1. Hinojosa-Gonzalez DE, Ferrigno AS, Martinez HR, Farias JS, Caro-Osorio E, Figueroa-Sanchez JA. Aneurysms of the lenticulostriate artery: a systematic review. World Neurosurg. 2021; 145:471–479.e10.

2. Narayan P, Workman MJ, Barrow DL. Surgical treatment of a lenticulostriate artery aneurysm. Case report. J Neurosurg. 2004; 100:340–342.
3. Fisher CM, Kistler JP, Davis JM. Relation of cerebral vasospasm to subarachnoid hemorrhage visualized by computerized tomographic scanning. Neurosurgery. 1980; 6:1–9.

4. Vargas J, Walsh K, Turner R, Chaudry I, Turk A, Spiotta A. Lenticulostriate aneurysms: a case series and review of the literature. J Neurointerv Surg. 2015; 7:194–201.

5. Samaniego EA, Roa JA, Hasan D. Vessel wall imaging in intracranial aneurysms. J Neurointerv Surg. 2019; 11:1105–1112.

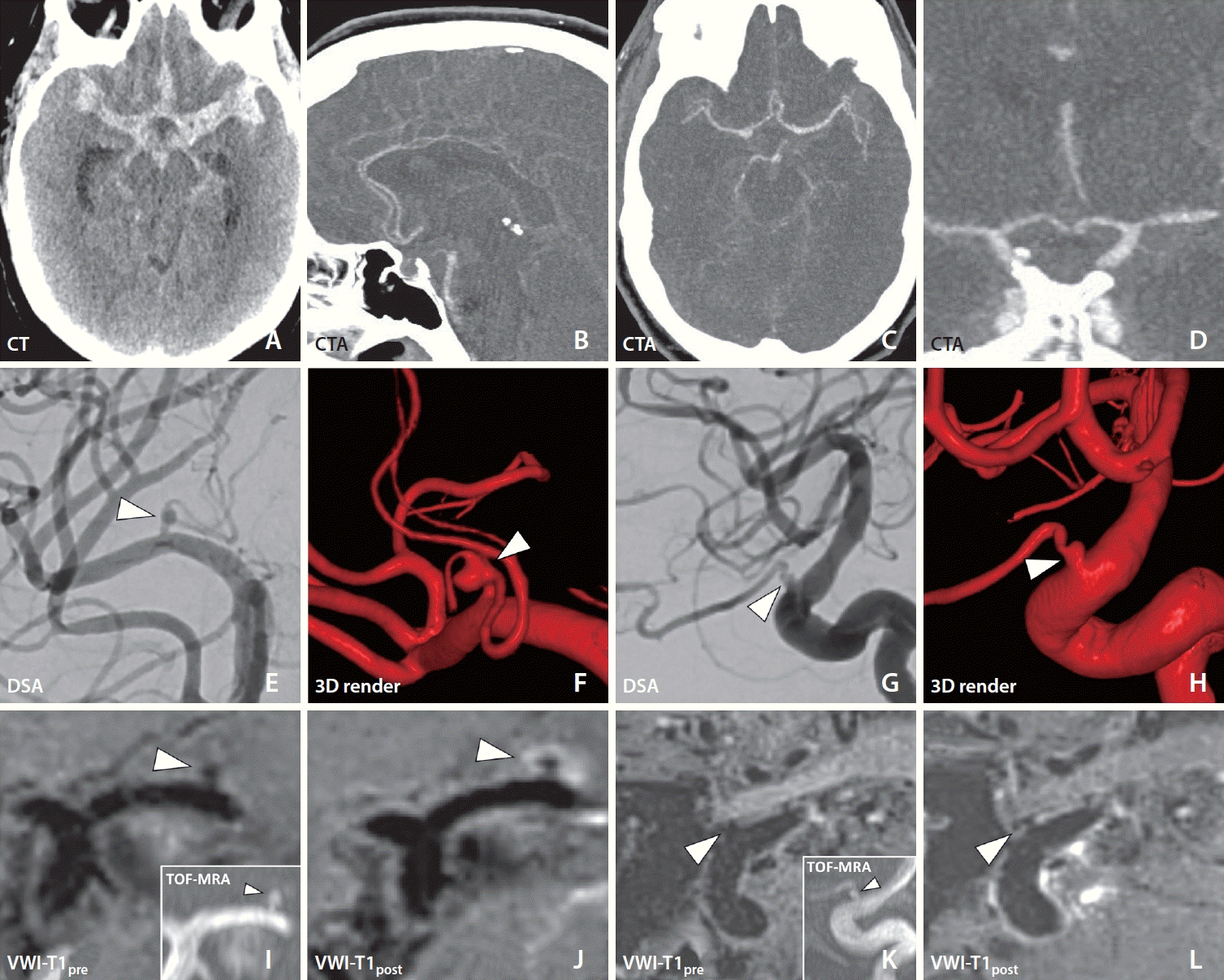
6. Cox M, Song JW, Nabavizadeh SA, Kung D, Loevner L, Choudhri O. Detection of angiographically occult ruptured basilar sidewall perforator aneurysm by vessel wall MR imaging. Neurohospitalist. 2021; 11:156–159.

7. Yoon W, Kim JH, Roh H, Kwon TH. Arterial wall imaging in angiographically occult spontaneous subarachnoid hemorrhage: new insight into the usual suspect. J Korean Neurosurg Soc. 2022; 65:245–254.

8. Jung HN, Suh SI, Ryoo I, Kim I. Usefulness of 3D high-resolution vessel wall MRI in diffuse nonaneurysmal SAH patients. Clin Neuroradiol. 2021; 31:1071–1081.

9. Hoh BL, Ko NU, Amin-Hanjani S, Chou SHY, Cruz-Flores S, Dangayach NS, et al. 2023 Guideline for the management of patients with aneurysmal subarachnoid hemorrhage: a guideline from the American Heart Association/American Stroke Association. Stroke. 2023; 54:e314–e370.

10. Heit JJ, Pastena GT, Nogueira RG, Yoo AJ, Leslie-Mazwi TM, Hirsch JA, et al. Cerebral angiography for evaluation of patients with CT angiogram-negative subarachnoid hemorrhage: an 11-year experience. AJNR Am J Neuroradiol. 2016; 37:297–304.

11. Ishihara H, Kato S, Akimura T, Suehiro E, Oku T, Suzuki M. Angiogram-negative subarachnoid hemorrhage in the era of three dimensional rotational angiography. J Clin Neurosci. 2007; 14:252–255.

12. Nguyen I, Caton MT, Tonetti D, Abla A, Kim A, Smith W, et al. Angiographically occult subarachnoid hemorrhage: yield of repeat angiography, influence of initial CT bleed pattern, and sources of diagnostic error in 242 consecutive patients. AJNR Am J Neuroradiol. 2022; 43:731–735.

13. Buscot MJ, Chandra RV, Maingard J, Nichols L, Blizzard L, Stirling C, et al. Association of onset-to-treatment time with discharge destination, mortality, and complications among patients with aneurysmal subarachnoid hemorrhage. JAMA Netw Open. 2022; 5:e2144039.

14. Santarosa C, Cord B, Koo A, Bhogal P, Malhotra A, Payabvash S, et al. Vessel wall magnetic resonance imaging in intracranial aneurysms: principles and emerging clinical applications. Interv Neuroradiol. 2020; 26:135–146.

15. Matouk CC, Mandell DM, Günel M, Bulsara KR, Malhotra A, Hebert R, et al. Vessel wall magnetic resonance imaging identifies the site of rupture in patients with multiple intracranial aneurysms: proof of principle. Neurosurgery. 2013; 72:492–496. discussion 496.

16. Yoshikawa K, Moroi J, Kokubun K, Furuya N, Yoshida Y, Kinoshita T, et al. Role of magnetic resonance vessel wall imaging in detecting and managing ruptured aneurysms among multiple intracranial aneurysms. Surg Neurol Int. 2021; 12:460.





 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download