Abstract
Notes
Conflicts of interest
Eujin Park, Myung Hyun Cho, Hyun Kyung Lee, Hee Gyung Kang, Jin-Soon Suh, and Eun Mi Yang are editorial board members of the journal but were not involved in the peer reviewer selection, evaluation, or decision process of this article. No other potential conflicts of interest relevant to this article were reported.
Author contributions
Conceptualization: HGK
Data curation: SWK, SJK, MB, YHA, MHC, HKL, KHH, YLK, MC
Funding acquisition: HGK
Investigation: JSS, EMY
Visualization: EP, HGK, EMY
Writing-original draft: EP, JSS, EMY
Writing-review & editing: EP, EMY
All authors read and approved the final manuscript.
References
Fig. 1.
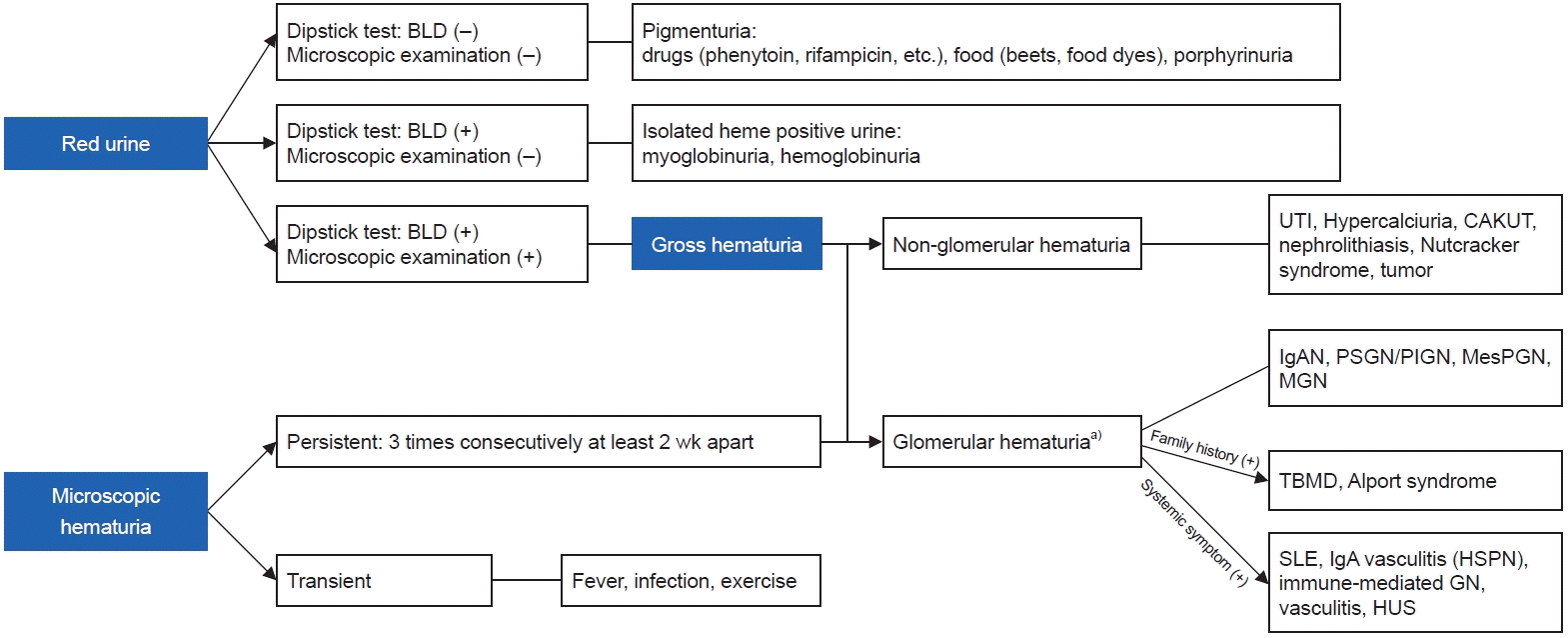
Fig. 2.
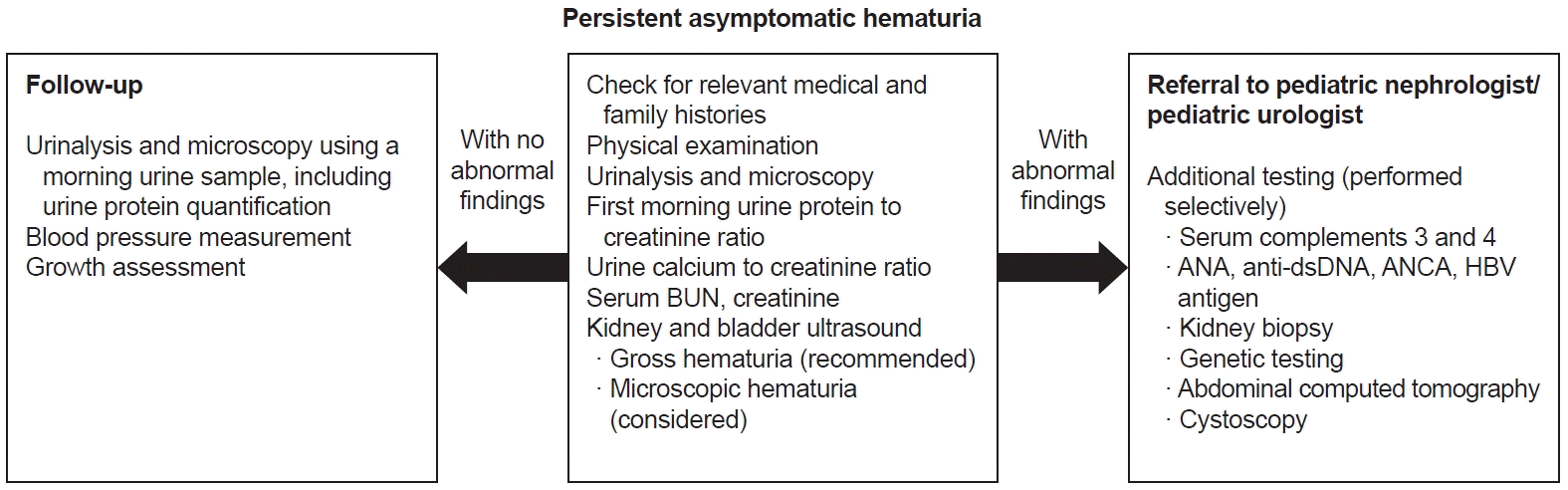
Table 1.
PSGN, poststreptococcal glomerulonephritis; PIGN, postinfectious glomerulonephritis; IgAN, immunoglobulin A (IgA) nephropathy; TBMD, thin basement membrane disease; MPGN, membranoproliferative glomerulonephritis; C3, complement 3; FSGS, focal segmental glomerulosclerosis; HSPN, Henosch–Schönlein purpura nephritis; CAKUT, congenital anomalies of kidney and urinary tract; ADPKD, autosomal dominant polycystic kidney disease.
a)Gross hematuria may occur.
Table 2.
| Cause | Bergstein et al. (2005) [5] | Youn et al. (2006) [6] | Greenfield et al. (2007) [7] | Mishra et al. (2022) [8] | Summary, No. (%)b) |
|---|---|---|---|---|---|
| No diagnosis/normal | 86 | 26 | 118 | 7 | 237 (33.3) |
| Hypercalciuria | 55 | 9 | 0 | 7 | 71 (10.0) |
| IgAN | 34 | 13 | a) | 2 | 49 (6.89) |
| PSGN | 21 | 3 | a) | 17 | 41 (5.77) |
| Other GN | 5 | 1 | a) | 11 | 17 (2.39) |
| Urinary tract infection | 1 | 8 | 48 | 3 | 60 (8.44) |
| Alport syndrome | 3 | 6 | 0 | 0 | 9 (1.27) |
| Urethrorrhagia | 0 | 8 | 52 | 0 | 60 (8.44) |
| Nephrolithiasis | 0 | 3 | 18 | 5 | 26 (3.66) |
| Exercise | 8 | 0 | 0 | 0 | 8 (1.13) |
| IgA vasculitis | 0 | 1 | 0 | 4 | 5 (0.70) |
| Sickle cell trait | 3 | 2 | 0 | 0 | 5 (0.70) |
| CAKUT | 5 | 0 | 45 | 0 | 50 (7.03) |
| ADPKD | 3 | 1 | 0 | 0 | 4 (0.56) |
| TBMD | 3 | 0 | 0 | 0 | 3 (0.42) |
| Chronic kidney disease | 0 | 0 | 0 | 2 | 2 (0.28) |
| Tumor | 1 | 1 | 7 | 0 | 9 (1.27) |
| Trauma | 0 | 0 | 48 | 0 | 48 (6.75) |
IgAN, immunoglobulin A nephropathy; PSGN, poststreptococcal glomerulonephritis; GN, glomerulonephritis; CAKUT, congenital anomalies of kidney and urinary tract; ADPKD, autosomal dominant polycystic kidney disease; TBMD, thin basement membrane disease.
a)The value of IgAN, PSGN, and other GN are 7; b)Greenfield et al. cases were excluded from the summary values for IgAN, PSGN, and other GN.
Table 3.
| Vehaskari et al. (1979) [11] | Trachtman et al. (1984) [12] | Piqueras et al. (1998) [13] | Lin et al. (2001) [14]a) | Bergstein et al. (2005) [5] | Lee et al. (2006) [15] | Moghtaderi et al. (2014) [16] | Guven et al. (2016) [17] | Summary No. (%) | |
|---|---|---|---|---|---|---|---|---|---|
| No diagnosis/ normal | 22 | 25 | 32 | 73 | 274 | 136 | 5 | 97 | 664 (54.9) |
| TBMD | 0 | 10 | 23 | 9 | 0 | 97 | 0 | 2 | 141 (11.7) |
| IgAN | 2 | 1 | 39 | 31 | 1 | 46 | 0 | 0 | 120 (9.92) |
| Hypercalciuria | 0 | 0 | 4 | 17 | 57 | 0 | 7 | 0 | 85 (7.02) |
| Lupus | 0 | 0 | 0 | 84 | 0 | 1 | 0 | 0 | 85 (7.02) |
| Other GN | 1 | 0 | 16 | 6 | 1 | 3 | 0 | 0 | 27 (2.23) |
| Hilar vasculopathy /vascular C3 | 0 | 5 | 15 | 0 | 0 | 0 | 0 | 0 | 20 (1.65) |
| UTI | 0 | 0 | 0 | 19 | 0 | 0 | 0 | 0 | 19 (1.57) |
| PSGN | 0 | 0 | 0 | 5 | 4 | 4 | 0 | 0 | 13 (1.07) |
| Nephrolithiasis | 0 | 0 | 0 | 0 | 0 | 0 | 13 | 0 | 13 (1.07) |
| CAKUT | 2 | 0 | 0 | 3 | 5 | 1 | 0 | 0 | 11 (0.91) |
| Alport syndrome | 0 | 1 | 2 | 0 | 0 | 1 | 0 | 2 | 6 (0.50) |
| IgA vasculitis | 0 | 0 | 0 | 3 | 0 | 0 | 0 | 0 | 3 (0.25) |
| Tumor | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 (0.08) |
| Blunt injury | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 1 (0.08) |
| Hemophilia | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 1 (0.08) |
TBMD, thin basement membrane disease; IgAN, immunoglobulin A (IgA) nephropathy; GN, glomerulonephritis; C3, complement 3; UTI, urinary tract infection; PSGN, poststreptococcal glomerulonephritis; CAKUT, congenital anomalies of kidney and urinary tract.
a)Lin's study was a school screening study of Taiwanese, where most were asymptomatic, but some lupus patients developed symptoms during follow-up.
Table 4.
| Cause | Vehaskari et al. (1979) [11] | Hisano and Ueda (1989) [18] | Lin et al. (2001) [14] | Lee et al. (2006) [15] | Guven et al. (2016) [17] | Summary, No. (%) |
|---|---|---|---|---|---|---|
| TBMD | 0 | 0 | 1 | 130 | 1 | 132 (31.1) |
| IgAN | 0 | 29 | 12 | 75 | 0 | 116 (27.3) |
| Other GNa) | 1 | 25 | 13 | 18 | 2 | 59 (13.9) |
| No diagnosis/normal | 4 | 0 | 7 | 40 | 1 | 52 (12.2) |
| Lupus | 0 | 1 | 30 | 0 | 0 | 31 (7.29) |
| IgA vasculitis | 0 | 10 | 0 | 0 | 0 | 10 (2.35) |
| Orthostatic proteinuria | 0 | 0 | 8 | 0 | 0 | 8 (1.88) |
| PSGN | 0 | 0 | 2 | 4 | 0 | 6 (1.41) |
| Alport syndrome | 0 | 1 | 0 | 3 | 1 | 5 (1.18) |
| VUR | 0 | 0 | 2 | 0 | 0 | 2 (0.47) |
| ATN | 0 | 0 | 1 | 0 | 0 | 1 (0.24) |
| Polyarthritis | 1 | 0 | 0 | 0 | 0 | 1 (0.24) |
| Hypercalciuria | 0 | 0 | 1 | 0 | 0 | 1 (0.24) |
| Blunt injury | 0 | 0 | 1 | 0 | 0 | 1 (0.24) |
TBMD, thin basement membrane disease; IgAN, immunoglobulin A (IgA) nephropathy; GN, glomerulonephritis; PSGN, poststreptococcal glomerulonephritis; VUR, vesicoureteral reflux; ATN, acute tubular necrosis.
a)Membranoproliferative glomerulonephritis, mesangial proliferative glomerulonephritis, focal segmental glomerulosclerosis, minimal change disease, complement 3 glomerulonephritis, hemolytic uremic syndrome.
Table 5.
eGFR, estimated glomerular filtration rate; Cr, creatinine; IDMS, isotope dilution mass spectrometry; CKiD, The Chronic Kidney Disease in Children Cohort Study; CysC, cystatin C; BUN, blood urea nitrogen.
a)If the cystatin C measurement is calibrated according to the International Federation for Clinical Chemistry and Laboratory Medicine calibration, use the cystatin C/1.17 value.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download