Abstract
Patients with chronic kidney disease (CKD) bear a significant financial burden and face numerous complications and higher mortality rates. The progression of CKD is associated with glomerular injury caused by glomerular hyperfiltration and oxidative stress. Factors such as uncontrolled hypertension, elevated urine protein levels, anemia, and underlying glomerular disease, contribute to CKD progression. In addition to conservative treatment, several medications are available to combat the progression of CKD to end-stage kidney disease. Renin-angiotensin-aldosterone system blockers could slow the progression of CKD by reducing glomerular hyperfiltration, lowering blood pressure, and decreasing inflammation. Mineralocorticoid receptor antagonists inhibit the mineralocorticoid receptor signaling pathway, thereby attenuating inflammation and fibrosis. Sodium-glucose cotransporter 2 inhibitors exhibit protective effects on the kidneys and against cardiovascular events. Tolvaptan, a selective vasopressin V2-receptor antagonist, decelerates the rate of increase in total kidney volume and deterioration of kidney function in patients with rapidly progressive autosomal dominant polycystic kidney disease. The protective effects of AST-120 remain controversial. Due to a lack of evidence regarding the efficacy and safety of these medications in children, it is imperative to weigh the benefits and adverse effects carefully. Further research is essential to establish the efficacy and safety profiles in pediatric populations.
Chronic kidney disease (CKD) is a condition characterized by abnormalities in kidney structure or function that persist for >3 months [1]. In patients with CKD, the glomerular filtration rate (GFR), a widely accepted indicator of kidney function that reflects the kidney’s excretory capacity, decreases to <60 mL/min/1.73 m2 [1]. The outcome of CKD can be predicted based on the cause, stage, and other clinical factors [1,2]. The staging of CKD is primarily based on the GFR and the degree of proteinuria, except in children aged <2 years. In this age group, the normal GFR is lower than that of children aged >2 years, and it may vary with changes in glomerular size and permeability [3].
Pediatric CKD occurs at a rate of 7.7 to 38 cases per million age-related populations, with variations depending on regions and races [4]. Considering the subtle or even limited manifestations of CKD, the actual incidence of CKD might have been underestimated. Children with CKD usually experience a decline in their kidney function at a rate of 1.5–4.3 mL/min/1.73 m2 per year; however, the rate varies depending on the cause of CKD [5-7]. Although the causes of pediatric CKD vary, the distribution based on age is similar globally. Congenital anomalies of the kidney and urology tract (CAKUT) are commonly encountered in infants, nephrotic syndrome in childhood, and glomerulonephropathies in adolescents [8-10]. Overall, CAKUT account for the largest portion of CKD cases (42%–59%), followed by glomerulopathy (5%–25%) and cystic disease (3.5%–14%) [4,8,10].
For years, treatment approaches for slowing the progression of CKD had relied on supportive methods such as control of proteinuria and blood pressure, anemia correction, and avoidance of exacerbating factors in addition to management of underlying disease [11]. Recently, a range of medications have been developed that are reportedly effective in slowing the deterioration of kidney function and ultimately delaying the need for kidney replacement therapy (KRT). Herein, I have reviewed the approved medications that are known to slow down the progression of CKD and explored their applicability in pediatric patients with CKD.
Despite the different underlying causes, CKD tends to progress similarly, with the kidney adapting to the loss of nephrons. As CKD advances, there is a reduction in the number of nephrons and overall kidney mass, whether acquired or congenital. In response, the surviving nephrons increase their filtration via the elevation of blood pressure and dilation of afferent vessels to address the existing excretory needs and maintain the total GFR. This compensation leads to intraglomerular hypertension and glomerular hyperfiltration in individual nephrons [12]. The increased filtration in a single nephron causes glomerular permeability changes, which leads to an increase in protein flux, including that of albumin. This process can contribute to mesangial proliferation, glomerular sclerosis, and ultimately, CKD progression [13,14]. In addition to hyperfiltration, mitochondrial dysfunction, caused by increased oxidative stress and uremic toxin, may contribute to the progression of CKD and its cardiovascular complications [15].
The duration until patients with CKD progress to end-stage kidney disease (ESKD) typically varies, ranging from 4.5 to 8.2 years, depending upon the underlying cause of CKD [6,16]. The progression tends to be more rapid in older patients (>12 years old), those with glomerular diseases, and those at higher CKD stages [2,6,16-18]. Additionally, patients with uncontrolled hypertension, higher urinary protein-to-creatinine ratio, anemia, hypocalcemia, and hyperphosphatemia are at a higher risk of experiencing a faster deterioration of kidney function [6,7,16,18].
Although the mechanism of CKD progression is not fully understood, the pathophysiology can be a target for medications that slow down the rate of decline in kidney function. By comprehending the processes and mechanisms involved in CKD progression, strategies and interventions can be developed to protect the kidneys from further damage.
Currently, there are no specific medications designed for the treatment of CKD. To prevent the progression of CKD, strict blood pressure control is recommended along with maintaining adequate levels of hemoglobin, bicarbonate, calcium, and vitamin D [11]. Additionally, although evidence is limited, regular physical activity is advised to lower blood pressure and body weight, and to improve mental health [19,20]. Given the diverse etiologies of CKD, adequate strategies that address the underlying mechanisms may be employed, such as immunosuppressants for glomerulonephritis [21].
Because children with CKD are prone to complications, it is crucial to implement intensive monitoring of growth and development, blood pressure, and nutrition. Regular assessments and timely interventions can help address these complications and contribute to the overall well-being of children with CKD. According to the CKD stage, the following recommendations have been made: protein intake should be within 100%–140% of the dietary reference intake (DRI), calcium within 100%–200% of the DRI, and phosphorous within 80%–100% of the DRI with frequent evaluation and monitoring [22].
In addition to controlling aggravating factors, the use of new medications could assist in delaying the progression of CKD. Based on the pathophysiology of CKD progression, several medications are currently used, ranging from angiotensin-converting enzyme inhibitors (ACEIs) and angiotensin receptor blockers (ARBs), which have been used from the 1980s, to sodium-glucose cotransporter 2 inhibitors (SGLT2-Is), which have recently been found to be promising in various disease.
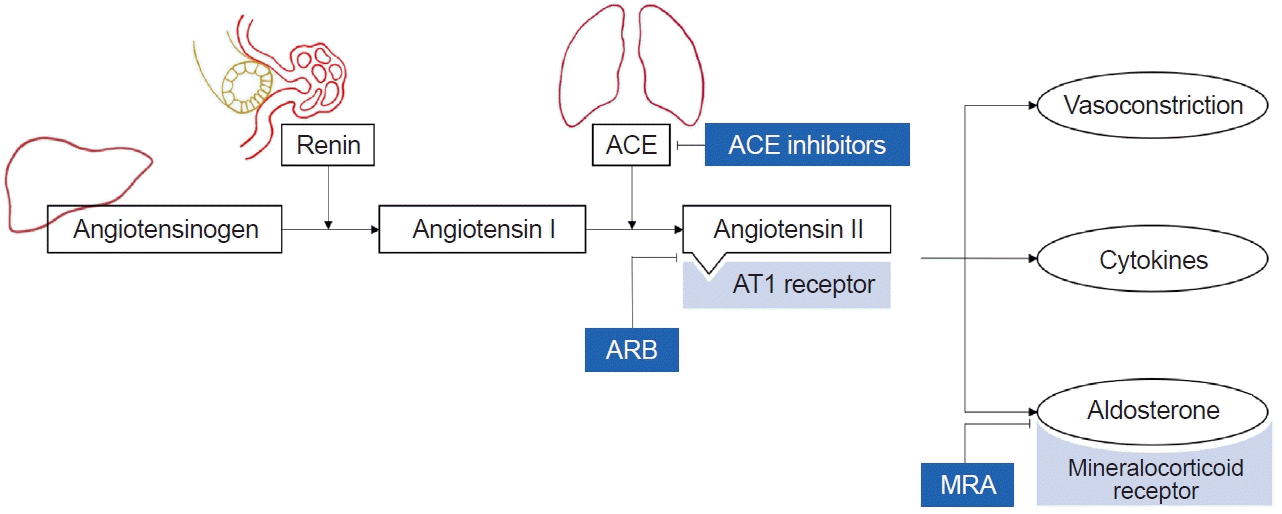
The renin-angiotensin-aldosterone system (RAAS), which preserves the fluid volume and electrolytes by reducing the urinary salt excretion, has been a therapeutic target for the treatment of vascular diseases [23]. ACEIs block the conversion of angiotensin I to angiotensin II, while ARBs selectively block angiotensin II type 1 receptors, responsible for the main effects of RAAS (Fig. 1) [23]. ACEIs and ARBs are known to preserve kidney function by reducing the glomerular permeability to proteins [24]. They alleviate glomerular hypertension and lower blood pressure, while also reducing the levels of cytokines related to inflammation [25,26]. Consequently, to lower blood pressure and proteinuria, guidelines recommend ACEIs or ARBs as first-line medications for blood pressure control in both adults and children [19,27].
The protective effects of ACEIs or ARBs on the kidneys have been reported in children, just as they have been reported in adults with CKD. The ESCAPE trial (ClinicalTrials.gov number, NCT00221845), a randomized clinical trial (RCT) examining the efficacy of blood pressure control, demonstrated that strict blood pressure control in children with CKD stages 2 to 4 and hypertension delay the deterioration of kidney function. After 5 years of ramipril use, the intensified control group showed a lower rate of 50% reduction in GFR or progression to ESKD compared to the conventional control group (70.1% vs. 58.3%; hazard ratio [HR], 0.6; 95% confidence interval [CI], 0.44–0.94; P=0.02) [28].
An observational study from Chronic Kidney Disease in Children, a pediatric multicenter cohort of 851 CKD pediatric patients in the United States, showed that 472 children with RAAS blockers had a significantly lower urine protein-to-creatinine ratio compared to those with discontinuation of RAAS blockers (0.45 vs. 1.54, P<0.05). Moreover, those who continued RAAS blockers exhibited a higher GFR (47 mL/min/1.73 m2 vs. 26 mL/min/1.73 m2, P<0.05) after a median follow-up of 4.1 years [29]. Additionally, the continuous use of RAAS blockers was associated with a lower risk of KRT compared to non-use (HR, 0.63; 95% CI, 0.46–0.87) [29].
Approximately 10% of individuals using ACEIs may experience a dry cough, and the occurrence of angioedema is rare [30]. Furthermore, both ACEIs and ARBs can cause hyperkalemia, hypotension, and decline in kidney function [30,31].
Certain clinical trials have reported the efficacy of dual blockade in patients with hypertension and/or proteinuria. However, dual therapy may increase the risk of hyperkalemia, hypotension, and increased creatinine levels when compared with monotherapy [32,33]. In children, no consistent results have been obtained regarding the effects of dual therapy in reducing proteinuria and/or delaying the progression of CKD [34].
Aldosterone, stimulated by angiotensin II and released from the adrenal cortex, binds to the mineralocorticoid receptor (Fig. 1). This binding increases sodium and water reabsorption while enhancing potassium excretion in kidney tubules, ultimately resulting in elevated blood pressure [35]. The increased activation of mineralocorticoid receptor signaling contributes to inflammation and fibrosis, which plays a role in the deterioration of target organs such as heart and kidney [35,36].
Steroidal mineralocorticoid receptor antagonists (MRAs), spironolactone or eplerenone, reportedly reduce proteinuria and blood pressure more than ACEIs and/or ARBs alone or a placebo [35,37]. Even in patients with ESKD, steroidal MRAs reduce the risk of mortality, including cardiovascular-related mortality [35,38]. However, the increased risk of hyperkalemia limits the use of steroidal MRAs in clinical practice, especially in patients with CKD, diabetes mellitus (DM), or heart failure [39]. Additionally, gynecomastia, stemming from the antiandrogenic effects of spironolactone, and the risk of acute kidney injury might increase with the use of steroidal MRAs [37].
Finerenone, the only approved non-steroidal MRA known for its enhanced selectivity and potency, was approved in the United States in 2021. In Korea, it was approved in February 2024 for adults with diabetic CKD stages 2 to 4 who have persistent proteinuria despite maximal treatment with ACEIs or ARBs. Based on the FIDELIO-DKD (ClinicalTrials.gov number, NCT02540993) and FIGARO-DKD (ClinicalTrials.gov number, NCT02545049) studies [40,41], FIDELITY analysis revealed the favorable effects of finerenone on cardiovascular and kidney outcomes in patients with diabetic kidney disease when compared to placebo. When 13,026 patients were randomized 1:1 and were followed up for a median 3.0 years, cardiovascular events were less frequent in patients treated with finerenone (n=825, 12.7%) compared to those treated with a placebo (n=939, 14.4%) (HR, 0.86; 95% CI, 0.78–0.95). Similarly, kidney events were significantly fewer in the finerenone group (n=360, 5.5%) compared to the placebo group (n=465, 7.1%) (HR, 0.77; 95% CI, 0.67–0.88) [42]. However, clinical trials in CKD patients without type 2 DM have not been conducted. An RCT to verify the antiproteinuric effect and safety of finerenone in children with CKD, FIONA (ClinicalTrials.gov number, NCT05196035) [43], is underway. Similar to previous MRAs, finerenone is also associated with hyperkalemia, which may necessitate discontinuation of the medications or hospitalization [42].
SGLT2-Is lower the blood glucose levels with less adverse effects in patients with DM by inhibiting glucose and sodium reabsorption in the proximal renal tubules [44]. Additionally, by increasing natriuresis and glycosuria, SGLT2-Is decrease plasma volume, intraglomerular hyperfiltration, and systemic blood pressure [45]. These effects lower proteinuria, which slows the rate of decline in GFR and decreases the risk of heart failure [45]. In addition to its protective effects against cardiovascular event in patients with DM [46], SGLT2-Is could also improve the kidney events, such as doubling of the serum creatinine level, initiation of KRT, or kidney disease-related death, in patients with DM [47,48].
The DAPA-CKD trial (ClinicalTrials.gov number, NCT03036150), the RCT involving 4,304 patients with a GFR of 25–75 mL/min/1.73 m2 and proteinuria, demonstrated that regardless of the presence of DM, the use of dapagliflozin improves the kidney events in patients with CKD (HR, 0.56; 95% CI, 0.45–0.68; median follow-up of 2.4 years) [49]. In patients with IgA nephropathy-induced CKD, 14.1% of whom had type 2 DM, the use of dapagliflozin over a median 2.1 years significantly improves the kidney events (HR, 0.29; 95% CI, 0.12–0.73) [50]. However, in 104 patients with CKD due to focal segmental glomerulosclerosis, who had a higher urinary protein-to-creatinine ratio of a median 1,248 mg/g compared to a median 949 mg/g in the total patients of the DAPA-CKD trial, the use of dapagliflozin over a median 2.4 years did not significantly improve the kidney events (HR, 0.62; 95% CI, 0.17–2.17) [49,51]. Furthermore, it did not demonstrate a notable difference in the change in GFR (−3.7 mL/min/1.73 m2 per year vs. −4.2 mL/min/1.73 m2 per year: difference 0.5; 95% CI, −0.9 to 2.0) [51]. In Korea, dapagliflozin and empagliflozin have been approved for adults with type 2 DM, chronic heart failure, and CKD. However, ipragliflozin and ertugliflozin have only been approved for lowering serum glucose in adults with type 2 DM.
In contrast to the number of studies conducted in adults, the number of studies in children is limited, with only one small-sized study involving eight patients. This study showed the antiproteinuric effect of dapagliflozin (2.1 g/m2/24 hr at baseline; 1.5 g/m2/24 hr after 12 weeks; P<0.05). However, there was a slight decrease in GFR (109.2±32.0 mL/min/1.73 m2 at baseline vs. 103.8±28.2 mL/min/1.73 m2 after 12 weeks, P=0.048) [52].
When treated with SGLT2-Is, patients are prone to urogenital infections, blood volume depletion, euglycemic diabetic ketoacidosis, bone fractures, lower limb amputation, and necrotizing fasciitis of perineum, known as Fournier’s gangrene [53,54]. An acute dip in GFR after medication initiation can occur [47-49], and this has been attributed to the vasoconstriction of the afferent arteriole because of natriuresis in the proximal tubule. Furthermore, the acute dip in GFR is reportedly not associated with long-term kidney injury [55].
Currently, many clinical trials to investigate the efficacy of SGLT2-Is in patients with various kidney disease are underway; dapagliflozin in patients with Alport syndrome (ClinicalTrials.gov number, NCT05944016) including adolescents aged 10 to 18 years old, empagliflozin in patients with polycystic kidney disease (ClinicalTrials.gov number, NCT06391450), and the combined use of SGLT2-Is and MRAs in patients with diabetic kidney disease (ClinicalTrials.gov number, NCT05254002) [56-58]. Clinical trials in pediatric CKD patients are slated to commence in 2025.
Autosomal dominant polycystic kidney disease (ADPKD) is one of most common monogenic causes of CKD, with approximately 50% of ADPKD patients progressing to ESKD [59]. While kidney function in children with ADPKD tends be preserved, it remains one of the major causes of pediatric CKD [4,8,59]. It is recommended to control hypertension with RAAS inhibitors in both adults and children with ADPKD and hypertension [60]. Because total kidney volume (TKV) in ADPKD correlates with the cyst burden, associated symptoms such as pain, hypertension, proteinuria, and hematuria, as well as deterioration of kidney function [60], TKV is investigated as a marker of the treatment effects.
Tolvaptan, a selective vasopressin V2 receptor antagonist, inhibits the binding of arginine vasopressin to V2 receptors. Consequently, it reduces intracellular cyclic adenosine monophosphate level, which is believed to be the main pathway of cystogenesis in ADPKD [61]. In the TEMPO 3:4 trial (ClinicalTrials.gov number, NCT00428948), tolvaptan treatment over 3 years was shown to decrease the rate of increase in TKV (2.80%/yr vs. 5.51%/yr, P<0.001) and slow the decline in GFR (−2.61 [mg/mL]−1 per year vs. −3.81 [mg/mL]−1 per year, P<0.001) compared to placebo in patients with ADPKD and a GFR > 60 mL/min/1.73 m2 [62]. Even in the later stages of CKD, the use of tolvaptan produces a slower decline in estimated GFR (−2.34 mL/min/1.73 m2 vs. −3.61 mL/min/1.73 m2, P<0.001) [63]. Based on these results, the European Renal Association recommended tolvaptan as the first-line medication for patients with ADPKD [64]. In Korea, tolvaptan was approved for adults with both CKD stage 2 to 3 and rapidly progressive ADPKD (Mayo class 1C to 1E in imaging tests). In an RCT conducted on pediatric patients with ADPKD (ClinicalTrials.gov number, NCT02964273), although the urine osmolarity and urine specific gravity decreased after 12 months of tolvaptan treatment, there were no significant changes in the TKV (tolvaptan vs. placebo: 2.3% vs. 5.8%, P=0.12) and GFR (1.9 mL/min/1.73 m2 vs. −1.8 mL/min/1.73 m2, P=0.11) [65]. Patients treated with tolvaptan are likely to experience adverse events related to increased urination, including thirst, polyuria, nocturia, and polydipsia. In addition, they may have elevated levels of liver enzyme levels [62,63,65].
AST-120 (KREMEZIN), a spherical adsorptive carbon, is used for the treatment of uremic symptoms and for delaying dialysis. It adsorbs uremic toxins and their precursors in the gastrointestinal tract. The efficacy of AST-120 in patients is controversial. Some studies have reported that the use of AST-120 could reduce the change in creatinine clearance and delay the initiation of dialysis [66,67]. However, other studies have suggested that while the use of AST-120 may lower serum or urine indoxyl sulfate, an assumed uremic toxin, it does not affect serum creatinine level and delay the need for dialysis [68-70]. Furthermore, increasing dosages or long-term use of AST-120 does not produce significant differences in the change in serum creatinine levels or GFR [68]. Moreover, AST-120 does not alleviate patients’ discomfort [68-70]. However, there have been no RCTs demonstrating the effects of AST-120 in children. Additionally, the pediatric use of AST-120 is not considered due to concerns about potential disturbance of nutrient absorption and discomfort. Therefore, further studies are necessary to determine the efficacy and safety of AST-120 use in pediatric CKD. AST-120 reportedly produces gastrointestinal complications (constipation, vomiting, and nausea), decreased appetite, and dermatological disease [66,68,70].
To preserve kidney function, various medications can be used in addition to classical supportive care in adults with CKD. However, in pediatric patients with CKD, there is a lack of evidence regarding the efficacy and safety of such drugs, except for ACEIs and ARBs. Therefore, before using such medications, their benefits and adverse effects should be considered.
References
1. Chapter 2: Definition, identification, and prediction of CKD progression. Kidney Int Suppl (2011). 2013; 3:63–72.
2. Warady BA, Abraham AG, Schwartz GJ, Wong CS, Munoz A, Betoko A, et al. Predictors of rapid progression of glomerular and nonglomerular kidney disease in children and adolescents: the Chronic Kidney Disease in Children (CKiD) Cohort. Am J Kidney Dis. 2015; 65:878–88.
3. Chapter 1: Definition and classification of CKD. Kidney Int Suppl (2011). 2013; 3:19–62.
4. Harambat J, van Stralen KJ, Kim JJ, Tizard EJ. Epidemiology of chronic kidney disease in children. Pediatr Nephrol. 2012; 27:363–73.

5. Furth SL, Abraham AG, Jerry-Fluker J, Schwartz GJ, Benfield M, Kaskel F, et al. Metabolic abnormalities, cardiovascular disease risk factors, and GFR decline in children with chronic kidney disease. Clin J Am Soc Nephrol. 2011; 6:2132–40.

6. Staples AO, Greenbaum LA, Smith JM, Gipson DS, Filler G, Warady BA, et al. Association between clinical risk factors and progression of chronic kidney disease in children. Clin J Am Soc Nephrol. 2010; 5:2172–9.

7. Kamath N, Iyengar A, George N, Luyckx VA. Risk factors and rate of progression of CKD in children. Kidney Int Rep. 2019; 4:1472–7.

8. Park PG, Kang HG, Park E, Ahn YH, Choi HJ, Han KH, et al. Baseline characteristics of participants enrolled in the KoreaN cohort study for Outcomes in patients With Pediatric Chronic Kidney Disease (KNOW-Ped CKD). Pediatr Nephrol. 2022; 37:3177–87.

9. Okuda Y, Soohoo M, Ishikura K, Tang Y, Obi Y, Laster M, et al. Primary causes of kidney disease and mortality in dialysis-dependent children. Pediatr Nephrol. 2020; 35:851–60.

10. Becherucci F, Roperto RM, Materassi M, Romagnani P. Chronic kidney disease in children. Clin Kidney J. 2016; 9:583–91.

11. Chapter 3: Management of progression and complications of CKD. Kidney Int Suppl (2011). 2013; 3:73–90.
12. Lopez-Novoa JM, Martinez-Salgado C, Rodriguez-Pena AB, Lopez-Hernandez FJ. Common pathophysiological mechanisms of chronic kidney disease: therapeutic perspectives. Pharmacol Ther. 2010; 128:61–81.

13. Brenner BM, Meyer TW, Hostetter TH. Dietary protein intake and the progressive nature of kidney disease: the role of hemodynamically mediated glomerular injury in the pathogenesis of progressive glomerular sclerosis in aging, renal ablation, and intrinsic renal disease. N Engl J Med. 1982; 307:652–9.
14. Denic A, Mathew J, Lerman LO, Lieske JC, Larson JJ, Alexander MP, et al. Single-nephron glomerular filtration rate in healthy adults. N Engl J Med. 2017; 376:2349–57.

15. Daenen K, Andries A, Mekahli D, Van Schepdael A, Jouret F, Bammens B. Oxidative stress in chronic kidney disease. Pediatr Nephrol. 2019; 34:975–91.

16. Cerqueira DC, Soares CM, Silva VR, Magalhaes JO, Barcelos IP, Duarte MG, et al. A predictive model of progression of CKD to ESRD in a predialysis pediatric interdisciplinary program. Clin J Am Soc Nephrol. 2014; 9:728–35.

17. Fathallah-Shaykh SA, Flynn JT, Pierce CB, Abraham AG, Blydt-Hansen TD, Massengill SF, et al. Progression of pediatric CKD of nonglomerular origin in the CKiD cohort. Clin J Am Soc Nephrol. 2015; 10:571–7.

18. Furth SL, Pierce C, Hui WF, White CA, Wong CS, Schaefer F, et al. Estimating time to ESRD in children with CKD. Am J Kidney Dis. 2018; 71:783–92.
19. Kidney Disease: Improving Global Outcomes (KDIGO) Blood Pressure Work Group. KDIGO 2021 clinical practice guideline for the management of blood pressure in chronic kidney disease. Kidney Int. 2021; 99(3S):S1–87.
20. Stevenson JK, Campbell ZC, Webster AC, Chow CK, Tong A, Craig JC, et al. eHealth interventions for people with chronic kidney disease. Cochrane Database Syst Rev. 2019; 8:CD012379.

21. Cirillo L, De Chiara L, Innocenti S, Errichiello C, Romagnani P, Becherucci F. Chronic kidney disease in children: an update. Clin Kidney J. 2023; 16:1600–11.

22. KDOQI Work Group. KDOQI clinical practice guideline for nutrition in children with CKD: 2008 update: executive summary. Am J Kidney Dis. 2009; 53(3 Suppl 2):S11–104.
23. Robles NR, Cerezo I, Hernandez-Gallego R. Renin-angiotensin system blocking drugs. J Cardiovasc Pharmacol Ther. 2014; 19:14–33.

24. Remuzzi A, Puntorieri S, Battaglia C, Bertani T, Remuzzi G. Angiotensin converting enzyme inhibition ameliorates glomerular filtration of macromolecules and water and lessens glomerular injury in the rat. J Clin Invest. 1990; 85:541–9.

25. Remuzzi G, Perico N, Macia M, Ruggenenti P. The role of renin-angiotensin-aldosterone system in the progression of chronic kidney disease. Kidney Int Suppl. 2005; 68(Suppl 99):S57–65.

26. Dandona P, Dhindsa S, Ghanim H, Chaudhuri A. Angiotensin II and inflammation: the effect of angiotensin-converting enzyme inhibition and angiotensin II receptor blockade. J Hum Hypertens. 2007; 21:20–7.

27. Drawz PE, Beddhu S, Bignall ONR 2nd, Cohen JB, Flynn JT, Ku E, et al. KDOQI US commentary on the 2021 KDIGO clinical practice guideline for the management of blood pressure in CKD. Am J Kidney Dis. 2022; 79:311–27.

28. ESCAPE Trial Group, Wuhl E, Trivelli A, Picca S, Litwin M, Peco-Antic A, et al. Strict blood-pressure control and progression of renal failure in children. N Engl J Med. 2009; 361:1639–50.

29. Abraham AG, Betoko A, Fadrowski JJ, Pierce C, Furth SL, Warady BA, et al. Renin-angiotensin II-aldosterone system blockers and time to renal replacement therapy in children with CKD. Pediatr Nephrol. 2017; 32:643–9.

30. Herman LL, Padala SA, Ahmed I, Bashir. K. Angiotensin-converting enzyme inhibitors (ACEI). In: StatPearls [Internet]. StatPearls Publishing; 2023. Available from: https://www.ncbi.nlm.nih.gov/books/NBK431051/.
31. Rodgers JE, Patterson JH. Angiotensin II-receptor blockers: clinical relevance and therapeutic role. Am J Health Syst Pharm. 2001; 58:671–83.

32. Makani H, Bangalore S, Desouza KA, Shah A, Messerli FH. Efficacy and safety of dual blockade of the renin-angiotensin system: meta-analysis of randomised trials. BMJ. 2013; 346:f360.

33. Azizi M, Menard J. Combined blockade of the renin-angiotensin system with angiotensin-converting enzyme inhibitors and angiotensin II type 1 receptor antagonists. Circulation. 2004; 109:2492–9.

34. Stotter BR, Ferguson MA. Should ACE inhibitors and ARBs be used in combination in children? Pediatr Nephrol. 2019; 34:1521–32.

35. Georgianos PI, Agarwal R. Mineralocorticoid receptor antagonism in chronic kidney disease. Kidney Int Rep. 2021; 6:2281–91.

36. Agarwal R, Kolkhof P, Bakris G, Bauersachs J, Haller H, Wada T, et al. Steroidal and non-steroidal mineralocorticoid receptor antagonists in cardiorenal medicine. Eur Heart J. 2021; 42:152–61.

37. Chung EY, Ruospo M, Natale P, Bolignano D, Navaneethan SD, Palmer SC, et al. Aldosterone antagonists in addition to renin angiotensin system antagonists for preventing the progression of chronic kidney disease. Cochrane Database Syst Rev. 2020; 10:CD007004.

38. Hasegawa T, Nishiwaki H, Ota E, Levack WM, Noma H. Aldosterone antagonists for people with chronic kidney disease requiring dialysis. Cochrane Database Syst Rev. 2021; 2:CD013109.

39. Thomsen RW, Nicolaisen SK, Hasvold P, Sanchez RG, Pedersen L, Adelborg K, et al. Elevated potassium levels in patients with chronic kidney disease: occurrence, risk factors and clinical outcomes: a Danish population-based cohort study. Nephrol Dial Transplant. 2018; 33:1610–20.
40. Bakris GL, Agarwal R, Anker SD, Pitt B, Ruilope LM, Rossing P, et al. Effect of finerenone on chronic kidney disease outcomes in type 2 diabetes. N Engl J Med. 2020; 383:2219–29.

41. Pitt B, Filippatos G, Agarwal R, Anker SD, Bakris GL, Rossing P, et al. Cardiovascular events with finerenone in kidney disease and type 2 diabetes. N Engl J Med. 2021; 385:2252–63.

42. Bayer. A study to learn more about how well the study treatment finerenone works, how safe it is, how it moves into, through, and out of the body, and the effects it has on the body when taken with an ace inhibitor or angiotensin receptor blocker in children with chronic kidney disease and proteinuria (FIONA). ClinicalTrials.gov identifier: NCT05196035 [cited May 11, 2024]. Available from: https://clinicaltrials.gov/study/NCT05196035.
43. Agarwal R, Filippatos G, Pitt B, Anker SD, Rossing P, Joseph A, et al. Cardiovascular and kidney outcomes with finerenone in patients with type 2 diabetes and chronic kidney disease: the FIDELITY pooled analysis. Eur Heart J. 2022; 43:474–84.

44. Chao EC, Henry RR. SGLT2 inhibition: a novel strategy for diabetes treatment. Nat Rev Drug Discov. 2010; 9:551–9.

45. Verma S, McMurray JJ. SGLT2 inhibitors and mechanisms of cardiovascular benefit: a state-of-the-art review. Diabetologia. 2018; 61:2108–17.

46. Zinman B, Wanner C, Lachin JM, Fitchett D, Bluhmki E, Hantel S, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015; 373:2117–28.

47. Wanner C, Inzucchi SE, Lachin JM, Fitchett D, von Eynatten M, Mattheus M, et al. Empagliflozin and progression of kidney disease in type 2 diabetes. N Engl J Med. 2016; 375:323–34.

48. Perkovic V, Jardine MJ, Neal B, Bompoint S, Heerspink HJL, Charytan DM, et al. Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. N Engl J Med. 2019; 380:2295–306.

49. Heerspink HJ, Stefansson BV, Correa-Rotter R, Chertow GM, Greene T, Hou FF, et al. Dapagliflozin in patients with chronic kidney disease. N Engl J Med. 2020; 383:1436–46.

50. Wheeler DC, Toto RD, Stefansson BV, Jongs N, Chertow GM, Greene T, et al. A pre-specified analysis of the DAPA-CKD trial demonstrates the effects of dapagliflozin on major adverse kidney events in patients with IgA nephropathy. Kidney Int. 2021; 100:215–24.

51. Wheeler DC, Jongs N, Stefansson BV, Chertow GM, Greene T, Hou FF, et al. Safety and efficacy of dapagliflozin in patients with focal segmental glomerulosclerosis: a prespecified analysis of the dapagliflozin and prevention of adverse outcomes in chronic kidney disease (DAPA-CKD) trial. Nephrol Dial Transplant. 2022; 37:1647–56.

52. Liu J, Cui J, Fang X, Chen J, Yan W, Shen Q, et al. Efficacy and safety of dapagliflozin in children with inherited proteinuric kidney disease: a pilot study. Kidney Int Rep. 2021; 7:638–41.

54. Qiu M, Ding LL, Zhang M, Zhou HR. Safety of four SGLT2 inhibitors in three chronic diseases: a meta-analysis of large randomized trials of SGLT2 inhibitors. Diab Vasc Dis Res. 2021; 18:14791641211011016.

55. Heerspink HJ, Cherney DZ. Clinical implications of an acute dip in eGFR after SGLT2 inhibitor initiation. Clin J Am Soc Nephrol. 2021; 16:1278–80.

56. Gross O. Phase 3 clinical trial with dapagliflozin in chronic kidney disease in adolescents and young adult patients (DOUBLE_PROTECT). ClinicalTrials.gov identifier: NCT05944016 [cited May 11, 2024]. Available from: https://clinicaltrials.gov/study/NCT05944016.
57. Hannover Medical School. Study of empagliflozin in patients with autosomal dominant polycystic kidney disease (EMPA-PKD). ClinicalTrials.gov identifier: NCT06391450 [cited May 11, 2024]. Available from: https://clinicaltrials.gov/study/NCT06391450.
58. Bayer. A study to learn how well the treatment combination of finerenone and empagliflozin works and how safe it is compared to each treatment alone in adult participants with long-term kidney disease (chronic kidney disease) and type 2 diabetes (CONFIDENCE). ClinicalTrials.gov identifier: NCT05254002 [cited May 11, 2024]. Available from: https://clinicaltrials.gov/study/NCT05254002.
59. Dell KM. The spectrum of polycystic kidney disease in children. Adv Chronic Kidney Dis. 2011; 18:339–47.

60. Chapman AB, Devuyst O, Eckardt KU, Gansevoort RT, Harris T, Horie S, et al. Autosomal-dominant polycystic kidney disease (ADPKD): executive summary from a Kidney Disease: Improving Global Outcomes (KDIGO) Controversies Conference. Kidney Int. 2015; 88:17–27.

61. Miyazaki T, Fujiki H, Yamamura Y, Nakamura S, Mori T. Tolvaptan, an orally active vasopressin V(2)-receptor antagonist: pharmacology and clinical trials. Cardiovasc Drug Rev. 2007; 25:1–13.

62. Torres VE, Chapman AB, Devuyst O, Gansevoort RT, Grantham JJ, Higashihara E, et al. Tolvaptan in patients with autosomal dominant polycystic kidney disease. N Engl J Med. 2012; 367:2407–18.

63. Torres VE, Chapman AB, Devuyst O, Gansevoort RT, Perrone RD, Koch G, et al. Tolvaptan in later-stage autosomal dominant polycystic kidney disease. N Engl J Med. 2017; 377:1930–42.

64. Gansevoort RT, Arici M, Benzing T, Birn H, Capasso G, Covic A, et al. Recommendations for the use of tolvaptan in autosomal dominant polycystic kidney disease: a position statement on behalf of the ERA-EDTA Working Groups on Inherited Kidney Disorders and European Renal Best Practice. Nephrol Dial Transplant. 2016; 31:337–48.

65. Mekahli D, Guay-Woodford LM, Cadnapaphornchai MA, Greenbaum LA, Litwin M, Seeman T, et al. Tolvaptan for children and adolescents with autosomal dominant polycystic kidney disease: randomized controlled trial. Clin J Am Soc Nephrol. 2023; 18:36–46.

66. Akizawa T, Asano Y, Morita S, Wakita T, Onishi Y, Fukuhara S, et al. Effect of a carbonaceous oral adsorbent on the progression of CKD: a multicenter, randomized, controlled trial. Am J Kidney Dis. 2009; 54:459–67.

67. Asai M, Kumakura S, Kikuchi M. Review of the efficacy of AST-120 (KREMEZIN®) on renal function in chronic kidney disease patients. Ren Fail. 2019; 41:47–56.

68. Cha RH, Kang SW, Park CW, Cha DR, Na KY, Kim SG, et al. A randomized, controlled trial of oral intestinal sorbent AST-120 on renal function deterioration in patients with advanced renal dysfunction. Clin J Am Soc Nephrol. 2016; 11:559–67.





 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download