1. Abe K, Yamaguchi T, Hori H, Sumi M, Horisawa S, Taira T, et al. Magnetic resonance-guided focused ultrasound for mesial temporal lobe epilepsy: a case report. BMC Neurol. 20:160. 2020.

2. Barbaro NM, Quigg M, Broshek DK, Ward MM, Lamborn KR, Laxer KD, et al. A multicenter, prospective pilot study of gamma knife radiosurgery for mesial temporal lobe epilepsy: seizure response, adverse events, and verbal memory. Ann Neurol. 65:167–175. 2009.

3. Barbaro NM, Quigg M, Ward MM, Chang EF, Broshek DK, Langfitt JT, et al. Radiosurgery versus open surgery for mesial temporal lobe epilepsy: the randomized, controlled ROSE trial. Epilepsia. 59:1198–1207. 2018.

4. Bartolomei F, Hayashi M, Tamura M, Rey M, Fischer C, Chauvel P, et al. Long-term efficacy of gamma knife radiosurgery in mesial temporal lobe epilepsy. Neurology. 70:1658–1663. 2008.

5. Chen N, Du SQ, Yan N, Liu C, Zhang JG, Ge Y, et al. Delayed complications after gamma knife surgery for intractable epilepsy. J Clin Neurosci. 21:1525–1528. 2014.

6. Cmelak AJ, Abou-Khalil B, Konrad PE, Duggan D, Maciunas RJ. Low-dose stereotactic radiosurgery is inadequate for medically intractable mesial temporal lobe epilepsy: a case report. Seizure. 10:442–446. 2001.

7. Cutsforth-Gregory JK, Lanzino G, Link MJ, Brown RD Jr, Flemming KD. Characterization of radiation-induced cavernous malformations and comparison with a nonradiation cavernous malformation cohort. J Neurosurg. 122:1214–1222. 2015.

8. Gross BA, Lin N, Du R, Day AL. The natural history of intracranial cavernous malformations. Neurosurg Focus. 30:E24. 2011.

9. Kang JY, Wu C, Tracy J, Lorenzo M, Evans J, Nei M, et al. Laser interstitial thermal therapy for medically intractable mesial temporal lobe epilepsy. Epilepsia. 57:325–334. 2016.

10. Kawai K, Suzuki I, Kurita H, Shin M, Arai N, Kirino T. Failure of low-dose radiosurgery to control temporal lobe epilepsy. J Neurosurg. 95:883–887. 2001.

11. Kawamura T, Onishi H, Kohda Y, Hirose G. Serious adverse effects of gamma knife radiosurgery for mesial temporal lobe epilepsy. Neurol Med Chir (Tokyo). 52:892–898. 2012.

12. Kim MJ, Chang KW, Park SH, Chang WS, Chang JH, Chang JW, et al. Predictive factors of radiation-induced changes following single-session gamma knife radiosurgery for arteriovenous malformations. J Clin Med. 10:2186. 2021.

13. Koike T, Yanagimachi N, Ishiguro H, Yabe H, Yabe M, Morimoto T, et al. High incidence of radiation-induced cavernous hemangioma in longterm survivors who underwent hematopoietic stem cell transplantation with radiation therapy during childhood or adolescence. Biol Blood Marrow Transplant. 18:1090–1098. 2012.

14. Lee EM, Kang JK, Kim SJ, Hong SH, Ko TS, Lee SA, et al. Gamma knife radiosurgery for recurrent or residual seizures after anterior temporal lobectomy in mesial temporal lobe epilepsy patients with hippocampal sclerosis: long-term follow-up results of more than 4 years. J Neurosurg. 123:1375–1382. 2015.

15. Lew SM, Morgan JN, Psaty E, Lefton DR, Allen JC, Abbott R. Cumulative incidence of radiation-induced cavernomas in long-term survivors of medulloblastoma. J Neurosurg. 104(2 Suppl):103–107. 2006.

16. McGonigal A, Sahgal A, De Salles A, Hayashi M, Levivier M, Ma L, et al. Radiosurgery for epilepsy: systematic review and International Stereotactic Radiosurgery Society (ISRS) practice guideline. Epilepsy Res. 137:123–131. 2017.

17. Nimjee SM, Powers CJ, Bulsara KR. Review of the literature on de novo formation of cavernous malformations of the central nervous system after radiation therapy. Neurosurg Focus. 21:e4. 2006.

18. Patet G, Bartoli A, Meling TR. Natural history and treatment options of radiation-induced brain cavernomas: a systematic review. Neurosurg Rev. 45:243–251. 2022.

19. Régis J, Rey M, Bartolomei F, Vladyka V, Liscak R, Schröttner O, et al. Gamma knife surgery in mesial temporal lobe epilepsy: a prospective multicenter study. Epilepsia. 45:504–515. 2004.

20. Srikijvilaikul T, Najm I, Foldvary-Schaefer N, Lineweaver T, Suh JH, Bingaman WE. Failure of gamma knife radiosurgery for mesial temporal lobe epilepsy: report of five cases. Neurosurgery. 54:1395–1402. discussion 1402-1404. 2004.

21. Trifiletti DM, Redmond KJ, Kim MM, Soltys SG, Milano MT, HattangadiGluth JA. Novel applications of stereotactic radiosurgery beyond oncology: prospective trials in functional radiosurgery. Int J Radiat Oncol Biol Phys. 115:4–6. 2023.

22. Vojtěch Z, Malíková H, Syrůček M, Krámská L, Šroubek J, Vladyka V, et al. Morphological changes after radiosurgery for mesial temporal lobe epilepsy. Acta Neurochir (Wien). 157:1783–1791. discussion 1791-1792. 2015.

23. Vojtech Z, Vladyka V, Kalina M, Nespor E, Seltenreichová K, Semnická J, et al. The use of radiosurgery for the treatment of mesial temporal lobe epilepsy and long-term results. Epilepsia. 50:2061–2071. 2009.

24. Winkler EA, Rutledge C, Ward M, Tihan T, Sneed PK, Barbaro N, et al. Radiation-induced cavernous malformation as a late sequelae of stereotactic radiosurgery for epilepsy. Cureus. 10:e2308. 2018.

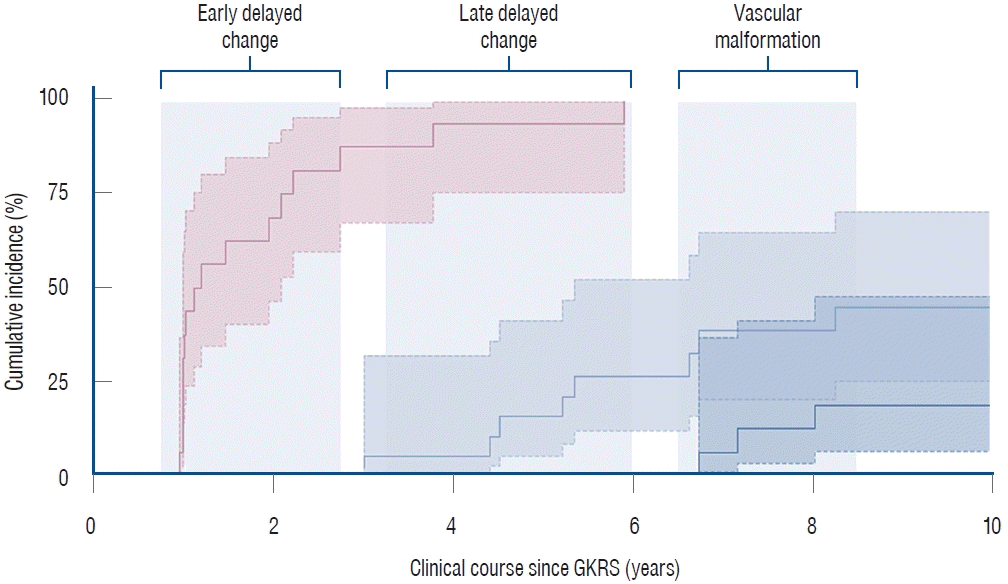
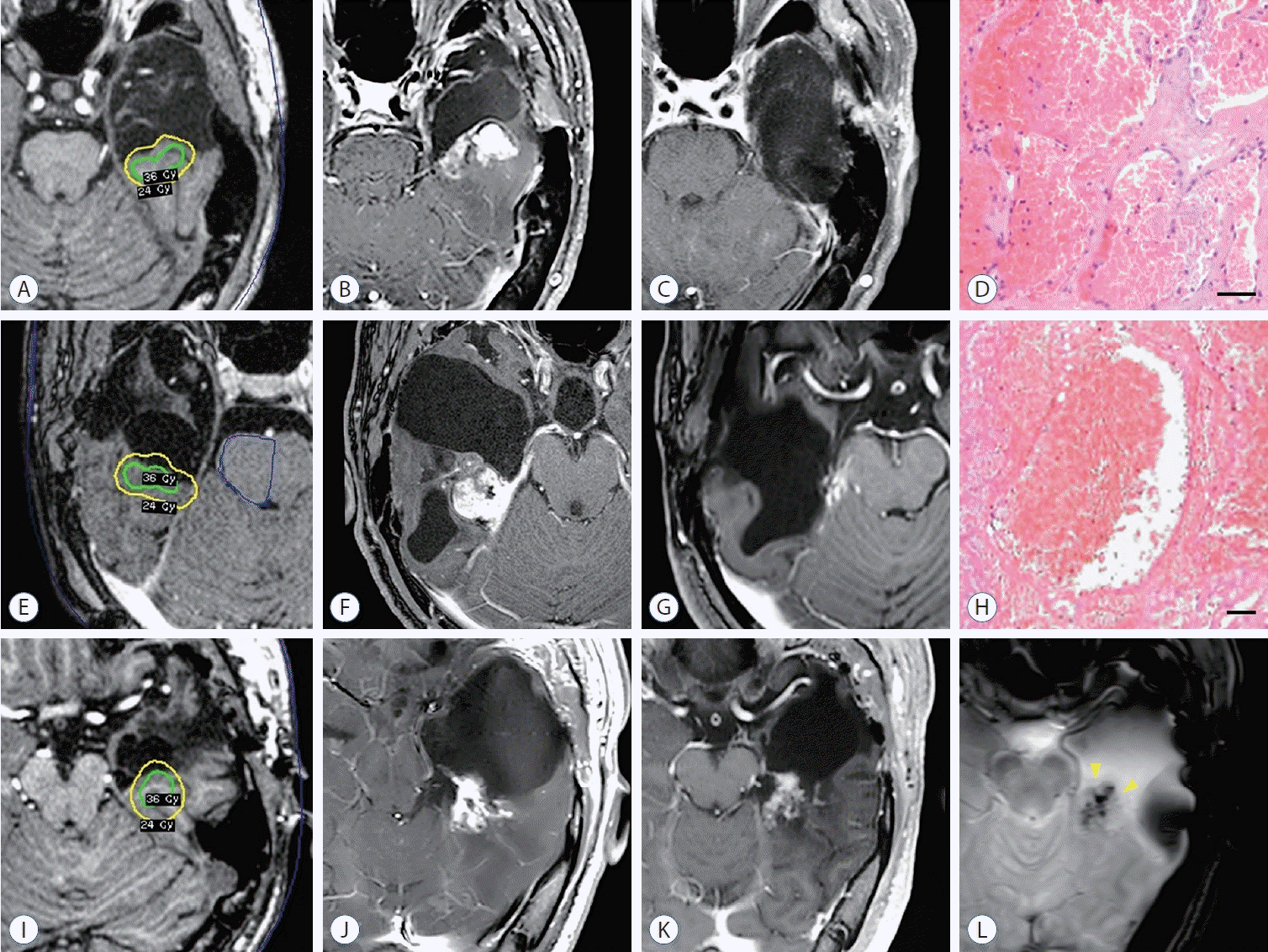




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download