Abstract
Lupus anticoagulant hypoprothrombinemia syndrome (LAHPS) is a rare entity characterized by the presence of lupus anticoagulant (LA) and prothrombin (factor II) deficiency. It may cause severe bleeding contrary to classical antiphospholipid syndrome. Here, we report a case of LAHPS presenting with a hemorrhagic ovarian cyst in a 17-year-old girl with systemic lupus erythematosus (SLE) nephritis. She had been followed up for 8 years. Her first manifestation of SLE was prolonged gingival bleeding after tooth extraction at 9 years of age. During the follow-up period, she had neither severe bleeding nor thrombotic complications despite a positive LA and a prolonged activated partial thromboplastin time (aPTT). At this visit, the patient presented with colicky abdominal pain, a hemorrhagic ovarian cyst, a prolonged prothrombin time, a prolonged aPTT, a low factor II level, and a positive LA, leading to the diagnosis of LAHPS. While a hemorrhagic ovarian cyst resolved completely in 3 months, she received oral pill, transfusions of red blood cells and plasma, and intravenous cyclophosphamide pulse therapy in combination with glucocorticoids due to persistent menorrhagia, anemia, prolonged aPTT, and lupus flaring. Thus, LAHPS needs to be considered in SLE patients with positive LA and prolonged aPTT.
Lupus anticoagulant hypoprothrombinemia syndrome (LAHPS) is a rare acquired disorder that presents with bleeding and/or thrombosis due to positive lupus anticoagulant (LA) and prothrombin (factor II) deficiency in patients with systemic lupus erythematosus (SLE), viral infections, or medications [1]. The incidence of LAHPS is higher in the pediatric age group, compared to adults and it affects females more often than males [2]. Anti-factor II antibodies (Abs) account for prothrombin deficiency in LAHPS and prothrombin is known to be one of the target antigens of antiphospholipid (aPL) Abs [3]. Patients with circulating aPL Abs usually have recurrent arterial and/or venous thrombotic events, whereas those with LAHPS are at a high risk of bleeding [4]. Here, we report a rare case of LAHPS associated with a hemorrhagic ovarian cyst in a 17-year-old girl with SLE nephritis who persistently showed LA and aPL Ab positivity.
A 17-year-old girl with SLE nephritis presented with squeezing colicky abdominal pain for a day. She had suffered from intermittent abdominal pain for 2 weeks. She had taken steroid (deflazacort; 30 mg/day, 0.5 mg/kg/day), mycophenolate mofetil (MMF; 1.5 g/day), enalapril, hydroxychloroquine, and calcitriol. The patient had been followed up for 8 years. Her first manifestations of SLE were recurrent persistent ecchymosis and prolonged gingival bleeding after tooth extraction at 9 years of age. First renal biopsy showed focal segmental proliferative lupus glomerulonephritis class IIIA (activity index 1, chronicity index 0) based upon the International Society of Nephrology and the Renal Pathology Society. During the follow-up period, she had neither severe bleeding nor thrombotic complications despite positive LA, aPL Ab, anti-cardiolipin (aCL) Ab, and anti-beta-2-glycoprotein I (anti-β2GP1) Ab with intermittent prolonged prothrombin time (PT) and activated partial thromboplastin time (aPTT). At this visit, her vital signs were within normal limits. Generalized abdominal tenderness was found without hepatosplenomegaly. Complete blood count showed white blood cells of 3,140/μL, hemoglobin of 10.4 g/dL, and platelets of 74,000/μL. Biochemical parameters showed blood urea nitrogen of 13.0 mg/dL, creatinine of 0.44 mg/dL, total CO2 of 18.7 mmol, amylase of 61.0 U/L, and C-reactive protein of 0.39 mg/dL. Coagulation studies revealed PT (18.3 seconds, reference 11.8–13.7 seconds) and aPTT (68.9 seconds, reference 29.3–40.5 seconds) prolongation with LA positivity. Levels of complements 3 and 4 and anti-double strand DNA were 53 mg/dL, 3.8 mg/dL, and 85 IU/mL (reference <7.0 IU/mL), respectively. She had positive findings for Abs of aCL immunoglobulin (Ig)G (>600 GPL U/mL, reference <10.0 GPL U/mL), anti-β2GP1 IgG (>600 U/mL, reference ≤20 SGU U/mL), and anti-β2GP1 IgM (30.1 U/mL, reference ≤20 U/mL). Urinalysis showed mild proteinuria (urine protein-to-creatinine ratio [UPCR] from 0.36 to 0.54) and hematuria (red blood cell [RBC] from 5–9 to 10–29/high power field). Abdominal ultrasonography and pelvic computed tomography revealed a 4.7 cm-sized hemorrhagic right ovarian cyst (Fig. 1A, B). In a gynecologic consultation, close observation and follow-up were advised. Due to abdominal discomfort and suspected SLE flaring, MMF dose was reduced (1 g/day) while steroid dose was increased (0.8 mg/kg/day). Tacrolimus (2 mg/day) was also added. She had menstrual bleeding 5 days later. Three months later, she complained of dizziness and persistent menorrhagia. RBC transfusion was performed at a hemoglobin level of 7.4 g/dL. Laboratory tests showed persistent prolonged aPTT (64.1 seconds) and PT (19.3 seconds), LA positivity, and positive Abs for aCL IgG (>419 GPL U/mL), anti-β2GP1 IgG (>533 U/mL), and anti-β2GP1 IgM (56.2 U/mL). Proteinuria was aggravated from 520 mg/day to 1,200 mg/day. However, pelvic ultrasonography showed decreasing-sized ovarian cystic lesion (<2 cm) (Fig. 1C). For controlling menorrhagia, combined oral contraceptive pill was given for 3 months. Repeated coagulation studies showed persistently prolonged aPTT (the longest 106.8 seconds) and low factor II level (the lowest 14%, reference 65%–125%). Factor VIII (the lowest 24%, reference 60%–150%) and factor XII (the lowest 29%, reference 60%–140%) were reduced but returned to normal ranges. Plasma mixing study revealed that PT and aPTT remained prolonged after mixing patient plasma with normal pooled plasma. A diagnosis of LAHPS associated with SLE was made, and doses of MMF (1.5 g/day) and tacrolimus (3 mg/day) were increased. Since then, however, she intermittently complained of epistaxis, menorrhagia, throbbing pain of finger tips, knee arthralgia, and headache. Proteinuria was also aggravated (UPCR, from 0.29 to 1.94). The patient was re-admitted for fresh frozen plasma transfusion and intravenous cyclophosphamide pulse therapy. A renal biopsy was performed again and the result revealed focal and segmental proliferative lupus glomerulonephritis class III A/C (activity index 4, chronicity index 1) (Fig. 2). The result of brain magnetic resonance imaging showed no pathologic findings. She received six courses of intravenous cyclophosphamide pulse therapy (monthly, 750 mg/dose). She is currently taking cyclosporine, deflazacort, hydroxychloroquine, and enalapril. Her general condition is being improved with decreased proteinuria (UPCR, 0.35). However, laboratory findings of prolongation of PT and aPTT, low factor II level, and positive LA persisted without bleeding or thrombotic complications (Fig. 3).
This is a rare case of LAHPS presenting with a hemorrhagic ovarian cyst in a 17-year-old girl with SLE nephritis who had been long followed up. LAHPS was diagnosed after the patient presented with recurrent bleeding episodes in association with coagulation abnormalities and LA positivity. Her bleeding symptoms were controlled following management of lupus flares.
LAHPS was first reported by Rapaport et al. [5] in 1960 in a pediatric SLE patient who suffered from severe bleeding and hypoprothrombinemia due to prothrombin activity inhibitors. Since then, numerous cases of LAHPS have been reported. However, many gaps still exist in our knowledge about the disease. Prothrombin or factor II is a vitamin K-dependent coagulation cofactor that is cleaved by factor Xa to form thrombin. Thrombin then induces platelet aggregation and is responsible for the activation of several other mediators in the coagulation cascade [6]. In LAHPS, the presence of anti-factor II Abs results in prothrombin deficiency which might be responsible for hemorrhagic complications. Meanwhile, LA, a subset of aPL Ab, can bind to phospholipid-associated proteins in coagulation complexes and disrupt phospholipid-dependent coagulation tests [7]. The presence of circulating LA is usually associated with venous and arterial thrombosis and pregnancy complications. It can also be associated with bleeding in cases with combined hypoprothrombinemia. Mazodier et al. [8] have found that in 74 patients with LAHPS, 55% of cases were related to autoimmune diseases, of which SLE and primary antiphospholipid antibody syndrome (APS) accounted for 38% and about 7%, respectively. Infectious diseases were associated with 33% of cases. Thrombotic and hemorrhagic complications in patients with LAHPS occur mainly in autoimmune disorders or lymphoma, but not in infectious diseases [8]. In a 2015 review paper of 89 patients (age, 1–86 years) with LAHPS, 55% of them were children younger than 16 years of age [2]. In another case series of pediatric SLE with LAHPS, the average age of presentation was 10.2 years, and girls were diagnosed with LAHPS four times more often than boys [9]. Bleeding characteristics of LAHPS patients varied from epistaxis to intracranial hemorrhage. Ecchymosis was the most common symptom at 44%, followed by epistaxis at 35%, hematuria at 15%, and gynecologic bleeding at 14% [2]. Our patient showed diverse bleeding manifestations from the initial phase of SLE development, which consisted of ecchymosis, persistent gingival bleeding, epistaxis, hemorrhagic ovarian cyst, and menorrhagia. We could not determine the presence of anti-factor II Ab because the test was unavailable in our hospital laboratory.
Together with bleeding, typical laboratory findings of LAHPS are prolonged aPTT with or without prolonged PT, positive LA, and deficiency of prothrombin. LAHPS might be caused by an immune complex with anti-prothrombin Ab. Non-neutralized prothrombin Abs can be attached to the inactive site of prothrombin to form antigen-Ab complexes, which are probably rapidly eliminated by the liver. This results in hypoprothrombinemia, eventually leading to bleeding tendencies [10]. Laboratory characteristics of 92 LAHPS patients published by Mulliez et al. [2] in 2015 showed that aPTT and PT were prolonged while thrombin time, fibrinogen, and platelet count were normal in most cases. Prolongation of aPTT and PT is mainly attributable to coagulation factor deficiency or the presence of an inhibitor. When a mixing study with normal pooled plasma was performed, aPTT and PT were normalized in factor-deficient patients. In contrast, aPTT and/or PT remained prolonged in LAHPS. In an analysis of 74 patients with LAHPS presented by Mazodier et al. [8], PT was prolonged by a mean of 1.7-fold in 66% and aPTT was prolonged by a mean of 2-fold in 88%. Factor II was decreased in 85%. Consistent with these findings, our patient showed prolongation of aPTT and PT, which did not return to normal following a mixing study. LA was persistently positive and factor II level remained low. LA might bind to prothrombin and cause it to be removed from circulation. LA is not an inhibitor of prothrombin, but a non-neutralizing Ab [11].
Meanwhile, in the setting of persistently positive aPL Abs, APS is characterized by an increased risk of thrombotic events and pregnancy morbidity [7]. Laboratory criteria for APS include positivity for LA, aCL IgG and/or IgM Ab, or anti-β2GP1 IgG and/or IgM Ab [7]. In our case, LA, aCL IgG/IgM, and anti-β2GPI IgG/IgM were all positive for more than 12 weeks. Since laboratory criteria were satisfied with a tingling sensation at the fingertips in June 2020, it might be possible for the development of vascular thrombosis and/or future pregnancy complications. Intriguingly, her mother who showed positive antinuclear Ab titer had a history of miscarriage. In a study by Tarr et al. [12], SLE patients with positive aPL Ab had a 50% chance of developing APS. Taking into account the high-risk profile of our patient, she should be monitored closely for the development of APS.
Currently, there are no standardized guidelines for the management of LAHPS. Immunosuppressants are used to clear inhibitors and control bleeding [9]. Corticosteroids can normalize PT and factor II levels in most cases. They should be considered as first-line treatment [8]. Other therapies include azathioprine, cyclophosphamide, intravenous Ig, plasma exchange, rituximab, and androgen danazol. Adjuvant treatment includes fresh frozen plasma, packed RBCs, vitamin K, platelet concentrate, prothrombin complex concentrate, and recombinant factor VIIa. However, the effectiveness of these treatments are difficult to evaluate because they have been used in combination and/or only in a small number of patients [8]. Looking at treatment methods of 77 LAHPS from 1960 to February 2014, fresh frozen plasma was used in 31% of cases and packed RBCs were used in 21% of cases as supportive care. In addition, 31% of LAHPS patients were given corticosteroids alone and 29% of patients were treated with corticosteroids in combination with other immunosuppressants [2]. In a review of 54 pediatric LAHPS as of June 2017, 46% of patients had complete resolution of their symptoms and 33% had improved only their clinical symptoms. Although 35% of patients improved spontaneously without treatment, 65% required treatment. Of treated patients, 68% of patients were given steroids (high-dose glucocorticoids and/or intravenous pulse methylprednisolone) and 26% were treated with immunosuppressive therapy. However, 4% of patients did not respond to treatment [13]. A collection of pediatric SLE-related LAHPS cases from the 1960s to 2021 demonstrated that a 13-year-old male patient with persistent bleeding after tooth extraction in 1984 improved with oral steroid and azathioprine treatment and a 12-year-old female patient in 2007 with epistaxis and menorrhagia improved with cyclophophamide, azathioprine, and steroid pulse therapy [9]. Looking at a total of 46 patients included in the analysis of this paper [9], mild to severe bleeding symptoms such as epistaxis, gingival bleeding, and intracerebral bleeding were reported, however, there was no reported case with a hemorrhagic ovarian cyst. Prior to the bleeding event, our patient was diagnosed with SLE nephritis. She was taking deflazacort, MMF, enalapril, hydroxychloroquine, and calcitriol. When the patient had abdominal pain, we suspected a flaring manifestation of SLE. Thus, we increased steroids and added tacrolimus. After 3 months, the patient complained of persistent menorrhagia and dizziness with hemoglobin level of 7.4 g/dL. RBC transfusion was performed and oral contraceptives were taken for 3 months to control menorrhagia. Recurrent bleeding symptoms, prolongation of PT and aPTT, low prothrombin level, and LA positivity could lead to the diagnosis of LAHPS associated with SLE in our patient. After being diagnosed with LAHPS, the patient took MMF and tacrolimus with increased doses. When proteinuria and clinical symptoms worsened, fresh frozen plasma transfusion and intravenous cyclophosphamide pulse therapy were administered. Since then, her symptoms have improved somewhat. She is currently taking cyclosporine, prednisolone, hydroxychloroquine, and enalapril. Hydroxychloroquine and low-dose aspirin are recommended for the prevention of blood clots in patients with aPL Ab-positive with SLE [14]. However, in our case, aPTT and PT are increased and recurrent bleeding events take place. Therefore, low-dose aspirin cannot be used at present.
To the best of our knowledge, this is the first case report of hemorrhagic ovarian cyst in a girl with SLE-related LAHPS. Additional research is needed as thrombosis and hemorrhagic tendencies may coexist when LAHPS is diagnosed in SLE patients.
Notes
Ethical statements
This report was approved by the Institutional Review Board (IRB) of Korea University Ansan Hospital before initiation. Informed consent was obtained from the patient at the request of IRB, although this was a retrospective chart review study involved no more than minimal risk (IRB No. 2022AS0098).
References
1. Chung CH, Park CY. Lupus anticoagulant-hypoprothrombinemia in healthy adult. Korean J Intern Med. 2008; 23:149–51.

2. Mulliez SM, De Keyser F, Verbist C, Vantilborgh A, Wijns W, Beukinga I, et al. Lupus anticoagulant-hypoprothrombinemia syndrome: report of two cases and review of the literature. Lupus. 2015; 24:736–45.

3. Atsumi T, Koike T. Clinical relevance of antiprothrombin antibodies. Autoimmun Rev. 2002; 1:49–53.

4. Montes de Oca MA, Babron MC, Bletry O, Broyer M, Courtecuisse V, Fontaine JL, et al. Thrombosis in systemic lupus erythematosus: a French collaborative study. Arch Dis Child. 1991; 66:713–7.

5. Rapaport SI, Ames SB, Duvall BJ. A plasma coagulation defect in systemic lupus erythematosus arising from hypoprothrombinemia combined with antiprothrombinase activity. Blood. 1960; 15:212–27.

6. Hoffman M, Meng ZH, Roberts HR, Monroe DM. Rethinking the coagulation cascade. Jpn J Thromb Hemost. 2005; 16:70–81.

7. Lackner KJ, Muller-Calleja N. Pathogenesis of the antiphospholipid syndrome revisited: time to challenge the dogma. J Thromb Haemost. 2016; 14:1117–20.

8. Mazodier K, Arnaud L, Mathian A, Costedoat-Chalumeau N, Haroche J, Frances C, et al. Lupus anticoagulant-hypoprothrombinemia syndrome: report of 8 cases and review of the literature. Medicine (Baltimore). 2012; 91:251–60.
9. Kocheril AP, Vettiyil GI, George AS, Shah S, Geevar T, Dave RG, et al. Pediatric systemic lupus erythematosus with lupus anticoagulant hypoprothrombinemia syndrome: a case series with review of literature. Lupus. 2021; 30:641–8.

10. Bajaj SP, Rapaport SI, Fierer DS, Herbst KD, Schwartz DB. A mechanism for the hypoprothrombinemia of the acquired hypoprothrombinemia-lupus anticoagulant syndrome. Blood. 1983; 61:684–92.

11. Laboratory Corporation of America Holdings (LabCorp). Lupus anticoagulants [Internet]. LabCorp; 2024 [cited 2024 Feb 16]. Available from: https://www.labcorp.com/resource/lupus-anticoagulants/.
12. Tarr T, Lakos G, Bhattoa HP, Soltesz P, Shoenfeld Y, Szegedi G, et al. Clinical thrombotic manifestations in SLE patients with and without antiphospholipid antibodies: a 5-year follow-up. Clin Rev Allergy Immunol. 2007; 32:131–7.

Fig. 1.
Abdominal ultrasonography and pelvic computed tomography. (A) A 4.9×4.1×5 cm heterogeneous mass (arrow) in the uterus on abdominal ultrasonography. (B) A hemorrhagic cyst (arrow) in the right ovary on pelvic computed tomography. (C) After 3 months, the size of right ovarian cystic lesion (arrow) is reduced on abdominal ultrasonography.
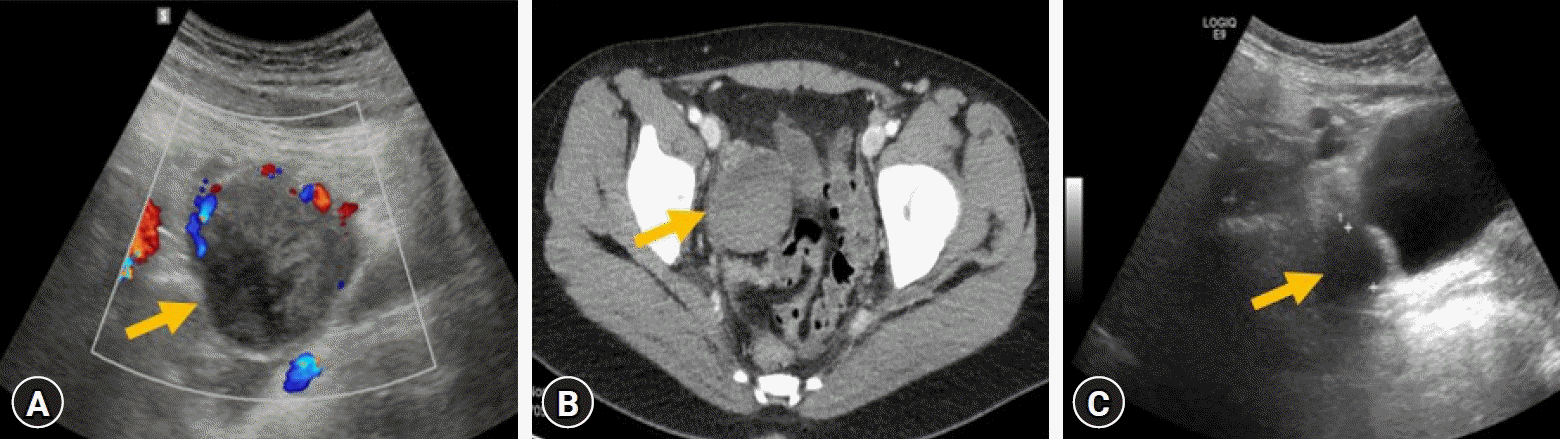
Fig. 2.
Second kidney biopsy findings on October 2020. (A) Immunofluorescence images showing a full house pattern in the mesangial region and capillary walls. (B-D) Segmental endocapillary hypercellularity (red arrow) and double contouring with subendothelial deposits in the glomerular capillary walls (blue arrow). (E-G) Electron microscopic findings with the presence of subendothelial and subepithelial deposits and foot process effacement (red arrow). B, C: Hematoxylin and eosin stain, ×400; D: Masson-Trichrome stain, ×400; E: ×2,000; F: ×5,000; G: ×10,000.
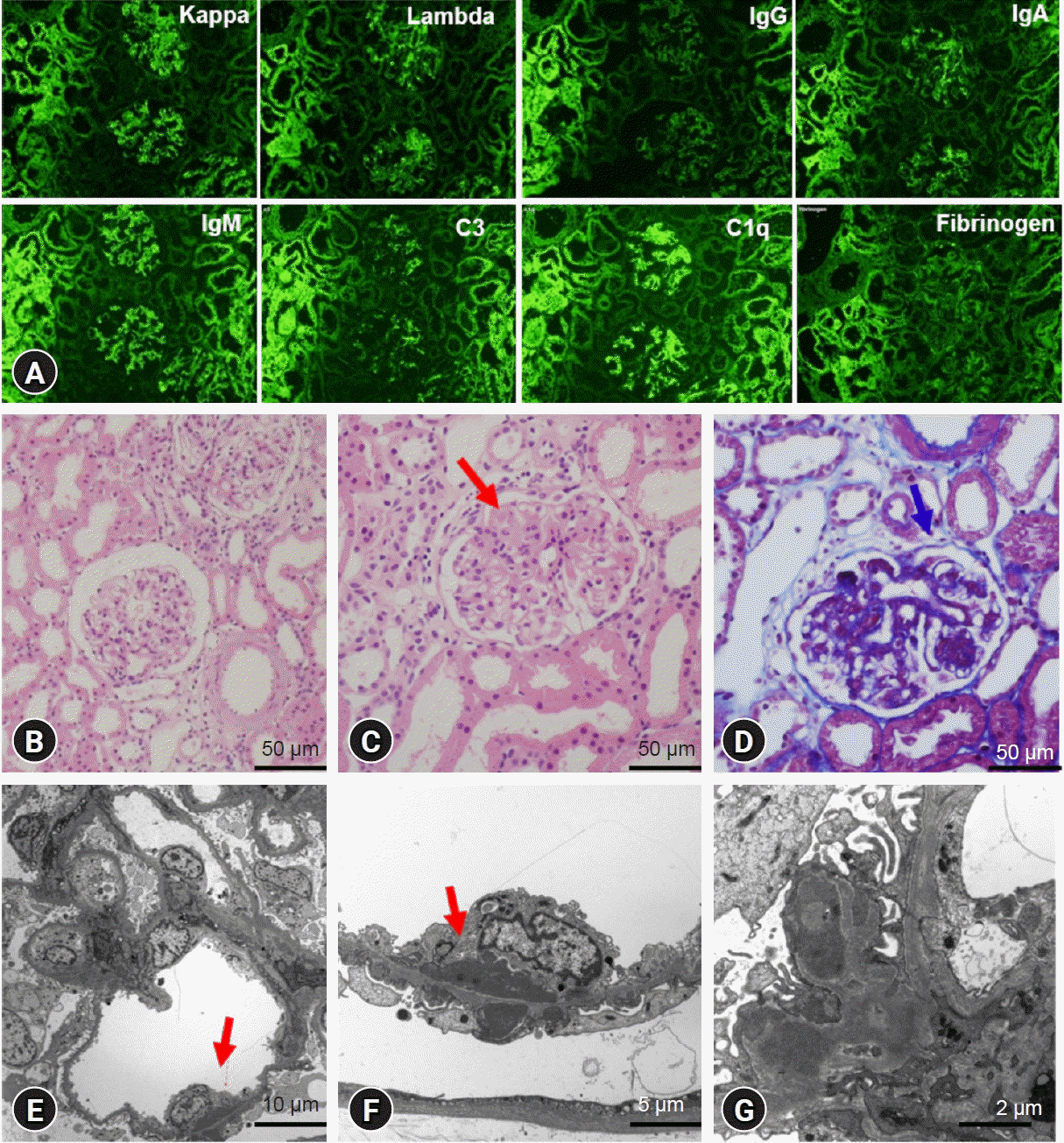
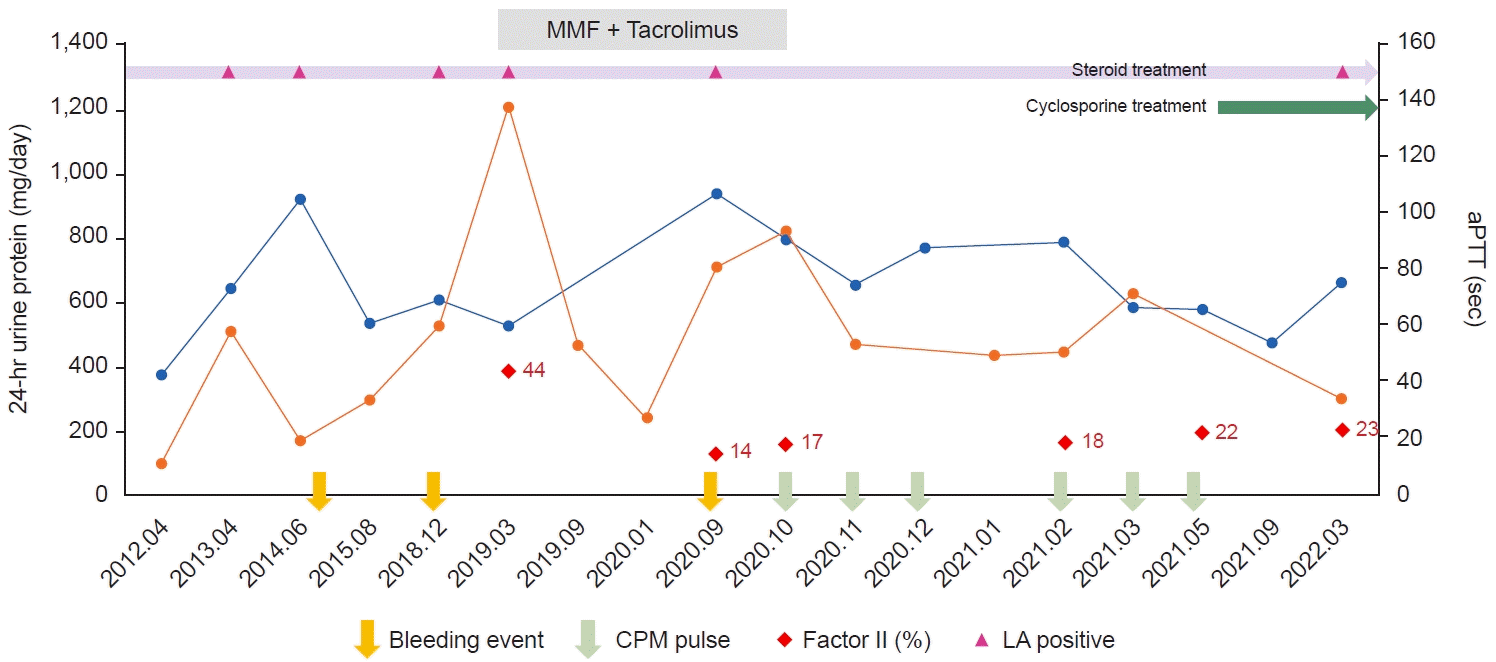
Fig. 3.
Clinical courses of the patient. Proteinuria (orange line), aPTT (blue line), factor II levels, the presence of LA, and bleeding episodes of the patient from April 2012 to March 2022. Epistaxis on July 2014 and September 2020, a hemorrhagic ovarian cyst on December 2018, and menorrhagia from December 2018.12 to April 2019. MMF, mycophenolate mofetil; aPTT, activated partial thromboplastin time; CPM, cyclophosphamide; LA, lupus anticoagulant.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download