Abstract
Vanrija humicola, a yeast belonging to Trichosporonaceae, is rarely pathogenic. All cases of isolation of V. humicola were retrospectively reviewed from 2021 to 2023. A total of four V. humicola were isolated from urine samples. Organisms cultured for 5 days at 25°C produced yellow, dry and cerebriform colonies, and were successfully identified as V. humicola using Bruker Biotyper MALDI-TOF. Two recent isolates were resistant to fluconazole, echinocandins, and flucytosine. In all 4 cases, V. humicola was sporadically isolated more than 14 days after admission. One case was presumed to be colonized. Of the other three cases that developed a urinary tract infection (UTI), only one with pancytopenia was treated for UTI by V. humicola with caspofungin, but expired 4 days later. V. humicola has emerged as a drug-resistant fungal pathogen of hospital-acquired UTI. Species identification and antifungal susceptibility testing of this organism are required for critical patients.
Go to : 
The genus Vanrija was initially characterized by Moore with Vanrija humicola designated as the type species [1,2]. Comprising basidiomycete fungi within the Trichosporonaceae family, Vanrija is widely distributed in the environment but an oppotunistic pathogen in severely debilitated hosts [3]. Among the nine recognized Vanrija species [4], V. humicola has previously been referred to as Cryptococcus humicola, Candida humicola, Cryptococcus humicolus, Torula humicola, Apiotrichum huicola, Azymoprocandida humicola, Mycotorula humicola, or Asterotremella humicola [5]. There is fewer than 10 cases published [2,3,6-9]. Systemic infections of V. humicola occur in immunocompromized hosts, such as central nervous system infections in HIV infected patients or cancer patients in literatures [9]. Until now, the only two reports regarding on urinary isolates were reported available in English [6,10], and there is still no report on a human infection in Korea. At our hospital, a tertiary care hospital in Seoul, Korea, V. humicola has been infrequently detected since it first appeared in 2021. This study therefore aims to elucidate the clinical significance of V. humicola isolated at our hospital.
All yeast isolates of clinical significance identified to species-level using Biotyper MALDI-TOF (Bruker Daltonics). The clinical characteristics of the patients with V. humicola were retrospectively reviewed through electronic medical records. Clinical relevance of V. humicola were evaluated with pathogenicity, infection types, risk factors of acquisition and epidemiological linkage between the cases. This study was approved by the Institutional Review Board of Asan Medical Center (No. 2024-0049) and the requirement for informed consent was waived. Two recent isolates were subcultured on blood agar plate (BAP), potato dextrose agar, and Sarbouraud dextrose agar (SDA) plate and colonial and microscopic growth was observed with incubation at both 25℃ and 37℃. Phenotypic tests were performed using the VITEK2 YST (bioMérieux), API 20C AUX (bioMérieux) and urease test tubes. Sequence analysis was carried for the internal transcribed spacer and D1/D2 regions of the 26S ribosomal DNA. Antifungal susceptibility test was performed using the Sensititre YeastOne YO10 AST (Thermo Fisher Scientific) with visual reading after 48 hours of incubation at 25℃.
All four cases recovered V. humicola from urine cultures after hospitalization for 14-100 days due to serious underlying and comorbid diseases. None of them did not cluster during hospitalization (Table 1). All except one showed symptoms or signs of cystitis as well as urine cultures produced pure culture of V. humicola > 100,000 CFU/mL. A man with a traumatic esophageal rupture had 3,000 CFU/mL of V. humicola isolated from his urine, and the urine analysis was normal, suggesting that he was colonized with V. humicola. The other three were immunocompromized, of whom two were with cancer chemotherapy and one with pancytopenia and prednisolone treatment. Two indwelled foley catheter for 24 and 45 days, respectively, and developed hematuria. Although one patient received voriconazole due to Candida krusei isolated from mediastinitis sites, the only patient to receive antifungal treatment targeting V. humicola infection was an 86-year-old man with pancytopenia who received 60 mg prednisolone daily for 25 days. The patient was empirically treated with 50 mg caspofungin for three days, but died 4 days after isolation of V. humicola, before the species identification was reported. Three remaining patients were discharged with resolution of chief complaints without any treatment targeted for V. humicola (Table 1).
All four isolates were identified to V. humicola first with a score of 1.37 - 1.85 by Biotyper, and ureasepositive when tested using the colonies on BAP. Culture growth of two recent isolates was better at 25℃ than 37℃. With an incubation at 25℃, it presented yellow, dry and cerebriform colonies with a fimbriate margin on day 5. Microscopic examination revealed budding yeasts of oval or ellipsoidal cell with pseudohyphae (Fig. 1). The VITEK2 YST card failed to identify species due to no active reaction, and API 20C AUX identified V. humicola with the first priority but not differentiated from Trichosporon mucoides. The colonies on SDA were better identified with the score values of 1.94 and 2.04, respectively, while those on BAP showed the score values of 1.41 and 1.37, respectively as a score value of Biotyper. Sequence analysis of both isolates yielded excellent identification of V. humicola (Table 2). Antifungal susceptibility of these two isolates showed high minimum inhibitory concentrations (MIC) to fluconazole, caspofungin, micafungin, anidulafungin and 5-FC (Table 2).
V. humicola was first described in 1975 in patients with conjunctivitis and ophthalmopathy [7]. Only two reports on urinary isolates have been published [2,10]. In a case of systemic infection, V. humicola is isolated from the blood, bone marrow, liver biopsy, lymph node, and urine [10]. In this study, all but one funguria of V. humicola were presumed to be a nosocomial urinary tract infection (UTI). Neither of VITEK2 YST or API 20 C identified V. humicola correctly, unlike previous reports where Candida humicola or Cryptococcus humicola were identified using the API 20 C or ID 32 C systems [2,10]. The biochemical profile of API 20C AUX showed that the two highest priority species, V. humicola and T. mucoides, were indistinguishable, which is not surprising given the fact that both species are closely related urease-positive yeasts [11]. Before introduction of MALDI-TOF in routine identification of yeast, this Candida-like yeast is probably underdetected due to relatively slow growth, although colony morphology is pathognomonic when it matured to yellow, dry and cerebriform colonies. Both mass spectrometric analysis and sequence analysis of 26S rRNA showed reliable identification. It is noteworthy that species identification by mass spectrometry was more successful for colonies grown on SDA than those grown on BAP, which is consistent with the previous study on culture yield of fungal keratitis [12]. Therefore, SDA-cultured colonies are preferred for appropriate identification of V. humicola by mass spectrometry.
Though three were compatible with UTI, only one of them treated with antifungal therapy and the other two were uneventful without treatment. Therefore, clinical outcome of V. humicola UTI may not be dependent on appropriate antifungal therapy. This is plausible considering that antifungal therapy is not always warranted for Candida UTI [13]. However, an elderly patient with pancytopenia receiving caspofungin was fatal. Considering that both isolates tested for antifungal susceptibility were resistant to all echinocandins, it was likely that this case was inadequately treated. Only three reports detailing the antifungal susceptibilities of V. humicola have been published to date [6,8,14]. It is susceptible to amphotericin B and itraconazole [6], but consistently resistant to echinocandin and 5-FC [14], like as two closely related genera, Cryptococcus and Trichosporon species, are intrinsically resistant to echinocandin [3]. Of a few fatal cases in previous reports, the isolate from a case with pulmonitis and meningitis showed high MIC across all tested antifungals, including fluconazole, itraconazole, caspofungin, and amphotericin B [8], and a case of meningitis in an AIDS patient is fatal despite fluconazole therapy over a 4-week period [9,10]. Therefore, antifungal susceptibility testing is required for appropriate therapy for the V. humicola infection of critically ill patient.
In conclusion, V. humicola emerged as a pathogen of UTI in Korea. This UTI presented as a nosocomial infection in hospitalized patients with immunocompromised conditions. For funguria of V. humicola, timely species identification based on MALDI-TOF mass spectrometry and antifungal susceptibility testing should be available in a clinical microbiology laboratory.
Ethics statement
This study was approved by the Institutional Review Board of Asan Medical Center (No. 2024-0049) and the requirement for informed consent was waived.
Conflicts of interest
Eun Jeong Won has been an associate editor of the Annals of Clinical Microbiology since January 2024. However, she was not involved in the review process of this article. No other potential conflict of interest relevant to this article was reported.
Acknowledgements
We acknowledge Dae Ryong Kim, Sook-Ja Park, and Mun Hui Jeong (Department of Laboratory Medicine, Asan Medical Center) for their technical assistance.
Funding
This research was supported by the Basic Science Research Program through the National Research Foundation of Korea, funded by the Korean Ministry of Education (Grant No. NRF-2022R1C1C1002741).
REFERENCES
1. Moore RT. Taxonomic proposals for the classification of marine yeasts and other yeast-like fungi including the smuts. Botanica Marina 1980;23:361-73.
2. Alvarez Gasca MA, Arguero Licea B, Pliego Castaneda A, Garcia Tena S. Fungal agents isolated from cancer patients. Rev Latinoam Microbiol 1998;40:15-24.
3. Morales-Lopez SE and Garcia-Effron G. Infections due to rare Cryptococcus species. A literature review. J Fungi 2021;7:279.
4. Liu XZ, Wang QM, Goker M, Groenewald M, Kachalkin AV, Lumbsch HT, et al. Towards an integrated phylogenetic classification of the Tremellomycetes. Stud Mycol 2015;81:85-147.
5. Schoch CL, Ciufo S, Domrachev M, Hotton CL, Kannan S, Khovanskaya R, et al. NCBI taxonomy: a comprehensive update on curation, resources and tools. Database (Oxford). 2020;2020:baaa062.
6. Garcia-Martos P, Noval JF, Garcia-Tapia A, Marin P, Puerto JL, Sepulveda A. Susceptibility to antifungal agents of Cryptococcus species of clinical interest. Med Clin (Barc) 2002;119:2113.
7. Nitzulescu V and Niculescu M. Cryptococcus species isolated from an ocular lesion. Arch Roum Pathol Exp Microbiol 1975;34:363-5.
8. Ramli SR, Leong MC, Khaithir TM, Aziz MN, Loons LC, Rafia MH. Cryptococcus humicolus meningitis: first case report in Malaysia. Southeast Asian J Trop Med Public Health 2012;43:1212-7.
9. Rogowska-Szadkowska D, Wiercinska-Drapalo A, Borzuchowska A, Prokopowicz D. Candida humicola infection of the central nervous system in an HIV-infected patient: a case report. Przegl Epidemiol 1997;51:465-9.
10. Shinde SM, Vanarse KS, Pandit AN. Systemic humicolus cryptococcosis. Indian Pediatr 2004;41:1162-4.
11. Sugita T, Takashima M, Nakase T, Ichikawa T, Ikeda R, Shinoda T. Two new yeasts, Trichosporon debeurmannianum sp. nov. and Trichosporon dermatis sp. nov., transferred from the Cryptococcus humicola complex. Int J Syst Evol Microbiol 2001;51:1221-8.
12. Reddy AK, Brahmaiah U, Narayen N, Reddy RK, Reddy RK, Chitta M, et al. Is blood agar an alternative to sabouraud dextrose agar for the isolation of fungi in patients with mycotic keratitis. Int Ophthalmol 2013;33:251-4.
13. Fisher JF, Sobel JD, Kauffman CA, Newman CA. Candida urinary tract infections--treatment. Clin Infect Dis 2011;52 Suppl 6:S457-66.
14. Bernal-Martinez L, Gomez-Lopez A, Castelli MV, Mesa-Arango AC, Zaragoza O, RodriguezTudela JL, et al. Susceptibility profile of clinical isolates of non-Cryptococcus neoformans/ non-Cryptococcus gattii Cryptococcus species and literature review. Med Mycol 2010;48:90-6.
Go to : 
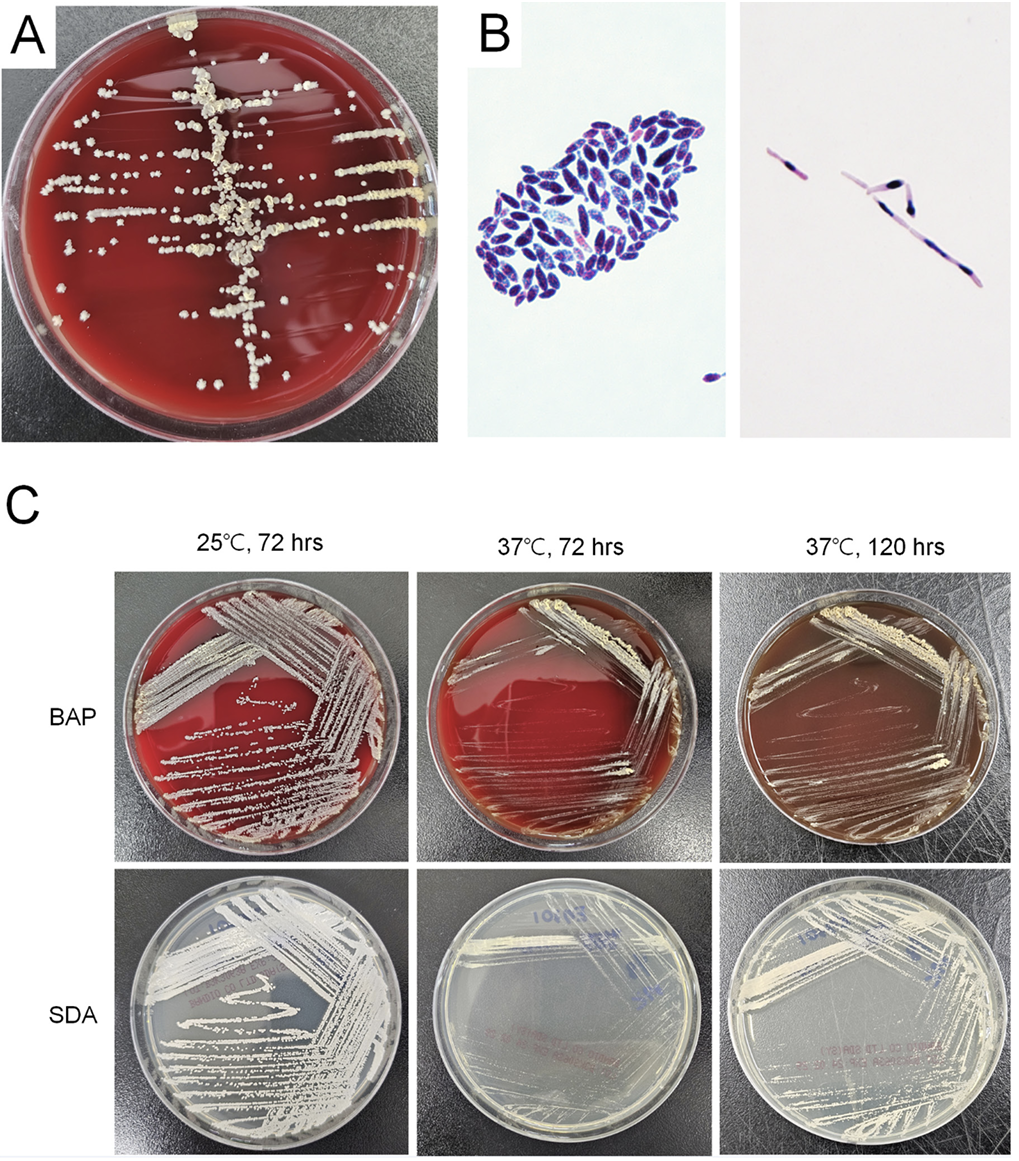 | Fig. 1.Culture findings of Vanrija humicola in this study. (A) After 5 days of growth on BAP, colonies are of yellow, dry and cerebriform with a fimbriate margin. (B) Microscopic examination showed budding yeasts of oval to ellipsoidal cells (2-8 × 3-12 µm) (Gram stain, ×1,000) (left side) and pseudohyphae (10-15 µm) (PAS stain, ×1,000) (right side). (C) Colonies on BAP and SDA grew more vigorously at 25℃, than at 37℃. BAP, blood agar plate; SDA, Sabouraud dextrose agar. |




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download