· In conclusion, adrenal hypoplasia congenita (AHC) is diagnosed due to adrenal crisis during the neonatal or infantile period at most of cases. However, it is important to consider adrenocorticotropic hormone when pigmentation or high ACTH are present, even if there is no adrenal insufficiency at an early age.
To the editor,
Adrenal hypoplasia congenita (AHC) is a rare form of primary adrenal insufficiency, typically an X-linked disorder caused by pathogenic variants in the NR0B1 gene, also known as DAX1 [1]. This gene encodes a protein crucial for the development and function of the adrenal glands and the hypothalamus-pituitary-gonadal axis [2]. AHC is characterized by adrenal insufficiency and hypogonadotropic hypogonadism (HH). Adrenal insufficiency often presents in infancy or early childhood with symptoms such as skin pigmentation, hypoglycemia, and salt-wasting dehydration [3].
We report an 8-year-old boy with X-linked AHC caused by a novel likely pathogenic variant in the NR0B1 gene, who initially presented with dark skin pigmentation in the ungual and periungual regions.
The patient had progressive dark pigmentation around the periungual area. He had no siblings and was born at full term with no significant medical or family history of adrenal diseases. His height was 133.5 cm, and weight was 26 kg with in normal range. A physical examination revealed dark pigmentation throughout the body, including the ungual and periungual areas. His testes size was less than 3 mL, but his bone age was advanced to 10 years and 6 months.
Blood tests indicated no electrolyte imbalance or hypoglycemia, but adrenocorticotropic hormone (ACTH) was elevated (21,310 pg/mL; reference range, 5–37 pg/mL), and cortisol was decreased (2.0 μg/dL; reference range, 4–11 μg/dL), indicating primary adrenal insufficiency. A rapid ACTH stimulation test showed no increase in cortisol or 17-hydroxyprogesterone. Very long-chain fatty acids were not remarkable, and no pathogenic variants were found in the Sanger sequencing of ABCD1 gene. These results suggest that adrenal insufficiency was not caused by adrenoleukodystrophy or 21-hydroxylase deficiency. Abdominal computed tomography showed no abnormalities in the adrenal glands.
Whole-genome sequencing identified a likely pathogenic hemizygous variant, c.134_135del (p.Asp45GlyfsTer26), in the NR0B1 gene, causing a frameshift and premature stop codon [4]. The patient's mother carried the same heterozygous variant but was asymptomatic. As a result, he was diagnosed with X-linked AHC.
NR0B1 is a nuclear transcription factor involved in the steroidogenic pathway in the hypothalamus, pituitary, and gonads [2]. Pathogenic variants in NR0B1 are estimated to affect 1 in 70,000 to 1 in 600,000 males [5]. These variants can lead to primary defects in adrenal gland development and HH, potentially causing absent or delayed puberty and infertility due to spermatogenesis abnormalities [6)].
Clinical features of adrenal insufficiency in AHC vary depending on the location of the NR0B1 pathogenic variants. Approximately 60% of affected males present with adrenal insufficiency in infancy, while 40% have an onset in childhood. Some cases may not show adrenal insufficiency until adulthood, with relatively mild symptoms [7]. Several cases of X-linked AHC have been reported in South Korea, typically presenting with adrenal crises in neonatal or infantile periods (Table 1) [8-13].
Previously reported cases had an onset of adrenal crisis at a neonatal or infantile period; however, the present case had no adrenal crisis until 8 years old but only developed skin pigmentation only. The normal structure of the adrenal gland and the absence of adrenal crisis suggest that this pathogenic variant could result in a partial loss of function for the gene NR0B1.
The disrupted hypothalamic-pituitary-gonadal axis in AHC leads to decreased follicle-stimulating hormone (FSH) and luteinizing hormone secretion, impairing gonadal function, delaying or preventing puberty, and causing infertility [14]. Treatment options include gonadotropin-releasing hormone pulsatile therapy, sequential gonadotropin therapy with FSH and human chorionic gonadotropin, and assisted reproductive technologies like testicular sperm extraction associated with intracytoplasmic sperm injection [15].
In this case, He is now 9 years and 10 months old and receiving an oral hydrocortisone treatment of 11 mg/m2. The serum ACTH levels have decreased gradually (Fig. 1). There is still pigmentation on the hands and feet, but it is improving compared to the first visit. However, he had not yet entered puberty in despite of the advanced bone age; therefore, he must be closely monitored for the onset of puberty.
In conclusion, AHC is diagnosed due to adrenal crisis during the neonatal or infantile period at most of cases. However, it is important to consider AHC when pigmentation or high ACTH are present, even if there is no adrenal insufficiency at an early age. Also, genetic analysis can useful in diagnosing adrenal insufficiency resulting from unknown causes.
Notes
ACKNOWLEDGMENTS
The authors thank to the all staff at the Department of Pediatrics of Ajou University Hospital.
References
1. Gupta P, Sharma R, Jain V. Adrenal hypoplasia congenitahypogonadotropic hypogonadism syndrome due to NR0B1 gene mutations. Indian J Pediatr. 2022; 89:587–90.

3. Reutens AT, Achermann JC, Ito M, Ito M, Gu WX, Habiby RL, et al. Clinical and functional effects of mutations in the DAX-1 gene in patients with adrenal hypoplasia congenita. J Clin Endocrinol Metab. 1999; 84:504–11.

4. Li H, Handsaker B, Wysoker A, Fennell T, Ruan J, Homer N, et al. The sequence alignment/map format and SAMtools. Bioinformatics. 2009; 25:2078–9.

5. Lin L, Gu WX, Ozisik G, To WS, Owen CJ, Jameson JL, et al. Analysis of DAX1 (NR0B1) and steroidogenic factor-1 (NR5A1) in children and adults with primary adrenal failure: ten years' experience. J Clin Endocrinol Metab. 2006; 91:3048–54.

6. Muscatelli F, Strom TM, Walker AP, Zanaria E, Recan D, Meindl A, et al. Mutations in the DAX-1 gene give rise to both X-linked adrenal hypoplasia congenita and hypogonadotropic hypogonadism. Nature. 1994; 372:672–6.

7. Achermann JC, Vilain EJ. NR0B1-related adrenal hypoplasia congenita [Internet]. Seattle (WA): University of Washington; c2001 [updated 2018 Jan 25]. Available from: https://www.ncbi.nlm.nih.gov/books/NBK1431/.
8. Choi JH, Shin YL, Kim GH, Kim Y, Park S, Park JY, et al. Identification of novel mutations of the DAX-1 gene in patients with X-linked adrenal hypoplasia congenita. Horm Res. 2005; 63:200–5.
9. Lee YW, Won JC, Ki CS, Lee HY, Ahn HS, Lee YK, et al. Clinical and genetic analysis of a Korean patient with late-onset X-linked adrenal hypoplasia congenita and hypogonadotropic hypogonadism: identification of a novel mutation in the NR0B1 gene. J Int Med Res. 2008; 36:357–61.

10. Park SH, Hong YH, Kim SS. Primary adrenal insufficiency in a newborn with adrenal hypoplasia congenita caused by a mutation of the DAX1 gene. Neonatal Med. 2016; 23:53–8.

11. Lee JY, Kim WD, Kim HS, Lee EK, Park HD. A novel mutation in the DAX1 gene in a newborn with adrenal hypoplasia congenita in Korea. J Genet Med. 2017; 14:27–30.

12. Choi HS, Kwon A, Chae HW, Suh J, Song KC, Lee JS, et al. Identification of a novel point mutation in DAX-1 gene in a patient with adrenal hypoplasia congenita. Ann Pediatr Endocrinol Metab. 2021; 26:126–9.

13. Shin C, Kim SE, Moon CJ, Yoo IH, Yim J, Cho WK, et al. A case of X-linked adrenal hypoplasia congenital (AHC) due to large deletion of NR0B1 (DAX1) and contiguous gene. Ann Clin Lab Sci. 2023; 53:667–70.
Fig. 1.
Serum cortisol and ACTH levels of the 8-year-old boy diagnosed with X-linked adrenal hypoplasia congenita due to NR0B1 likely pathogenic variant. Before treatment, an elevated ACTH (21,310 pg/mL) and low cortisol (2.0 μg/dL) were noted. After treatment with hydrocortisone, gradually decreased ACTH was noted. ACTH, adrenocorticotropic hormone.
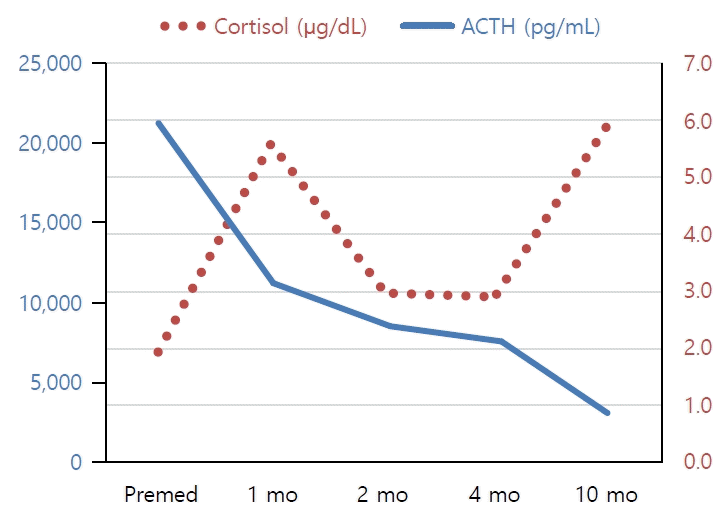
Table 1.
Molecular and clinical features of X-linked adrenal hypoplasia congenita reported in South Korea
| Variable | Present case | Choi et al., 2005 [8] | Choi et al., 2005 [8] | Choi et al., 2005 [8] | Lee et al., 2008 [9] | Park et al., 2016 [10] | Lee et al., 2017 [11] | Choi et al., 2021 [12] | Shin et al., 2023 [13] |
|---|---|---|---|---|---|---|---|---|---|
| Sex | Male | Male | Male | Male | Male | Male | Male | Male | Male |
| Age at diagnosis (yr) | 8 | 5 | 3 | 7 | 23 | 0.1 | 0.1 | 17 | 0.1 |
| Birth weight (g) | 3,020 | 2,960 | 3,450 | - | - | 3,160 | 3,520 | 3,900 | 3,220 |
| Pathogenic variant location | c.134_135del | c.1156_1157delCT | c.134G>A | Complete deletion of NR0B1 | c.959_960insT | c.543delA | c.844C>T | c.881T>C | Complete deletion of NR0B1 |
| Types of pathogenic variant | Frameshift | Frameshift | Nonsense | Microdeletion of Xp21.1 | Frameshift | Frameshift | Nonsense | Missense | Large deletion of Xp21.2 |
| Family history | None | None | None | Sibling (died in infancy due to adrenal crisis) | None | None | None | None | Silbling (adrenal insufficiency but no genetic confirmation) |
| Adrenal crisis (age) | No | Yes (3 days) | Yes (24 days) | Yes (3 days) | Yes (13 years) | Yes (18 days) | Yes (21 days) | Yes (2 months) | Yes (7 days) |
| Hypogonadotropic hypogonadism | - | - | - | - | Yes | - | - | Yes | - |




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download