INTRODUCTION
Bacteriophages are viruses that selectively kill bacteria by infecting them and reproduce inside them without any negative effect on human cells. They are very commonly found in soil, sea water, oceanic and terrestrial surfaces. They are classified according to their morphological characteristics, content of nucleic acid, the site where they can mostly be found, and the bacterial species that they can kill (1). Phages are found in a variety of morphologies such as filamentous phages, phages with a lipid-containing envelope and phages with lipids in the particle shell. They have either DNA or RNA as genome, which can be single or double stranded, and contain information on the proteins that constitute the particles (2). Bacteriophages have shown usefulness for the treatment of respiratory infections, gastrointestinal infections, skin infections, eye infections, fracture-related infections, urinary tract infections etc. (3, 4, 5).
Bacteriophages are very specific to particular species regarding their hosts. They usually infect a single bacterial species or a specific strain within a species. After attachment of a bacteriophage to a susceptible host, bacterial lysis occurs through a series of steps.
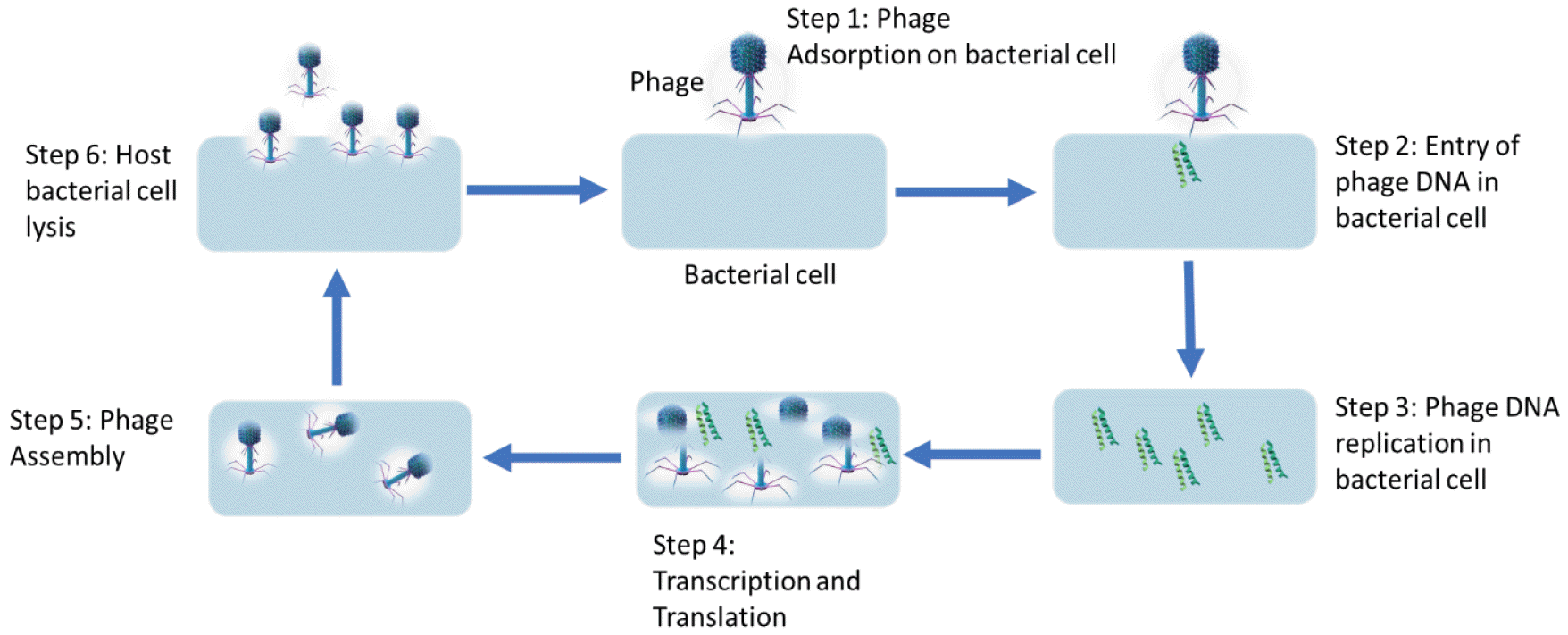
Bacteriophage’s action involves six stages: Stage 1 involves adsorption onto the bacterial cell’s surface, where they occupy specific viral sites on the host. In stage two, phage releases its DNA into the bacterial cells. In stage three, the DNA is replicated in bacterial cells. Gene transcription and translation occurs in stage four.
The new phage particles are formed in stage five, also known as phage assembly. In the final stage, the host lyses and phage bursts out, causing the destruction of the host bacterial cells and the release of new phages, which can infect neighboring cells (Fig. 1) (6). One of the most important features of bacteriophages in infecting a host cell is the viral receptor-binding protein (RBP). These proteins, also known as spikes or fibers, are different in each phage with different morphology. These proteins are called tail fiber or tail spike proteins in tailed phages. RBPs bind to the polysaccharides part or protein portion on the bacterial surface (7, 8).
Although there are no FDA approved phages for human use in the market, several phages indicated for different conditions have been a part of clinical trials. Several candidates are presently in phase 3 clinical trials such as PreforPro phage for vaginal infection (manufactured by Deerland enzymes) and Pyobacteriophage for acute tonsillitis (Tashkent Padiatric Medical Institute). Many others are in phase 2 and phase 1, such as Phage bank for Diabetic foot osteomyelitis (by Adaptive Phage Therapeutics, Inc.), Bactelide for pressure ulcer infection (by Phagelux Inc.) etc. (9). Its market size was valued at 39.81 million USD and is expected to grow 17% by 2030, to approximately $84 million annually (10, 11). The attraction gained by bacteriophages is due to the increasing antimicrobial resistance.
ADVANTAGES OF BACTERIOPHAGES OVER ANTIBIOTICS
In comparison to antibiotics, bacteriophages have several advantages. Bacteriophages are significantly safer and better tolerated, as they selectively replicate in the target bacterium but do not infect mammalian cells (12). They are made up of protein and nucleic acid which make them inherently non-toxic. Several studies done in the past in Eastern Europe support this conclusion. No reports of significant adverse events following phage administration have been published. They have minimal effect on natural flora in contrast to most broad-spectrum antibiotics which can significantly affect natural flora (13).
There is very little chance of resistance with these agents as they are bactericidal. Bacteria infected by lytic phages are unable to regain their original viability. In contrast, certain antibiotics which are bacteriostatic, such as tetracycline, readily permit evolution in bacteria and eventually result in resistance. However, some reports have shown that bacteria may develop resistance to the attack of phages, hence, it is important to use phages therapy cautiously (14). The administration of bacteriophages is relatively easier. No repeated administrations are required in case of bacteriophages as they remain in the human body for several days. Moreover, they can increase in number by themselves. The term ‘auto dosing’ is used as phage itself contributes to its dose (13). The bacteriophages can be discovered rapidly from waste materials/sewages. However, isolation is technically more challenging. The process is still relatively easier as compared to discovery and development of a new antibiotic molecule (15).
APPLICATION OF BACTERIOPHAGES IN BACTERIAL INFECTIONS
Bacteriophages have been used to treat skin infections, gastrointestinal infections, respiratory infections, urinary tract infections and systemic infections. Phages have shown their effect against antibiotic-resistant infections caused by ESKAPE pathogens (Enterococcus faecium, Staphylococcus aureus, Klebsiella pneumoniae, Acinetobacter baumannii, Pseudomonas aeruginosa, and Enterobacter spp.) (16).
Skin infections: The Skin is the largest organ of the human body. It provides a physical barrier to several pathogens. Any cuts or burns may expose the body to the pathogenic microorganisms present in the environment (17). Phage therapy was used historically for injured soldiers with wound infections. It is reported that phage therapy saved more lives as compared to many other treatments. However, even after excellent results, the phage treatment was discontinued due to the discovery of penicillin (12). More recently, a phage named PaVOA (belongs to the Myoviridae family) was isolated from hospital sewage to study its effect as antibacterial agent. In this study, rabbit skin infected with P. aeruginosa was treated with a phage PaVOA. The effect was enhanced by the addition of calcium and magnesium ions. The phage showed superior effect as compared to ceftriaxone. The phage showed stability between -4°C and 20°C of temperature and was also resistant to acid-base and UV light. These phages were inactivated in the blood and hence, did not cause any animal deaths (18). Another study showed the activity of bacteriophages against P. aeruginosa. In this study, wound dressings were prepared by electrospinning biocompatible fibers loaded with bacteriophages. The stability was up to 4 weeks when stored at -20°C. These fibers were also found to be active against S. aureus (19). A clinical study involving 87 male patients with deep purulent skin infections caused by Staphylococcus showed positive results in all patients treated with phage therapy (20).
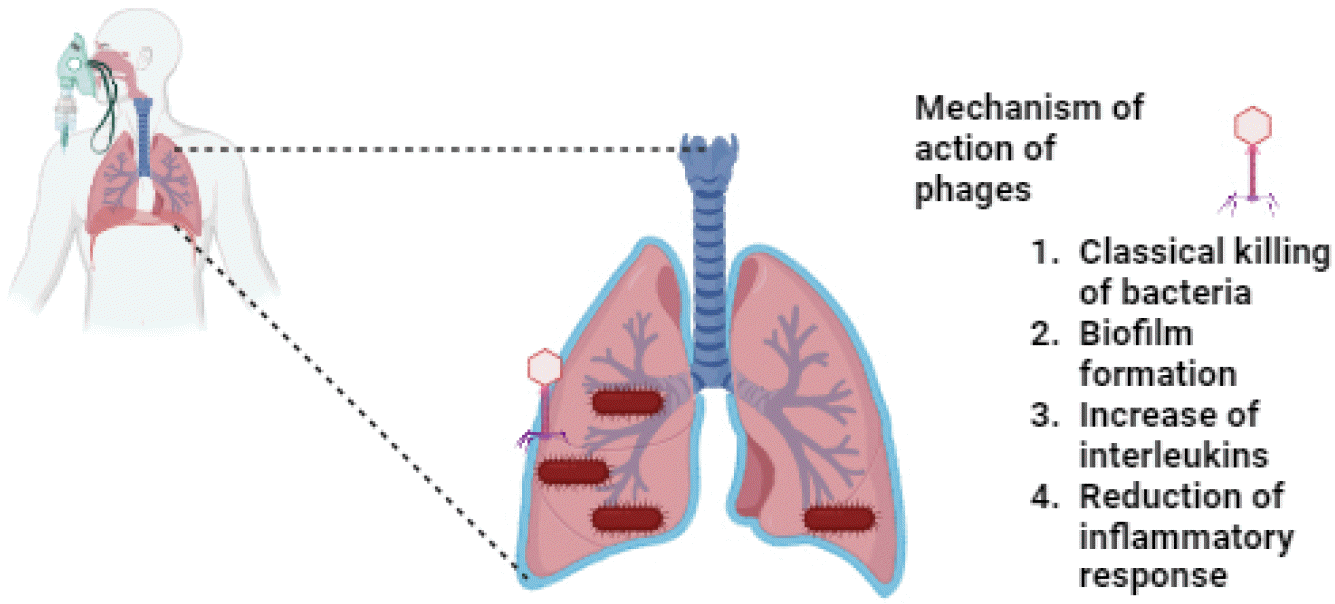
Respiratory infections: Inhalation phage therapy was first reported in the 1960s in European nations. Gradually, it was used by other nations against antibiotic resistant bacteria. In one study, PELP20 named phage was tested against lung infections caused by P. aeruginosa in a murine model. After 24 hours of phage treatment, 3-log reduction in P. aeruginosa CFU were observed. It indicates that PELP20 can penetrate and kill bacteria within a biofilm-associated cystic fibrosis (CF) lung-like environment. The results showed that phage therapy has potential for treatment of established and recalcitrant chronic respiratory tract infections (21). Phage treatment has shown promising results in the treatment of chronic MDR P. aeruginosa life-threatening lung infection. There was significant improvement in the condition resulting in hospital discharge and discontinuation of systemic antibiotic treatments. The phage therapy helped in the reduction of chronic inflammation and prevented infectious exacerbations (Fig. 2) (22).
Pabary et al. studied the effect of phage therapy against acute P. aeruginosa lung infection in BALB/c mice. Both bacterial challenge and phage were administered intranasally. The infection was completely removed in all mice (23). Semler et al. used phages KS12 and KS4-M against Burkholderia cepacia strain. The results showed that bacterial burden was significantly reduced after 2 days of treatment (24).
Gastrointestinal infections: Phages are present in the large intestine and their levels range between 108 and 8×1010 phage virions per gram of feces, measured by phage particles count (25). Most of the phages present in the human intestine remain stable in the gastrointestinal tract. This has led to the establishment of a global intestinal phagosome which is useful to show a correlation between phages and healthy status of humans (26). Bacteriophages have been found to increase the immune response by stimulating the formation of interferons and interleukins. They are sensed by the receptors in the intestine for the production of these biomolecules (27).
Phage therapy has been found useful for treatment against Clostridioides difficile in ulcerative colitis and invasive adherent Escherichia coli in Crohn’s disease (26). Three phages, ICP1, ICP2, and ICP3, with specific effects against V. cholerae were found to be effective against V. cholerae-induced diarrhea after 24 hours administration (28). FDA has also approved the clinical trial phase 1/2a for the treatment of Crohn’s disease using a set of bacteriophages. Escherichia coli has been implicated in the pathogenesis of Crohn’s disease, and bacteriophages specific to this bacterium have been used for treatment (29).
Miscellaneous infections: Enterococcus faecium is involved in root canal infections, persistent endodontic infections, urinary infections, and bacteremia. Bacteriophage vB_EfaH_163 was isolated from human fecal matter and was tested against vancomycin resistant E. faecium using G. mellonella model. The results shows that vB_EfaH_163 could infect several E. faecium strains of different origin (30). Another E. faecalis based phage named bacteriophage Vb_EfaM_LG1 was studied in combination with cefotaxime. The phage-antibiotic combination showed tremendous synergistic effect against E. faecalis. It also disrupts the biofilm efficiently and prevents the resistance (31). In a clinical case report, A MDR A. baumannii infection was treated with bacteriophages. A 77-year-old man who suffered from an assault, subdural hematoma, and traumatic brain injury, developed a postoperative infection with cerebritis, subdural and epidural empyema, requiring debridement. The intraoperative cultures grew MDR A. baumannii which were found to be resistant to all antibiotics. After treatment, the respiratory, blood, and urine cultures were negative (32). Klebsiella phages were isolated from human feces and a cocktail was used against K. pneumonia (Kp). Different phages including KP13-2, KP13-3, KP13-7, KP13-8, KP13-14, KP13-15, KP13-16, KP13-20, KP13-26, and KP13-27 were used to make a cocktail of 2-5 phages for further use. The results showed that the cocktail of phages reduced the Kp levels in fecal samples and attenuated hepatobiliary injuries and liver fibrosis (33).
Some of the bacteriophages used for different types of infections are given in Table 1.
Table 1.
Summary of phages and their application in different infectious diseases
| S. No | Phage | Infection Type | Route of administration | Application Details | Target Bacteria | Ref. |
|---|---|---|---|---|---|---|
| 1. | Pakpunavirus Phage vFB297 | Respiratory infection | Nebulizer | Effective in Human lung infection | Pseudomonas aeruginosa | (22) |
| 2. | Myoviridae Phage PaVOA | Skin Infection | Topical | Isolated from hospital sewage; tested on rabbit skin; superior effect compared to ceftriaxone; stable under various conditions. | Pseudomonas aeruginosa | (18) |
| 3. | PELP20 | Respiratory infection | Natural respiratory inhalation route | Tested on murine lung infections; showed a 3-log reduction in bacterial CFU within 24 hours; effective in CF lung-like environment. | Pseudomonas aeruginosa | (34) |
| 4. | Cocktail of T4 phage and KEP10 phage | Various Infections | Urethral route in rats | Effective in Urinary Tract Infection | E. coli | (35, 36) |
| 5. | Phage K1F-GFP | Various Infections | In-vitro study: Human epithelial cells | Effective in Urinary Tract Infection | E. coli | (37) |
| 6. | Phages ICP1, ICP2, and ICP3 | Gastrointestinal infections | Oral gavage in mice | Effective against V. cholerae-induced diarrhea within 24 hours of administration. | Salmonella enterica | (28, 38) |
| 7. | Phage named φK1-5 | Various Infections | In-vitro assay: Plaque assay technique | Effective in Urinary Tract Infection | K1 or K5 strains of E. coli. | (39) |
| 8 | Bacteriophage vB_EfaH_163 | Various infections | Using G. mellonella Model | Isolated from human feces; effective against vancomycin-resistant E. faecium in the G. mellonella model | Enterococcus faecium | (30) |
| 9 | Vb_EfaM_LG1 | Various infections | Spot agar assay | Combined with cefotaxime; showed synergistic effect and disrupted biofilm formation. | Enterococcus faecium | (31) |
| 10 | Klebsiella phages (e.g., KP13-2, KP13-3, etc.) | Various infections | Oral route in mice | Cocktail reduced Kp levels in fecal samples; attenuated hepatobiliary injuries and liver fibrosis. | K. pneumonia | (33) |
| 11 | KS12, KS4-M | Respiratory infections | Aerosol route | Reduced bacterial burden significantly after 2 days of treatment. | Burkholderia cepacian | (24) |
| 12 | PreforPro | Vaginal infections | Oral route | In phase 3 clinical trials for vaginal infections; manufactured by Deerland Enzymes. | Various | (9) |
| 13 | Pyobacteriophage | Tonsillitis | Inhalation route | In phase 3 clinical trials for acute tonsillitis; tested by Tashkent Pediatric Medical Institute. | Various | (9) |
| 14 | PhageBank | Diabetic foot osteomyelitis | Topical + intravenous | In phase 2 clinical trials; developed by Adaptive Phage Therapeutics, Inc. | Various | (9) |
| 15 | Bactelide | Pressure ulcer infection | Transdermal | In phase 1 clinical trials; developed by Phagelux Inc | Various |
FORMULATIONS OF BACTERIOPHAGES
In the 1940s, Eli Lilly produced seven phage lysates and jelly-based preparations including Neiso-lysate, Staphylo-lysate, Ento-lysate, Colo-jel and Ento-jel) targeted against streptococci, staphylococci, Escherichia coli, and other bacterial strains. However, due to the safety concerns of these phage preparations several controversies arise (35). After several decades, when the phages were restudied, the focus on their formulation aspect was also redeemed.
Formulation development is important as the functions of bacteriophages can be affected due to denaturation and protein misfolding. Moreover, studies have shown that phages are sensitive to organic solvents, pH, temperature, and salinity. The stability is better if they are stored at 4°C. Use of certain substances can improve the stability of phages such as gelatine, magnesium ions and glycerol. Most phage formulations rely on some of the forms of encapsulation including emulsification, freeze-drying, spray-drying and liposome encapsulation. These techniques help to increase the shelf life of the phages from a few days to several months (40).
Some of the types of formulations meant to be used for respiratory tract infection include intravenous, nebulization and dry powder inhalation (41). Lyophilization increases the shelf life of bacteriophages significantly. In one of the studies three lytic bacteriophages Escherichia phage ECP311, Klebsiella phage KPP235 and Enterobacter phage ELP140 were lyophilized, and the stability was increased to up to 10 months. A metered dose liquids spray formulation for topical delivery was developed with two types of anti-Pseudomonas phages, PEV1 (myovirus) and PEV31 (podovirus) against Pseudomonas aeruginosa. The formulation contains 35% ethanol in water containing non-ionic polymers. Both PEV1 and PEV31 remained biologically stable in the optimized formulations during storage at 4°C for eight weeks (42).
In general, for gastrointestinal infections, oral formulations are favored. Phages in the water-based liquid suspensions have shown to survive gastric environments and were recovered in the feces. Moreover, the formulation of liquid phage suspensions is easy as they are prepared in sterile buffers such as phosphate-buffered saline. Topical liquid, semi-solid, and liposome-encapsulated formulations, are favored for wound dressings. In case of liquid preparations, the liquid is applied with the help of gauze to the affected site. Semisolid formulations can be applied directly on the skin. For respiratory infections, formulations of phage can be either stable liquid formulations for intranasal instillation or nebulization, or as a solid powder in an inhalable form. Intravenous route is preferred for the treatment of systemic infections and intravesical instillation for genitourinary infection (43). Topical and transdermal products are used to treat skin infections, intrarectal for intestinal infections. According to one report, around 35% of formulations are of oral formulation, 13% injection, 15% rectal, 16% topical, 10% topical and 3% inhalation (44).
Although no bacteriophage formulation is approved by FDA for human use, several bacteriophage formulations are approved for animal health. Some of the commercially available phages which are approved by USFDA for animal health include Ecolicide PX (contamination on animal fur), Ecolicide (contamination in pet food), SalmoGuard (poultry feed additive), Staphage Lysate (for skin infection in dogs) (45, 46). Several other products also exist which are approved by other regulatory authorities of different countries.
LIMITATIONS OF BACTERIOPHAGES
Although there are several advantages of Bacteriophages, there exist some limitations too. Firstly, phages are active against a narrow range of bacterial strains which is because of their inherent specificity towards certain strains. Hence, it becomes difficult to find the right phage for a specific bacterial infection (47). The second concern is related to the stability of phages. Phages can be sensitive to environmental factors such as temperature, pH, and ultraviolet light. This sensitivity can affect their stability and effectiveness, particularly in clinical settings where precise conditions may not always be controllable. Storage and transportation of phage-based therapies also require careful handling to maintain their viability (48). However, several techniques such as lyophilization have been used to increase the stability of Bacteriophages to up to several months. Biofilm penetration and emergence of resistance is another major challenge for Bacteriophages. Just like with antibiotics, bacteria can develop resistance to phages. This can occur through various mechanisms, such as mutations in surface receptors that the phage uses to bind to the bacterial cell. Once resistance develops, the phage may become ineffective in treating the infection, necessitating the search for alternative phages (49).
ETHICS RELATED TO BACTERIOPHAGES
Although bacteriophages have shown usefulness in several infections, there are certain issues concerning the regulatory authorities regarding their use. Firstly, reliance on imported sources of phages in terms of equity is a matter of concern. Moreover, to find the source for high-quality phages is difficult, especially for those new to the therapy. Accessibility of phages is presently not organized and is largely driven by networking between clinicians and phage laboratories (50). Access is commonly through non-commercial routes, international sources or on payment per patient basis. Due to the possible delay to receive phages, there is a risk of infections getting worse (51).
The use of phages for hard-to-treat infections is still a matter of debate, as the healthcare system often hesitates to allocate their efforts, time, and resources towards unlicensed therapies. Also, the cost of phages is not fixed; instead, it has a wide range and may not be affordable for every patient.
The current manufacturing of phages is mainly done by non-GMP conditions which raises concerns related to quality, consistency and ultimately safety. The regulatory authorities need to ensure that quality standards are met for phage treatments to avoid any suboptimal quality treatments offered to patients. Some reports have mentioned adverse effects with high concentration of phages, which could be due to the presence of pyrogens possibly associated with manufacturing (52).
CONCLUSION
The use of bacteriophages against bacterial infections holds significant promise as an alternative to antibiotics. They have high specificity, better safety, better tolerability, easy development and relatively low chances of resistance. Studies have shown their application as an antibacterial alternative for infections related to skin, gastrointestinal tract, respiratory tract and urinary tract. Research is also performed for the development of the most suitable formulation for delivery of bacteriophages with better compliance and shelf life. Several phages are under phase 3 clinical trials for their antibacterial action, and are expected to be approved for clinical use. Ongoing key scientific considerations and advancing research in the upcoming years will be crucial in realizing the full therapeutic potential of phage-based interventions for bacterial infections.




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download