examination, and who underwent laparotomy or laparoscopic surgery for the treatment of adenomyosis, were enrolled in the study. Unlike other studies that recruited healthy women as controls, our study used adenomyotic and healthy nonadenomyotic myometria obtained from the same individual. The levels of CHI3L1, MMP-9, and MCP-1 in the biopsy samples were determined using enzyme-linked immunoassay kits, according to the manufacturer’s protocol.
Results
A highly significant increase in the levels of CHI3L1, MMP-9, and MCP-1 was found in adenomyotic tissues compared to non-adenomyotic tissues (P<0.001). A significant positive correlation was found between CHI3L1 and MMP-9 levels (r=0.463; P=0.008), CHI3L1 and MCP-1 levels (r=0.594; P<0.001), and MCP-1 and MMP-9 levels (r=0.680; P<0.001) in adenomyotic tissues.
The diagnosis and management of adenomyosis, a common gynecological disorder, poses significant challenges. Adenomyosis is characterized by invasion of the endometrial glands and stroma into the myometrium, resulting in diffuse uterine enlargement and a variety of symptoms, including pelvic pain, abnormal uterine bleeding, subfertility, and also adverse pregnancy outcomes such as hypertensive disorder during pregnancy, gestational diabetes mellitus, postpartum hemorrhage, placental abruption, preterm birth, and delivery of a small-for-gestational-age infant [1-3]. Adenomyosis has long been regarded as an “internal endometriosis”; however, despite its similarity to endometriosis in terms of ectopic endometrial tissue, the pathogenesis and clinical course of this condition remain unknown [1,2].
Adenomyosis affects women of reproductive age with a prevalence ranging from 5% to 70%. Hysterectomy is the most common surgical procedure for treating adenomyosis, with an average frequency of 20-30% [4]. Most cases (70-80%) are reported in women between the ages of 40 years and 50 years. Approximately 5-25% of adenomyosis cases are found in patients aged <39 years, and only 5-10% are found in women aged >60 years [5]. Naftalin et al. [6] found that 206 of 985 patients (20.9%) in the United Kingdom were diagnosed with adenomyosis, and the prevalence increased to 32% among women aged 40-49 years. Another study in Italy reported an adenomyosis prevalence of 34% in 156 women aged 18-30 years [7]. The incidence rate in Asia cannot be fully represented; however, a study conducted in Korea among women aged 11-52 years revealed an increase in adenomyosis prevalence from 1.4 per 1,000 in 2002 to 7.5 per 1,000 in 2016 [8]. Of the 220 women examined in Thailand, 47 (21.4%) were diagnosed with definite adenomyosis [9]. A large-scale study conducted in the United States from 2006 to 2015 reported the overall incidence of adenomyosis as 1.03% (28.9%) per 10,000 women per year. The overall prevalence in 2015 was 0.8% and was highest in women aged 41-45 years (1.5%) [10].
Previous studies have elucidated the pathogenesis of adenomyosis through several theories, including endometrial invagination of the myometrium with a tissue injury and repair (TIAR) mechanism, development of adult stem cells or de novo growth of the Müllerian duct, and invasion from outside-to-inside the uterus [11-13]. Supraphysiological estrogen production (hyperestrogenism) due to local paracrine activity in the eutopic and ectopic endometrium contributes to the development of adenomyosis [11,14]. Progesterone resistance in adenomyosis causes abnormal endometrial proliferation during the secretory phase of menstrual cycle. Hyperestrogenism also causes increased uterine sensitivity to oxytocin, which increases the mechanical pressure that breaks junctional zone cells. TIAR mechanisms are activated in response to tissue microtraumas. Local inflammatory mediators, such as interleukin-1 beta, cyclooxygenase-2, and prostaglandin E2, are produced and activate steroidogenic acute regulatory proteins as well as P450 aromatase, which causes a hyperestrogenic positive feedback mechanism. Repeated micro-traumatization tears the myometrium and activates matrix metalloproteases (MMPs), particularly MMP-2 and -9, aiding endometrial invagination into the myometrium. In the de novo growth theory, the Müllerian duct regresses and resides in the eutopic and ectopic endometrium. Müllerian cells possess stem cell properties that enable them to divide rapidly and differentiate into endometrial and stromal cells. Micro-traumatization of the basal endometrium and junctional zone causes Müllerian cells to invade the myometrium and differentiate into the endometrium [11]. The outside-to-inside invasion theory states that mature endometrial cells from retrograde menstruation have the potential to infiltrate the uterine perimetrium and break through the outer layer of the myometrium, which later develops into intramyometrial endometrial implants (focal adenomyosis) [12,13].
Chitinase-3-like protein-1 (CHI3L1) is a 40 kDa glycoprotein that can bind chitin without chitinase enzymatic activity owing to mutations in its active domains. CHI3L1 is produced during chronic inflammation and has the ability to bind several receptors, including receptor for advanced glycation end products, syndecan-1 (Sdc1)/alpha-v beta-3, and interleukin 13 receptor alpha 2, eventually inducing inflammasome formation, apoptosis, carcinogenesis, and angiogenesis. This protein also increases the production of interleukins (IL), including IL-6, IL-8, IL-12, IL-18, interferon-γ, tumor necrosis factor-α, chemokine ligands (CXCL) 9 and 11, and monocyte chemoattractant protein-1 (MCP-1)/ chemokine (C-C motif) ligand 2 (CCL2) [15,16].
MCP-1, also known as CCL2, attracts and activates mononuclear cells, resulting in their aggregation in the epithelial-mesenchymal transition zone of the adenomyotic endometrium [17]. Several studies showed positive regulation of MCP-1 towards phosphatidylinositol 3-kinase/protein kinase B (Akt), mitogen-activated protein kinase (MAPK)/extracellular-signal-regulated kinase 1/2 (ERK1/2)/MMP-9, wingless-related integration site/β-catenin, C-C motif chemokine receptor 2, reticular activating system, rapidly accelerated fibrosarcoma-1, MAPK kinase, ERK, and nuclear factor kappa B pathways, resulting in cell proliferation, migration, and angiogenesis [18,19].
MMPs are a family of zinc-dependent endopeptidases that play a role in physiological and pathophysiological tissue remodeling. MMPs cleave the structural elements of the extracellular matrix. MMP family members were categorized based on their substrate specificity. MMP-9, also known as gelatinase B, is a member of the gelatinase family. The main substrate of MMP-9 is gelatin; however, it can also cleave collagen types IV, V, VII, X, and XIV; elastin; fibrillin; and osteonectin. Numerous cells, including neutrophils, macrophages, and fibroblasts, secrete MMP-9 [20]. Several studies have shown that MMP-9 plays an important role in the pathophysiology of adenomyosis by assisting endometrial invasion into myometrial tissue and remodeling vascular endothelial tissue during adenomyotic implant angiogenesis [21,22].
Despite significant roles in many pathological conditions, the role of CHI3L1, MMP-9, and MCP-1 in adenomyosis remains poorly understood. Studies related to adenomyosis are still extremely limited, and there is a pressing need for better understanding of the factors involved in the development of this condition. Therefore, this study aimed to investigate the potential correlations between CHI3L1, MMP-9, and MCP-1/CCL2 in the context of adenomyosis pathogenesis. The results of this study are expected to provide new insights into the mechanisms of adenomyosis pathogenesis, and may contribute to the development of molecular diagnostics and more effective therapies for this condition.
This is a cross-sectional study. This study was approved by ethical approval number LB.02.01/X.6.5/187/2023. Patients aged between 20 years and 49 years, who visited the Department of Obstetrics and Gynecology between May and July 2023 and had moderate-to-severe adenomyosis (adenomyotic area >25% of the myometrium) based on transvaginal ultrasound and histological diagnosis were enrolled in the study. The transvaginal ultrasound findings for adenomyosis were as follows: 1) the presence of ectopic endometrial glands and stroma or myometrial cysts and fluid-filled striations (Fig. 1A); 2) muscular hyperplasia/hypertrophy, which appeared as fan-shaped shadowing (Fig. 1B); and 3) increased vascularity (Fig. 1C). Adenomyosis was histologically diagnosed based on the presence of endometrial stroma and glandular tissue within the smooth muscle of the myometrium (Fig. 2). Patients were excluded if they 1) received hormonal therapy in the last 3 months; 2) received anti-inflammatory therapy; 3) had an active infection; 4) had a uterine malignancy; or 5) had co-existing adenomyosis and uterine leiomyoma.
The number of patients recruited was determined using the following formula.
Where: n=number of participants; r=expected correlation coefficient; α=probability of type I error-incorrectly rejecting the null hypothesis; β=probability of type II error-incorrectly failing to reject the null hypothesis; Zα=the z-score at α level; Z1-β=the z-score at β level.
By adjusting r to 0.5, a type I error rate (α) of 5%, and a type II error rate (β) of 20%, a minimum of 29 participants were enrolled. Finally, 32 participants were recruited, with a 10% dropout rate. Written and verbal informed consent was obtained from all patients before treatment was administered.
Adenomyotic and normal myometrial biopsies were obtained from the same patient via laparotomy or laparoscopic surgery. The tissues were then cut to a size of 1 cm3, washed with ice-cold phosphate-buffered saline (PBS) at pH 7.4, and weighed. Tissues were homogenized and sonicated in PBS at a ratio of 1:9 (tissue [g]: PBS volume [mL]) using an ultrasonic homogenizer (Omni Ruptor 4000 Ultrasonic Homogenizer; Omni International, Kennesaw, GA, USA). The homogenate was then centrifuged for 5 minutes at 5,000×g using a 15 mL/50 mL centrifuge (Eppendorf Centrifuge 5810 R; Eppendorf, Hamburg, Germany). The supernatant was collected and used for further analyses.
CHI3L1 (Cat No. BZ-08123602-EB), MMP-9 (Cat No. BZ-08126390-EB), and MCP-1 (Cat No. BZ-08120384-EB) levels were measured using BioenzyTM enzyme-linked immunosorbent assay kits (Bioenzy, Jakarta, Indonesia) according to the manufacturer’s protocol. Briefly, a standard solution was prepared by diluting 120 µL standard solution to 120 µL standard diluent to generate a 240 ng/mL standard stock solution, which was then serially diluted at 1:2 using the standard diluent. Subsequently, 50 µL of the standard solution was pipetted into each well plate. The sample homogenate supernatant (40 µL) was then added to each well. Subsequently, 10 µL of anti-CHI3L1/MMP-9/MCP-1 antibody was added to the sample wells. This was followed by the addition of streptavidin-horseradish peroxidase conjugate (50 µL) to the standard and sample wells. The plates were then incubated at 37°C for 60 minutes. The supernatant was discarded, and the wells were washed five times with washing buffer. Substrates A (50 µL) and B (50 µL) were then added to each well and incubated for 10 minutes at 37°C in the dark. A stop solution 50 µL was then added, and the absorbance of the solution was measured at 450 nm using a microplate spectrophotometer (xMarkTM Microplate Absorbance Spectrophotometer, Bio-Rad; Hercules, CA, USA).
Statistical analyses were conducted using the IBM SPSS Statistics 26.0 (IBM, Armonk, NY, USA). Differences in biomarker levels between adenomyotic and non-adenomyotic myometria were tested using a paired t-test for normally distributed data or the Wilcoxon signed-rank test if the data were not normally distributed. P-value <0.05 indicated statistically significant difference between two groups. Pearson’s or Spearman’s correlation analysis was conducted to determine the correlation between each biomarker. Data normality was tested using the Shapiro-Wilk test. P-value <0.05 indicated that the data were not normally distributed.
Thirty-two patients agreed to participate in the study, and their characteristics are shown in Table 1. Most participants were aged between 30 years and 40 years, had delivered more than once, and were obese.
CHI3L1, MMP-9, and MCP-1 levels in adenomyotic and non-adenomyotic tissues are shown in Table 2. CHI3L1, MMP-9, and MCP-1 levels were significantly higher in adenomyotic tissues than in non-adenomyotic tissues.
Correlation analyses between CHI3L1, MMP-9, and MCP-1 levels are shown in Fig. 3. Positive, strong, and significant correlations were found between CHI3L1 and MMP-9 (r=0.463; P=0.008), CHI3L1 and MCP-1 (r=0.594; P<0.001), and MCP-1 and MMP-9 (r=0.680; P<0.001) in adenomyotic tissues.
Many participants in this study were in the 30-40 years age group. This result is in line with those of previous epidemiological studies, which found that the prevalence of adenomyosis is higher in women of older reproductive age. An epidemiological study conducted in the United States of America from 2006 to 2015 found that the highest incidence of adenomyosis occurred in women aged 41-45 years (69.1 per 10,000 women in 2008). The total prevalence until 2015 was 0.8%, and the highest prevalence was observed in women aged 41-45-year-old women (1.5%) [10]. A study in Indonesia by Fitrina et al. [23] showed the highest prevalence of adenomyosis (70.7%) in patients aged >35 years. This phenomenon is likely due to increased estrogen exposure in the older reproductive age group [5].
A higher prevalence of adenomyosis was observed in the postpartum women (56.3%). Fitrina et al. [23] also found that patients with adenomyosis were mainly multiparous (51.7%). Upson and Missmer [7] stated that parity is a risk factor for adenomyosis because trophoblast invasion of the inner myometrium during pregnancy can disrupt the endometrium-myometrium boundary.
In the current study, the percentage of patients with adenomyosis who were obese was 50% and of those who were overweight was 25%. Obesity may be a risk factor for adenomyosis because more adipose tissue produces higher aromatase levels, resulting in increased estrogen levels [24]. Nevertheless, this condition does not affect the current results, as our samples were taken directly from adenomyotic and non-adenomyotic tissues, which are not affected by systemic inflammation caused by metabolic conditions such as obesity.
Significantly higher CHI3L1, MMP-9, and MCP-1 levels were found in adenomyotic uterine tissue than in non-adenomyotic tissue, although both were obtained from the same patient. This finding is consistent with previous studies that have shown the roles of CHI3L1, MMP-9, and MCP-1 in uterine pathologies. Tuten et al. [25] found that CHI3L1 serum levels were significantly higher in patients with moderate-to-severe endometriosis than in those with minimal-to-mild endometriosis. Guo et al. [26] found a significant increase in CHI3L1 serum levels in patients with uterine leiomyoma compared to healthy controls and a positive correlation between CHI3L1 and leiomyoma masses. Vannuccini et al. [22] found that both eutopic and ectopic endometria of adenomyosis expressed significantly higher levels of MMP-2, MMP-9, and vascular endothelial growth factor (VEGF) than the normal endometrium, with a positive correlation between VEGF and metalloproteinase expression. An et al. [27] found a significant increase in MCP-1 levels in the eutopic endometrial epithelia of patients with adenomyoma compared to that in patients with normal endometrium.
As hypothesized, this study established a strong positive correlation among CHI3L1, MMP-9, and MCP-1 levels. This finding emphasizes the involvement of these chemokines in the development of adenomyosis through an inflammatory process, which has not yet been studied. Yeo et al. [15] reported that, in various types of cancer and endothelial cells, CHI3L1 increases the expression levels of MCP-1/CCL2, CXCL2, and MMP-9 through its receptor, Sdc1. The same receptor-molecule mechanism may also be involved in adenomyosis; however, this hypothesis needs to be proven.
This study presents several novel findings in comparison to those of other studies. To our knowledge, this study is the first to determine the relationship between adenomyosis and increased CHI3L1, MCP-1, and MMP-9 levels. Additionally, instead of using blood serum as a sample [25,26], we utilized tissue biopsy, which is a more accurate representation to study the pathophysiology of adenomyosis. Finally, unlike other studies that recruited healthy women as controls [25,26], we used adenomyotic and healthy non-adenomyotic myometrial tissues from the same individual. This approach can reduce sampling bias, as biomarker levels can be affected by an individual’s condition. By using biopsies from the same individual, one can be more certain of the roles of CHI3L1, MMP-9, and MCP-1 in adenomyosis pathophysiology.
This study has several limitations. First, the study design was cross-sectional and could not depict the temporal relationship between the development of adenomyosis and increase in CHI3L1, MMP-9, and MCP-1 levels. A prospective cohort study would be more suitable for describing this relationship. Finally, the reference range for CHI3L1 level in adenomyotic tissues remains unknown.
Our results suggest that increased levels of CHI3L1, MMP-9, and MCP-1 are specific to the adenomyotic tissue. These findings strengthen previous evidence that suggests these molecules could serve as potential serum biomarkers in patients with adenomyosis [25,26]. However, in clinical settings, it is more practical to use blood serum biomarkers than uterine biopsy. Thus, further research would be beneficial to determine the correlation between adenomyotic uterine biopsy markers and elevated serum biomarker levels. However, we hypothesize that these markers might not be sufficient for primary diagnosis in the absence of ultrasound findings. Nonetheless, an elevation in the serum levels of these markers can suggest that the patient will most likely experience adenomyosis. Whether these three biomarkers can serve as specific indicators of adenomyosis, and not of other conditions, such as endometriosis or leiomyoma, requires further study.
Notes
Ethical approval
This study was approved by the ethics committee of Dr. Hasan Sadikin Central Public Hospital (ethical approval number LB.02.01/X. 6.5/187/2023).
References
1. Taylor HS, Pal L, Sell E. Speroff’s clinical gynecologic endocrinology and infertility. 9th ed. Philadelphia: Wolters Kluwer;2019.
2. Guo SW. The pathogenesis of adenomyosis vis-à-vis ndometriosis. J Clin Med. 2020; 9:485.
3. Jung YM, Wi W, Koo HS, Shim SH, Oh SY, Lee SM, et al. The timing of adenomyosis diagnosis and its impact on pregnancy outcomes: a national population-based study. Obstet Gynecol Sci. 2024; 67:270–8.

4. Taran FA, Stewart EA, Brucker S. Adenomyosis: epidemiology, risk factors, clinical phenotype and surgical and interventional alternatives to hysterectomy. Geburtshilfe Frauenheilkd. 2013; 73:924–31.

5. Vercellini P, Consonni D, Dridi D, Bracco B, Frattaruolo MP, Somigliana E. Uterine adenomyosis and in vitro fertilization outcome: a systematic review and meta-analysis. Hum Reprod. 2014; 29:964–77.

6. Naftalin J, Hoo W, Pateman K, Mavrelos D, Holland T, Jurkovic D. How common is adenomyosis? A prospective study of prevalence using transvaginal ultrasound in a gynaecology clinic. Hum Reprod. 2012; 27:3432–9.
8. Lee JE, Shim S, Chung YJ, Kim MR, Jung CY, Kim S. Epidemiology and recent treatment of leiomyoma, adenomyosis and endometriosis with administrative data in Korean women: a national population-based study. Korean J Obstet Gynecol. 2009; 105:305.
9. Naphatthalung W, Cheewadhanaraks S. Prevalence of endometriosis among patients with adenomyosis and/or myoma uteri scheduled for a hysterectomy. J Med Assoc Thai. 2012; 95:1136–40.
10. Yu O, Schulze-Rath R, Grafton J, Hansen K, Scholes D, Reed SD. Adenomyosis incidence, prevalence and treatment: United States population-based study 2006-2015. Am J Obstet Gynecol. 2020; 223:94. e1-94.e10.

11. García-Solares J, Donnez J, Donnez O, Dolmans MM. Pathogenesis of uterine adenomyosis: invagination or metaplasia? Fertil Steril. 2018; 109:371–9.
12. Rossi M, Vannuccini S, Capezzuoli T, Fambrini M, Vannuzzi V, Donati C, et al. Mechanisms and pathogenesis of adenomyosis. Curr Obstet Gynecol Rep. 2022; 11:95–102.

13. Benagiano G, Brosens I. History of adenomyosis. Best Pract Res Clin Obstet Gynaecol. 2006; 20:449–63.

14. Rižner TL. The important roles of steroid sulfatase and sulfotransferases in gynecological diseases. Front Pharmacol. 2016; 7:30.

15. Yeo IJ, Lee CK, Han SB, Yun J, Hong JT. Roles of chitinase 3-like 1 in the development of cancer, neurodegenerative diseases, and inflammatory diseases. Pharmacol Ther. 2019; 203:107394.

16. Pouyafar A, Heydarabad MZ, Mahboob S, Mokhtarzadeh A, Rahbarghazi R. Angiogenic potential of YKL-40 in the dynamics of tumor niche. Biomed Pharmacother. 2018; 100:478–85.
17. Gschwandtner M, Derler R, Midwood KS. More than just attractive: how CCL2 influences myeloid cell behavior beyond chemotaxis. Front Immunol. 2019; 10:2759.

18. Tang CH, Tsai CC. CCL2 increases MMP-9 expression and cell motility in human chondrosarcoma cells via the Ras/Raf/MEK/ERK/NF-κB signaling pathway. Biochem Pharmacol. 2012; 83:335–44.

19. Peng Z, Pang H, Wu H, Peng X, Tan Q, Lin S, et al. CCL2 promotes proliferation, migration and angiogenesis through the MAPK/ERK1/2/MMP9, PI3K/AKT, Wnt/β‑catenin signaling pathways in HUVECs. Exp Ther Med. 2022; 25:77.

20. Yabluchanskiy A, Ma Y, Iyer RP, Hall ME, Lindsey ML. Matrix metalloproteinase-9: many shades of function in cardiovascular disease. Physiology (Bethesda). 2013; 28:391–403.

21. Kobayashi H. Molecular targets for nonhormonal treatment based on a multistep process of adenomyosis development. Reprod Sci. 2023; 30:743–60.
22. Vannuccini S, Tosti C, Carmona F, Huang SJ, Chapron C, Guo SW, et al. Pathogenesis of adenomyosis: an update on molecular mechanisms. Reprod Biomed Online. 2017; 35:592–601.

23. Fitrina M, Bayuaji H, Madjid TH, Armawan E. Karakteristik pasien adenomiosis dengan gambaran ultrasonografi di rumah sakit dr. hasan sadikin bandung periode 2015-2016. Indones J Obstet Gynecol Sci. 2018; 1:35–9.

24. Tan IF, Horne AW. Obesity and chronic pelvic pain. In : Mahmood TA, Arulkumaran S, Chervenak FA, editors. Obesity and gynecology. 2nd ed. Philadelphia: Elsevier;2020. p. 281–91.
25. Tuten A, Kucur M, Imamoglu M, Oncul M, Acikgoz AS, Sofiyeva N, et al. Serum YKL-40 levels are altered in endometriosis. Gynecol Endocrinol. 2014; 30:381–4.

26. Guo W, Wang J, Wei H. Serum YKL-40 level positively correlates with uterine leiomyomas. Reprod Sci. 2016; 23:1559–64.
27. An M, Li D, Yuan M, Li Q, Zhang L, Wang G. Interaction of macrophages and endometrial cells induces epithelial-mesenchymal transition-like processes in adenomyosis. Biol Reprod. 2017; 96:46–57.
Fig. 1.
Transvaginal ultrasound findings of adenomyosis. (A) Presence of myometrial cyst. (B) Presence of myometrial hypertrophy as fan-shaped shadow. (C) Increase of uterine vascularity depicted as patterns of penetrating vessels at color Doppler ultrasonography.
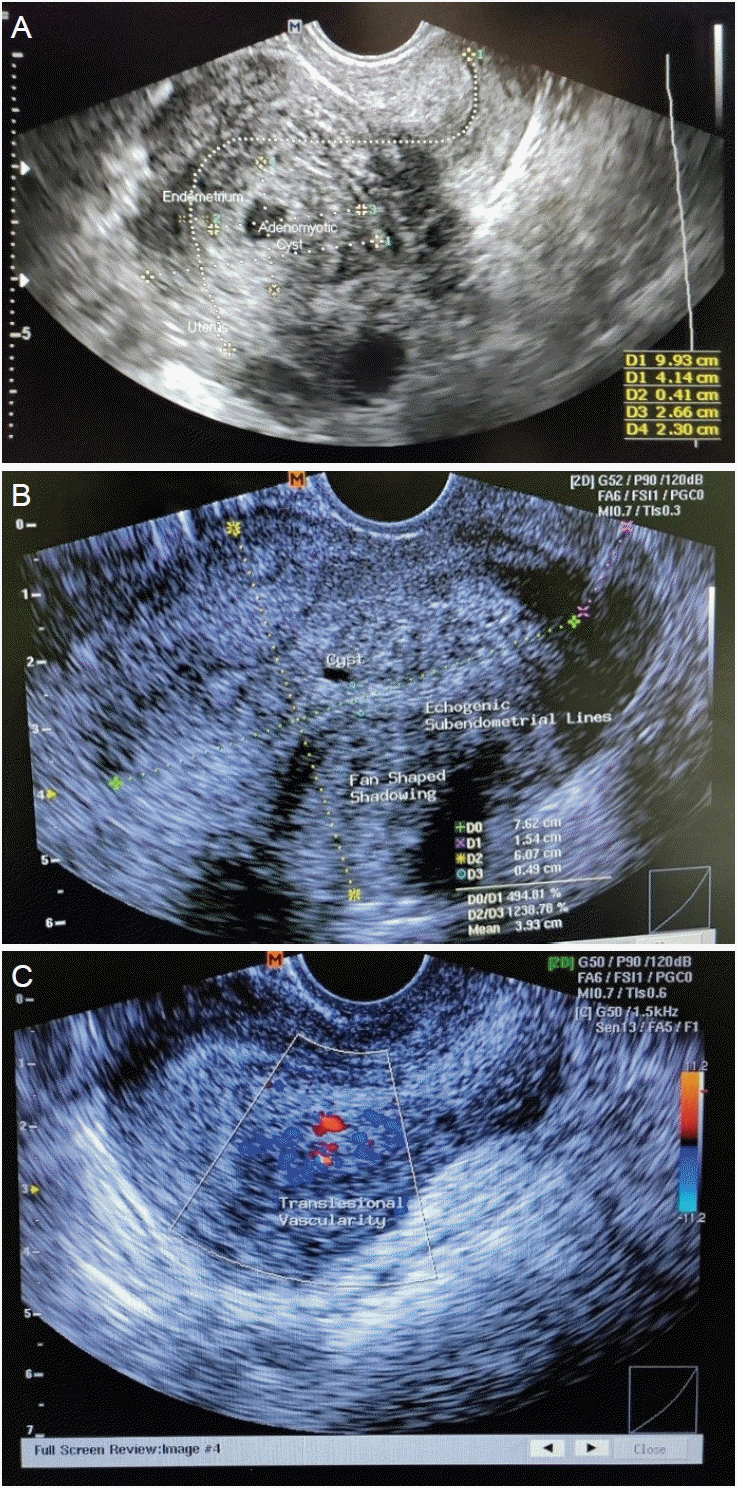
Fig. 2.
Histological finding of adenomyosis. (A) Normal myometrium; (B) stromal and glandular areas within the myometrium showing signs of adenomyosis. These histological findings used ×100 times enlargement rate.
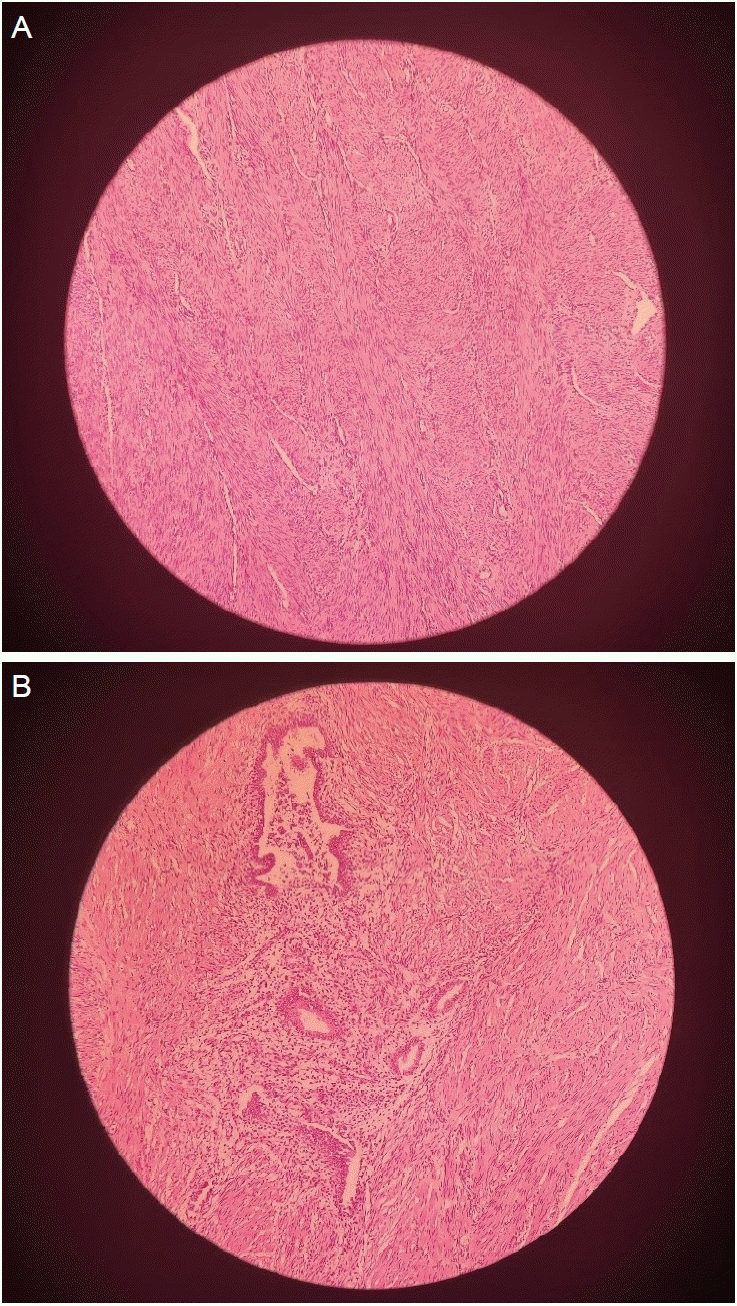
Fig. 3.
Correlation analyses between CHI3L1, MMP-9, and MCP-1 levels in adenomyotic tissues. (A) Correlation between CHI3L1 and MMP-9 levels. (B) Correlation between CHI3L1 and MCP-1 levels. (C) Correlation between MMP-9 and MCP-1 levels. CHI3L1, chitinase-3-like protein-1; MMP-9, matrix metalloproteinase-9; MCP-1, monocyte chemoattractant protein-1.
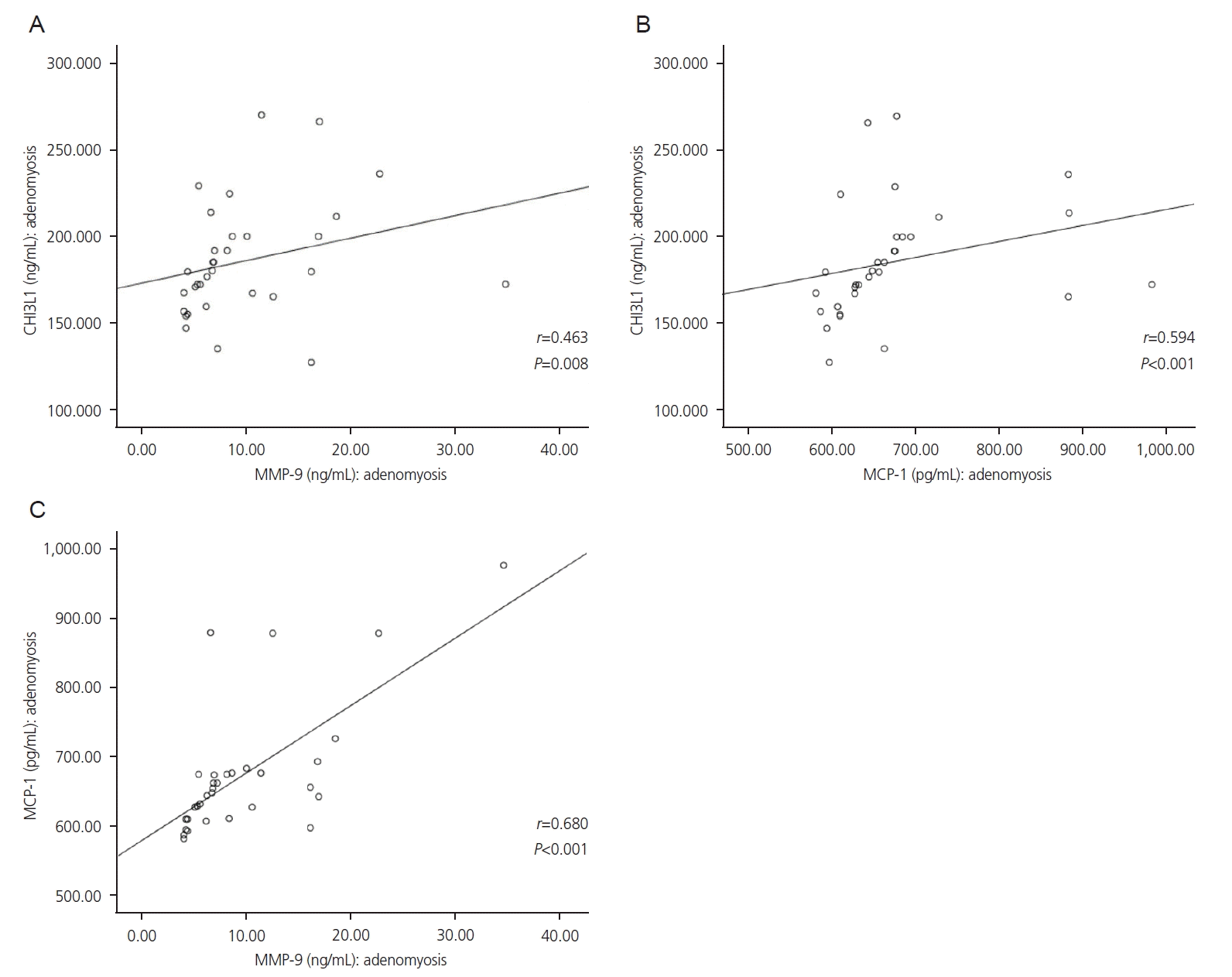
Table 1.
Characteristics of patients
Table 2.
CHI3L1, MMP-9, and MCP-1 levels from adenomyotic and non-adenomyotic tissue biopsy samples
| Biomarker |
Tissue |
P-value | |
|---|---|---|---|
| Adenomyosis (n=32) | Non-adenomyosis (n=32) | ||
| CHI3L1 level (ng/mL) | |||
| Mean±SD | 188.31±32.97 | 132.81±10.44 | <0.001a) |
| Median (range) | 182.21 (130.70-271.41) | 134.29 (109.16-149.99) | |
| MMP-9 level (ng/mL) | |||
| Mean±SD | 9.76±6.62 | 3.49±1.43 | <0.001b) |
| Median (range) | 6.96 (4.13-34.26) | 3.41 (1.12-7.00) | |
| MCP-1 level (pg/mL) | |||
| Mean±SD | 675.58±95.22 | 532.76 (54.94) | <0.001b) |
| Median (range) | 652.76 (583.74-976.94) | 545.85 (417.64-627.82) | |




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download