INTRODUCTION
Kidney stone disease is a common urological condition that affects about 15% of the world's population [
1]. In the past few decades, the prevalence of kidney stones in both sexes has been steadily rising due to the drastic changes in lifestyle and dietary patterns, and the increasing number of individuals with obesity and metabolic syndrome, in addition to advances in radiological diagnosis [
2]. Kidney stones are usually recurrent with a relapse rate of approximately 50% in 5 to 10 yrs and 75% in 20 yrs [
34] The costs associated with diagnosis and treatment place a huge burden on the healthcare system [
5]. Moreover, since the workforce population is at high risk, the condition also imposes a considerable burden on society as a whole [
6]. Therefore, identifying the modifiable risk factors for kidney stones can help in the formulation of public health policies to prevent kidney stones and reduce the related socioeconomic burden.
Increased dietary fiber intake offers multiple health benefits [
78], and improves cardiovascular and cerebrovascular health [
9]. A relatively higher intake of dietary fiber was found to lower the risk of obesity, metabolic syndrome, and diabetes [
1011], all of which are established risk factors for kidney stones. However, studies on the association between dietary fiber intake and kidney stone risk are few, and the results have been conflicting [
12131415]. Furthermore, smaller population sizes have been a common limitation in recent studies examining this association. Therefore, we drew data from the National Health and Nutrition Examination Survey (NHANES) to explore the association between dietary fiber intake and kidney stones in a representative sample of the US population [
16]. The results were adjusted for known risk factors to provide additional evidence on the independent effect of dietary fiber intake on kidney stones.
DISCUSSION
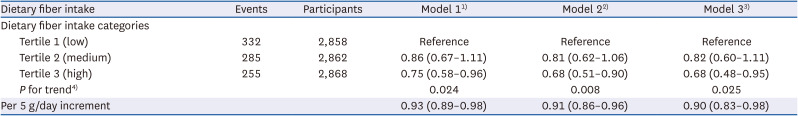
In this study, a high intake of dietary fiber compared to low intake was associated with a significantly lower risk of kidney stones. The association between dietary fiber intake and kidney stones was evident in different subgroups of sex, age, obesity, and diabetes. These findings suggest that, after adjusting for traditional risk factors, dietary fiber intake was an independent predictive factor of kidney stones. To the best of our knowledge, this is the first study using a nationally representative sample to explore the association and the dose–response relationship between dietary fiber intake and kidney stones.
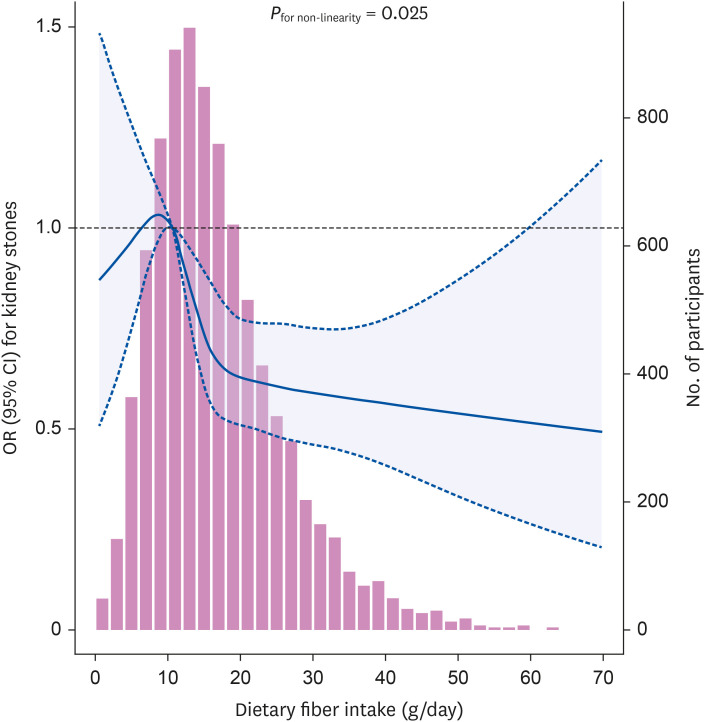
However, we found that the dose–response relationship between dietary fiber intake and kidney stones was non-linear. As the intake of dietary fiber gradually increased from approximately 10 g/day, the risk of kidney stones decreased significantly. When the intake of dietary fiber intake increased from 10 g/day to 15 g/day, the risk of kidney stones decreased dramatically. The risk continued to decline when dietary fiber intake continued to increase over 15 g/day, but the trend of the association was less prominent. These findings suggest that for adults with a dietary fiber intake of approximately 10 g/day, a slight increase in the intake may have an obvious preventive effect on kidney stones. In the study population, the CI of the association between dietary fiber intake below 10 g/day and kidney stones was wide and included the null effect, which may be due to the small sample size in this range of fiber intake.
Few studies have explored the effect of dietary fiber on kidney stones, and the results of these studies have been controversial. Some studies have demonstrated that a fiber-rich diet is associated with an increase in the excretion of oxalic acid which could promote the formation of kidney stones [
1213]. However, consistent with our results, a study conducted in the United Kingdom found that fiber intake was associated with a significantly reduced risk of kidney stones (hazard rate [HR] per 10 g/day, 0.82; 95% CI, 0.77–0.87) [
23]. A study by Sorensen
et al. [
14] involving 83,922 postmenopausal women with a self-reported history of the presence of kidney stones found that women in the highest dietary fiber intake quintile (≥ 21.9 g/day) were 22% less likely (HR, 0.78; 95% CI, 0.67–0.92) to report an incident kidney stone event compared to women with the lowest fiber intake quintile (< 10.6 g/day). A study by Turney
et al. [
15] involving 51,336 adults found that when compared with individuals with a median dietary fiber intake of 13.3 g/day, the HR (95% CI) for incident kidney stones in those with a median dietary fiber intake of 27.0 g/day was 0.72 (0.52–1.00), ssuggesting a lower risk of developing kidneys stones. Consistent with their study, we also found that adults with a fiber intake ≥ 19.1 g/day were associated with a 32% lower risk of kidney stones compared with those with a fiber intake < 12.1 g/day. Moreover, this study found that with every 5 g/day increment in fiber intake, the subject was 10% less likely to develop kidney stones. Of note, we adjusted various traditional risk factors in the multivariate regression model, including age, sex, race and ethnicity, smoking status, alcohol consumption, physical activity, BMI, hypertension, diabetes, dyslipidemia, daily water intake, and total energy intake. Despite this, the association between dietary fiber intake and kidney stones was still significant. Together, these results suggest that an adequate intake of dietary fiber is an independent protective factor against the development of stones.
The AHA recommends a dietary fiber intake of at least 25 g/day for adults to prevent cardiovascular disease [
22]. The present study first evaluated the significance of this cutoff point in preventing kidney stones. The risk of kidney stones for adults who achieved a fiber intake of 25 g/day decreased by approximately 30% compared to the reference group. Our results highlight the benefits of adherence to the recommendations of the AHA to prevent kidney stones.
Although many questions remain on the link between dietary fiber intake and the development of kidney stones, there are potential mechanisms that may explain how fiber intake contributes to kidney stone prevention. It has been suggested that calcium oxalate crystals could be mechanically trapped within the fiber network [
24]. Through this binding mechanism, the excretion of calcium oxalate crystals increases, which reduces the risk of stone formation [
12]. In addition, adequate dietary fiber intake has been proven to reduce the risk of hypercalciuria [
1225]. By combining phytic acid with dietary calcium in the intestine, dietary fiber promotes the formation of calcium phytate and increases its excretion [
26]. However, foods with a high content of dietary fiber are usually rich in oxalic acid, and an increase in oxalic acid absorption increases oxaluria, which could raise the risk of stone formation. Therefore, prospective studies exploring the underlying mechanisms of the effect of dietary fiber on stone prevention are needed.
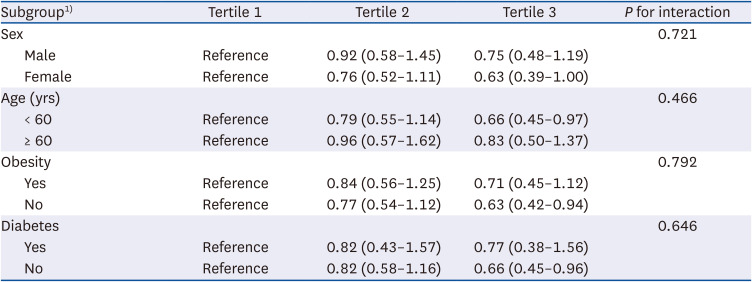
The male sex, age, obesity, and diabetes are well-known risk factors for kidney stone disease [
24]. This study found no significant interactions between the aforementioned factors and dietary fiber intake in the development of kidney stones. Among males, in participants aged 60 yrs and above, and participants with obesity and diabetes, the observed association between dietary fiber intake and kidney stones included a null effect, potentially attributable to the reduced statistical power caused by the relatively small sample sizes within these subgroups.
The strengths of this study include the analysis of the association in various subgroups, further assessment of the dose–response pattern of the association, and adjustment for confounders such as demographic characteristics, lifestyle information, and comorbidities. However, several limitations should be mentioned. First, recall bias may have been introduced because the assessment of kidney stones was based on the self-reported history in NHANES. However, this method has been proven to be reliable in previous studies, with 97% of the patients who reported having stones being confirmed to have a history of the condition in their medical records [
2728]. Second, the causal association between dietary fiber intake and kidney stones could not be evaluated due to the cross-sectional design. Further studies using a prospective cohort design will clarify this causal relationship. Third, unfortunately, the NHANES data did not have information on the composition of kidney stones. Therefore, we could not further explore the association between fiber intake and different types of stones, which may have helped provide valuable evidence and clues to the physiological mechanisms involved in the role of dietary fiber and stones of different compositions.
In conclusion, in this nationally representative population of the US, an increase in dietary fiber intake was associated with a decrease in the risk of kidney stones. These findings suggest that adults who maintain an adequate dietary fiber intake have a lower risk of developing kidney stones. Our results provide evidence of the need for a nutrition management strategy for the prevention of kidney stones. Also, increasing dietary fiber intake should be emphasized in public health policies to prevent kidney stones.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download