1. van Deursen VM, Urso R, Laroche C, et al. Co-morbidities in patients with heart failure: an analysis of the European Heart Failure Pilot Survey. Eur J Heart Fail. 2014; 16:103–111. PMID:
24453099.

2. Cho JY, Cho DH, Youn JC, et al. Korean Society of Heart Failure guidelines for the management of heart failure: definition and diagnosis. Korean Circ J. 2023; 53:195–216. PMID:
37161680.
3. Lee JH, Hwang KK. End-of-life care for end-stage heart failure patients. Korean Circ J. 2022; 52:659–679. PMID:
36097835.

4. Vest AR, Chan M, Deswal A, et al. Nutrition, obesity, and cachexia in patients with heart failure: a consensus statement from the Heart Failure Society of America Scientific Statements Committee. J Card Fail. 2019; 25:380–400. PMID:
30877038.

5. Damluji AA, Alfaraidhy M, AlHajri N, et al. Sarcopenia and cardiovascular diseases. Circulation. 2023; 147:1534–1553. PMID:
37186680.

6. Pandey A, Kitzman D, Reeves G. Frailty is intertwined with heart failure: mechanisms, prevalence, prognosis, assessment, and management. JACC Heart Fail. 2019; 7:1001–1011. PMID:
31779921.
7. Tanaka S, Yamashita M, Saito H, et al. Multidomain frailty in heart failure: current status and future perspectives. Curr Heart Fail Rep. 2021; 18:107–120. PMID:
33835397.
8. Afilalo J, Alexander KP, Mack MJ, et al. Frailty assessment in the cardiovascular care of older adults. J Am Coll Cardiol. 2014; 63:747–762. PMID:
24291279.

9. Fried LP, Tangen CM, Walston J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001; 56:M146–M156. PMID:
11253156.

10. McDonagh J, Martin L, Ferguson C, et al. Frailty assessment instruments in heart failure: a systematic review. Eur J Cardiovasc Nurs. 2018; 17:23–35. PMID:
28471241.

11. Oksuzyan A, Juel K, Vaupel JW, Christensen K. Men: good health and high mortality. Sex differences in health and aging. Aging Clin Exp Res. 2008; 20:91–102. PMID:
18431075.

12. Denfeld QE, Habecker BA, Camacho SA, et al. Characterizing sex differences in physical frailty phenotypes in heart failure. Circ Heart Fail. 2021; 14:e008076. PMID:
34428925.
13. Zhang Y, Zhang J, Ni W, et al. Sarcopenia in heart failure: a systematic review and meta-analysis. ESC Heart Fail. 2021; 8:1007–1017. PMID:
33576177.

14. Clark BC, Manini TM. Sarcopenia =/= dynapenia. J Gerontol A Biol Sci Med Sci. 2008; 63:829–834. PMID:
18772470.

15. Chandrashekhar Iyer L, Vaishali K, Babu AS. Prevalence of sarcopenia in heart failure: a systematic review. Indian Heart J. 2023; 75:36–42. PMID:
36567064.

16. Maeda D, Matsue Y, Kagiyama N, et al. Sex differences in the prevalence and prognostic impact of physical frailty and sarcopenia among older patients with heart failure. Nutr Metab Cardiovasc Dis. 2022; 32:365–372. PMID:
34893406.

17. Beaudart C, McCloskey E, Bruyère O, et al. Sarcopenia in daily practice: assessment and management. BMC Geriatr. 2016; 16:170. PMID:
27716195.
18. Valentova M, Anker SD, von Haehling S. Cardiac cachexia revisited: the role of wasting in heart failure. Heart Fail Clin. 2020; 16:61–69. PMID:
31735316.
19. Maekawa E, Noda T, Maeda D, et al. Prognostic impact of cachexia by multi-assessment in older adults with heart failure: FRAGILE-HF cohort study. J Cachexia Sarcopenia Muscle. 2023; 14:2143–2151. PMID:
37434419.

20. Piepoli MF, Kaczmarek A, Francis DP, et al. Reduced peripheral skeletal muscle mass and abnormal reflex physiology in chronic heart failure. Circulation. 2006; 114:126–134. PMID:
16818813.

21. von Haehling S, Morley JE, Anker SD. An overview of sarcopenia: facts and numbers on prevalence and clinical impact. J Cachexia Sarcopenia Muscle. 2010; 1:129–133. PMID:
21475695.

22. Evans WJ, Morley JE, Argilés J, et al. Cachexia: a new definition. Clin Nutr. 2008; 27:793–799. PMID:
18718696.
23. Lv S, Ru S. The prevalence of malnutrition and its effects on the all-cause mortality among patients with heart failure: a systematic review and meta-analysis. PLoS One. 2021; 16:e0259300. PMID:
34710169.

24. Donini LM, Scardella P, Piombo L, et al. Malnutrition in elderly: social and economic determinants. J Nutr Health Aging. 2013; 17:9–15. PMID:
23299371.

25. Sze S, Pellicori P, Zhang J, Weston J, Clark AL. The impact of malnutrition on short-term morbidity and mortality in ambulatory patients with heart failure. Am J Clin Nutr. 2021; 113:695–705. PMID:
33236050.

26. Mitnitski AB, Mogilner AJ, Rockwood K. Accumulation of deficits as a proxy measure of aging. Sci World J. 2001; 1:323–336.

27. Sanchez-Rodriguez D, Marco E, Cruz-Jentoft AJ. Defining sarcopenia: some caveats and challenges. Curr Opin Clin Nutr Metab Care. 2020; 23:127–132. PMID:
31789867.
28. Cruz-Jentoft AJ, Bahat G, Bauer J, et al. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing. 2019; 48:16–31. PMID:
30312372.

29. Chen LK, Woo J, Assantachai P, et al. Asian Working Group for Sarcopenia: 2019 consensus update on sarcopenia diagnosis and treatment. J Am Med Dir Assoc. 2020; 21:300–307.e2. PMID:
32033882.

30. Cruz-Jentoft AJ, Baeyens JP, Bauer JM, et al. Sarcopenia: European consensus on definition and diagnosis: report of the European Working Group on sarcopenia in older people. Age Ageing. 2010; 39:412–423. PMID:
20392703.
31. Chen LK, Liu LK, Woo J, et al. Sarcopenia in Asia: consensus report of the Asian Working Group for Sarcopenia. J Am Med Dir Assoc. 2014; 15:95–101. PMID:
24461239.

32. Bhasin S, Travison TG, Manini TM, et al. Sarcopenia definition: the position statements of the Sarcopenia Definition and Outcomes Consortium. J Am Geriatr Soc. 2020; 68:1410–1418. PMID:
32150289.
33. Cawthon PM, Manini T, Patel SM, et al. Putative cut-points in sarcopenia components and incident adverse health outcomes: an SDOC analysis. J Am Geriatr Soc. 2020; 68:1429–1437. PMID:
32633824.

34. Morishita T, Uzui H, Sato Y, Mitsuke Y, Tada H. Associations between cachexia and metalloproteinases, haemodynamics and mortality in heart failure. Eur J Clin Invest. 2021; 51:e13426. PMID:
33111322.

35. Zopf Y, Schink K, Reljic D, et al. Assessing cachexia in older patients: different definitions - but which one is the most practical for clinical routine? Arch Gerontol Geriatr. 2020; 86:103943. PMID:
31561063.

36. Emami A, Saitoh M, Valentova M, et al. Comparison of sarcopenia and cachexia in men with chronic heart failure: results from the Studies Investigating Co-morbidities Aggravating Heart Failure (SICA-HF). Eur J Heart Fail. 2018; 20:1580–1587. PMID:
30160804.

37. Janovska P, Melenovsky V, Svobodova M, et al. Dysregulation of epicardial adipose tissue in cachexia due to heart failure: the role of natriuretic peptides and cardiolipin. J Cachexia Sarcopenia Muscle. 2020; 11:1614–1627. PMID:
33084249.
38. Gaggin HK, Belcher AM, Gandhi PU, Ibrahim NE, Januzzi JL Jr. Serial echocardiographic characteristics, novel biomarkers and cachexia development in patients with stable chronic heart failure. J Cardiovasc Transl Res. 2016; 9:429–431. PMID:
27631883.

39. Fujimoto Y, Maeda D, Kagiyama N, et al. Prevalence and prognostic impact of the coexistence of cachexia and sarcopenia in older patients with heart failure. Int J Cardiol. 2023; 381:45–51. PMID:
36934990.

40. Jensen GL, Cederholm T, Correia MI, et al. GLIM criteria for the diagnosis of malnutrition: a consensus report from the Global Clinical Nutrition Community. JPEN J Parenter Enteral Nutr. 2019; 43:32–40. PMID:
30175461.
41. Hu Y, Zhang C, Zou C, Yang H, Chen Y, Liang T. Anthropometric measures and physical examination could be used to assess phenotypic GLIM (Global leadership initiative on malnutrition) criteria in heart failure patients. Nutr Metab Cardiovasc Dis. 2023; 33:2419–2427. PMID:
37788948.

42. Lee SY, Gallagher D. Assessment methods in human body composition. Curr Opin Clin Nutr Metab Care. 2008; 11:566–572. PMID:
18685451.
43. White JV, Guenter P, Jensen G, et al. Consensus statement: Academy of Nutrition and Dietetics and American Society for Parenteral and Enteral Nutrition: characteristics recommended for the identification and documentation of adult malnutrition (undernutrition). JPEN J Parenter Enteral Nutr. 2012; 36:275–283. PMID:
22535923.

44. Cederholm T, Bosaeus I, Barazzoni R, et al. Diagnostic criteria for malnutrition - an ESPEN consensus statement. Clin Nutr. 2015; 34:335–340. PMID:
25799486.

45. Driggin E, Cohen LP, Gallagher D, et al. Nutrition assessment and dietary interventions in heart failure: JACC review topic of the week. J Am Coll Cardiol. 2022; 79:1623–1635. PMID:
35450580.
46. Beltrami M, Fumagalli C, Milli M. Frailty, sarcopenia and cachexia in heart failure patients: different clinical entities of the same painting. World J Cardiol. 2021; 13:1–10. PMID:
33552398.

47. Valdiviesso R, Amaral TF, Moreira E, et al. Associations of medicine use and ejection fraction with the coexistence of frailty and sarcopenia in a sample of heart failure outpatients: a cross-sectional study. BMC Cardiovasc Disord. 2023; 23:594. PMID:
38053018.
48. Sze S, Zhang J, Pellicori P, Morgan D, Hoye A, Clark AL. Prognostic value of simple frailty and malnutrition screening tools in patients with acute heart failure due to left ventricular systolic dysfunction. Clin Res Cardiol. 2017; 106:533–541. PMID:
28204965.

49. Matsue Y, Kamiya K, Saito H, et al. Prevalence and prognostic impact of the coexistence of multiple frailty domains in elderly patients with heart failure: the FRAGILE-HF cohort study. Eur J Heart Fail. 2020; 22:2112–2119. PMID:
32500539.

50. Fujimoto Y, Maeda D, Kagiyama N, et al. Prognostic implications of six-minute walking distance in patients with heart failure with preserved ejection fraction. Int J Cardiol. 2023; 379:76–81. PMID:
36914073.

51. Maeda D, Matsue Y, Kagiyama N, et al. Inaccurate recognition of own comorbidities is associated with poor prognosis in elderly patients with heart failure. ESC Heart Fail. 2022; 9:1351–1359. PMID:
35088546.

52. Fujimoto Y, Matsue Y, Maeda D, et al. Association and prognostic value of multi-domain frailty defined by cumulative deficit and phenotype models in patients with heart failure. Can J Cardiol. 2024; 40:677–684. PMID:
38007218.
53. Konishi M, Kagiyama N, Kamiya K, et al. Impact of sarcopenia on prognosis in patients with heart failure with reduced and preserved ejection fraction. Eur J Prev Cardiol. 2021; 28:1022–1029. PMID:
33624112.

54. Hirose S, Matsue Y, Kamiya K, et al. Prevalence and prognostic implications of malnutrition as defined by GLIM criteria in elderly patients with heart failure. Clin Nutr. 2021; 40:4334–4340. PMID:
33551220.

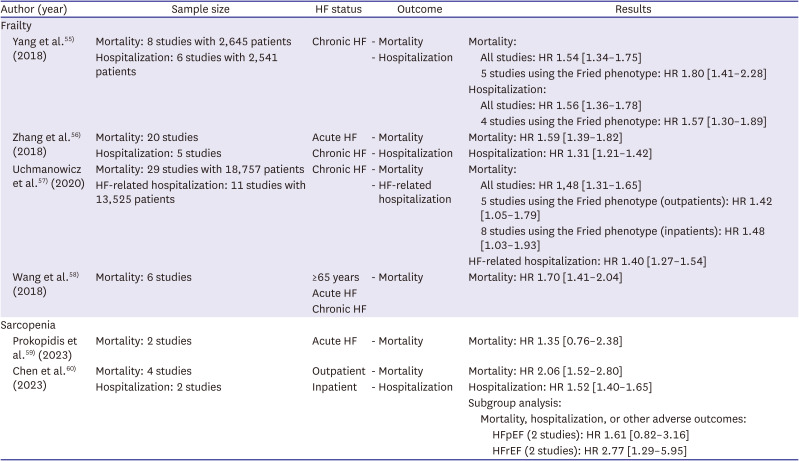
55. Yang X, Lupón J, Vidán MT, et al. Impact of frailty on mortality and hospitalization in chronic heart failure: a systematic review and meta-analysis. J Am Heart Assoc. 2018; 7:e008251. PMID:
30571603.

56. Zhang Y, Yuan M, Gong M, Tse G, Li G, Liu T. Frailty and clinical outcomes in heart failure: a systematic review and meta-analysis. J Am Med Dir Assoc. 2018; 19:1003–1008.e1. PMID:
30076123.

57. Uchmanowicz I, Lee CS, Vitale C, et al. Frailty and the risk of all-cause mortality and hospitalization in chronic heart failure: a meta-analysis. ESC Heart Fail. 2020; 7:3427–3437. PMID:
32955168.
58. Wang X, Zhou C, Li Y, Li H, Cao Q, Li F. Prognostic value of frailty for older patients with heart failure: a systematic review and meta-analysis of prospective studies. BioMed Res Int. 2018; 2018:8739058. PMID:
30426017.

59. Prokopidis K, Triantafyllidis KK, Kechagias KS, Mitropoulos A, Sankaranarayanan R, Isanejad M. Are sarcopenia and its individual components linked to all-cause mortality in heart failure? A systematic review and meta-analysis. Clin Res Cardiol. 2023.

60. Chen R, Xu J, Wang Y, et al. Prevalence of sarcopenia and its association with clinical outcomes in heart failure: an updated meta-analysis and systematic review. Clin Cardiol. 2023; 46:260–268. PMID:
36644878.

61. Oguri M, Ishii H, Yasuda K, Sumi T, Takahashi H, Murohara T. Combined prognostic value of malnutrition using GLIM criteria and renal insufficiency in elderly heart failure. ESC Heart Fail. 2022; 9:704–711. PMID:
34783197.

62. Lin H, Zhang H, Lin Z, Li X, Kong X, Sun G. Review of nutritional screening and assessment tools and clinical outcomes in heart failure. Heart Fail Rev. 2016; 21:549–565. PMID:
26920682.
63. Li H, Cen K, Sun W, Feng B. Prognostic value of geriatric nutritional risk index in elderly patients with heart failure: a meta-analysis. Aging Clin Exp Res. 2021; 33:1477–1486. PMID:
32766928.

64. Dong CH, Chen SY, Zeng HL, Yang B, Pan J. Geriatric nutritional risk index predicts all-cause mortality in patients with heart failure: a systematic review and meta-analysis. Clinics (Sao Paulo). 2021; 76:e2258. PMID:
33787674.

65. Hu Y, Yang H, Zhou Y, et al. Prediction of all-cause mortality with malnutrition assessed by nutritional screening and assessment tools in patients with heart failure: a systematic review. Nutr Metab Cardiovasc Dis. 2022; 32:1361–1374. PMID:
35346547.

66. Osório AF, Ribeiro ÉC, Parahiba SM, Forte GC, Clausell NO, Souza GC. Prognostic value of nutritional screening tools in hospitalized patients with decompensated heart failure: a systematic review and meta-analysis. Nutr Res. 2023; 120:1–19. PMID:
37871448.

67. Li H, Zhou P, Zhao Y, Ni H, Luo X, Li J. Prediction of all-cause mortality with malnutrition assessed by controlling nutritional status score in patients with heart failure: a systematic review and meta-analysis. Public Health Nutr. 2021; 25:1–8.
68. Chen MY, Wen JX, Lu MT, et al. Association between prognostic nutritional index and prognosis in patients with heart failure: a meta-analysis. Front Cardiovasc Med. 2022; 9:918566. PMID:
35757355.

69. Zhang X, Su Y. Low prognostic nutritional index predicts adverse outcomes in patients with heart failure: a systematic review and meta-analysis. Angiology. 2024; 75:305–313. PMID:
36826172.

70. Loncar G, Fülster S, von Haehling S, Popovic V. Metabolism and the heart: an overview of muscle, fat, and bone metabolism in heart failure. Int J Cardiol. 2013; 162:77–85. PMID:
21982619.

71. Hadad C, Damez C, Bouquillon S, et al. Neutral pentosides surfactants issued from the butadiene telomerization with pentoses: preparation and amphiphilic properties. Carbohydr Res. 2006; 341:1938–1944. PMID:
16697984.

72. Anker SD, Laviano A, Filippatos G, et al. ESPEN. ESPEN Guidelines on Parenteral Nutrition: on cardiology and pneumology. Clin Nutr. 2009; 28:455–460. PMID:
19515464.
73. Hersberger L, Dietz A, Bürgler H, et al. Individualized nutritional support for hospitalized patients with chronic heart failure. J Am Coll Cardiol. 2021; 77:2307–2319. PMID:
33958128.

74. Houston DK, Nicklas BJ, Ding J, et al. Dietary protein intake is associated with lean mass change in older, community-dwelling adults: the health, aging, and body composition (Health ABC) study. Am J Clin Nutr. 2008; 87:150–155. PMID:
18175749.

75. Abizanda P, López MD, García VP, et al. Effects of an oral nutritional supplementation plus physical exercise intervention on the physical function, nutritional status, and quality of life in frail institutionalized older adults: the ACTIVNES study. J Am Med Dir Assoc. 2015; 16:439.e9–439.e16.

76. Ueno K, Kaneko H, Itoh H, et al. Effectiveness and approach of rehabilitation in patients with acute heart failure: a review. Korean Circ J. 2022; 52:576–592. PMID:
35929052.

77. Kim SE, Yoo BS. Treatment strategies of improving quality of care in patients with heart failure. Korean Circ J. 2023; 53:294–312. PMID:
37161744.
78. Nagatomi Y, Ide T, Higuchi T, et al. Home-based cardiac rehabilitation using information and communication technology for heart failure patients with frailty. ESC Heart Fail. 2022; 9:2407–2418. PMID:
35534907.

79. Greenberg B. Medical management of patients with heart failure and reduced ejection fraction. Korean Circ J. 2022; 52:173–197. PMID:
35257531.

80. Hyun J, Cho JY, Youn JC, et al. Korean Society of Heart Failure guidelines for the management of heart failure: advanced and acute heart failure. Korean Circ J. 2023; 53:452–471. PMID:
37525390.

81. McDonagh TA, Metra M, Adamo M, et al. 2021 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J. 2021; 42:3599–3726. PMID:
34447992.

82. Yancy CW, Jessup M, Bozkurt B, et al. 2017 ACC/AHA/HFSA focused update of the 2013 ACCF/aha guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association Task Force on clinical practice guidelines and the Heart Failure Society of America. Circulation. 2017; 136:e137–e161. PMID:
28455343.
83. Tsutsui H, Isobe M, Ito H, et al. JCS 2017/JHFS 2017 guideline on diagnosis and treatment of acute and chronic heart failure - digest version. Circ J. 2019; 83:2084–2184. PMID:
31511439.

84. Vaduganathan M, Docherty KF, Claggett BL, et al. SGLT-2 inhibitors in patients with heart failure: a comprehensive meta-analysis of five randomised controlled trials. Lancet. 2022; 400:757–767. PMID:
36041474.

85. Hamada T, Kubo T, Kawai K, et al. Kochi YOSACOI study. Frailty interferes with the guideline-directed medical therapy in heart failure patients with reduced ejection fraction. ESC Heart Fail. 2023; 10:223–233. PMID:
36193578.

86. Sze S, Pellicori P, Zhang J, Weston J, Squire IB, Clark AL. Effect of frailty on treatment, hospitalisation and death in patients with chronic heart failure. Clin Res Cardiol. 2021; 110:1249–1258. PMID:
33399955.

87. Kondo T, Adachi T, Kobayashi K, et al. Physical frailty and use of guideline-recommended drugs in patients with heart failure and reduced ejection fraction. J Am Heart Assoc. 2023; 12:e026844. PMID:
37301739.
88. Abe T, Jujo K, Maeda D, et al. The interaction between physical frailty and prognostic impact of heart failure medication in elderly patients. ESC Heart Fail. 2023; 10:1698–1705. PMID:
36824014.

89. Onder G, Penninx BW, Balkrishnan R, et al. Relation between use of angiotensin-converting enzyme inhibitors and muscle strength and physical function in older women: an observational study. Lancet. 2002; 359:926–930. PMID:
11918911.

90. Goldwater DS, Pinney SP. Frailty in advanced heart failure: a consequence of aging or a separate entity? Clin Med Insights Cardiol. 2015; 9(Suppl 2):39–46.

91. Kim J, Grotegut CA, Wisler JW, et al. The β-arrestin-biased β-adrenergic receptor blocker carvedilol enhances skeletal muscle contractility. Proc Natl Acad Sci U S A. 2020; 117:12435–12443. PMID:
32414934.

92. Greene SJ, Butler J, Albert NM, et al. Medical therapy for heart failure with reduced ejection fraction: the CHAMP-HF registry. J Am Coll Cardiol. 2018; 72:351–366. PMID:
30025570.
93. Brunner-La Rocca HP, Linssen GC, Smeele FJ, et al. Contemporary drug treatment of chronic heart failure with reduced ejection fraction: the CHECK-HF registry. JACC Heart Fail. 2019; 7:13–21. PMID:
30606482.

94. Pitt B. The role of mineralocorticoid receptor antagonists (MRAs) in very old patients with heart failure. Heart Fail Rev. 2012; 17:573–579. PMID:
21996778.

95. Abe T, Jujo K, Kametani M, et al. Prognostic impact of additional mineralocorticoid receptor antagonists in octogenarian heart failure patients. ESC Heart Fail. 2020; 7:2711–2724. PMID:
32860346.

96. Sanders NA, Supiano MA, Lewis EF, et al. The frailty syndrome and outcomes in the TOPCAT trial. Eur J Heart Fail. 2018; 20:1570–1577. PMID:
30225878.

97. Dewan P, Jackson A, Jhund PS, et al. The prevalence and importance of frailty in heart failure with reduced ejection fraction - an analysis of PARADIGM-HF and ATMOSPHERE. Eur J Heart Fail. 2020; 22:2123–2133. PMID:
32353205.
98. Yabe D, Shiki K, Homma G, et al. Efficacy and safety of the sodium-glucose co-transporter-2 inhibitor empagliflozin in elderly Japanese adults (≥65 years) with type 2 diabetes: a randomized, double-blind, placebo-controlled, 52-week clinical trial (EMPA-ELDERLY). Diabetes Obes Metab. 2023; 25:3538–3548. PMID:
37622398.

99. Butt JH, Dewan P, Merkely B, et al. Efficacy and safety of dapagliflozin according to frailty in heart failure with reduced ejection fraction: a post hoc analysis of the DAPA-HF trial. Ann Intern Med. 2022; 175:820–830. PMID:
35467935.

100. Coats AJ, Butler J, Tsutsui H, et al. Efficacy of empagliflozin in heart failure with preserved ejection fraction according to frailty status in EMPEROR-preserved. J Cachexia Sarcopenia Muscle. 2024; 15:412–424. PMID:
38158636.





 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download