INTRODUCTION
With the advent of the detachable balloon, transarterial endovascular surgery became the mainstay treatment of direct carotid-cavernous fistula (dCCF) since 1980 [
1]. Following the unavailability of the detachable balloon in the market, coils have been replaced, and transarterial and/or transvenous coil embolization has become the standard treatment of dCCF [
2]. Despite the high efficacy of coiling for fistulae occlusion, the preservation of the internal carotid artery (ICA) and its reconstruction are not usually possible and can lead to complete ICA occlusion [
3].
Alternately, new methods have been applied for better occlusion of the fistula with preservation of the ICA, such as stent-assisted coiling, balloon-assisted coiling, embolization with liquid embolic agents like Onyx, flow-diverters, and covered stents [
2]. Over the last 2 decades, covered stents have emerged as a novel method to reconstruct ICA walls as well as the occlusion of the CCF [
4]. In the current study, we present initial and long-term angiographic outcomes of dCCF treatment with the BeGraft-covered stent (Bentley InnoMed GmbH) to evaluate its efficacy and safety.
CASE SERIES
Endovascular Procedure
All patients received a diagnostic 4-vessel digital subtraction angiography (DSA). All procedures were performed under general anesthesia using the Siemens Artis Zee floor system (Siemens). The 6F long sheath or 6F guiding catheter was placed into the affected ICA. Subsequently, a microwire was navigated into the ipsilateral middle cerebral artery. The BeGraft was advanced over the microwire into the desired location in the ICA across the fistula. The stent was deployed with staged balloon inflation to achieve a suitable apposition between the stent and the vessel wall. If endoleak occurred, 1 or multiple balloon inflations were performed by applying a higher bursting pressure or using a larger balloon. If the endoleak remained despite re-dilatation, the leak was closed by another endovascular approach.
Antiplatelet Regimen
For all patients, after the insertion of a 6 Fr sheath, 3,000 IU of heparin was administered, followed by an infusion of 1,000 IU of heparin every hour into the procedure. Patients received a loading dose of dual antiplatelet therapy (DAPT), including 325 mg acetylsalicylic acid (ASA) and 180 mg ticagrelor, before anesthesia induction. Immediately after stent delivery, 180 µg/kg eptifibatide was injected intravenously, and the same dose was repeated after an interval of 10 minutes. After the procedure, an infusion of eptifibatide (2 µg/kg/min, maximum dose of 7.5 mg/h) was administrated and continued for 4 to 6 hours. DAPT maintenance was started 4 to 6 hours after the procedure, including 90 mg ticagrelor twice a day plus 80 mg ASA daily. DAPT was continued for 3 to 6 months and then was converted to single antiplatelet therapy, including ASA 160 mg daily, for another 6 to 9 months.
Patient Population
The database of our center was reviewed, and the details of other treatment strategies, such as embolization with coils and embolic agents, were conveyed to patients and their relatives, and patients made the final treatment decision. This study was approved by the IRB of the center and informed consent was obtained from all patients before the operation. The demographics of patients, their clinical presentation, the procedural techniques, pre- and post-procedural complications, immediate angiographic outcomes, and post-procedural clinical and imaging outcomes were collected.
The study covered 4 patients, including 1 female and 3 males, with a median age of 38.5 years (interquartile range [IQR], 26–56). The median duration of symptoms before diagnosis was 5 months (IQR, 3.25–19.25). All dCCFs were traumatic and caused by motor vehicular accidents in 3 patients (75%) and falling down in 1 patient (25%). On admission, all patients were alert without any neurological deficits. They prominently presented with ocular symptoms, including proptosis and chemosis. One had decreased visual acuity. In all patients, only the left eye was involved. One patient, 26 years old, was misdiagnosed and had 2 craniotomy procedures before being referred to our center.
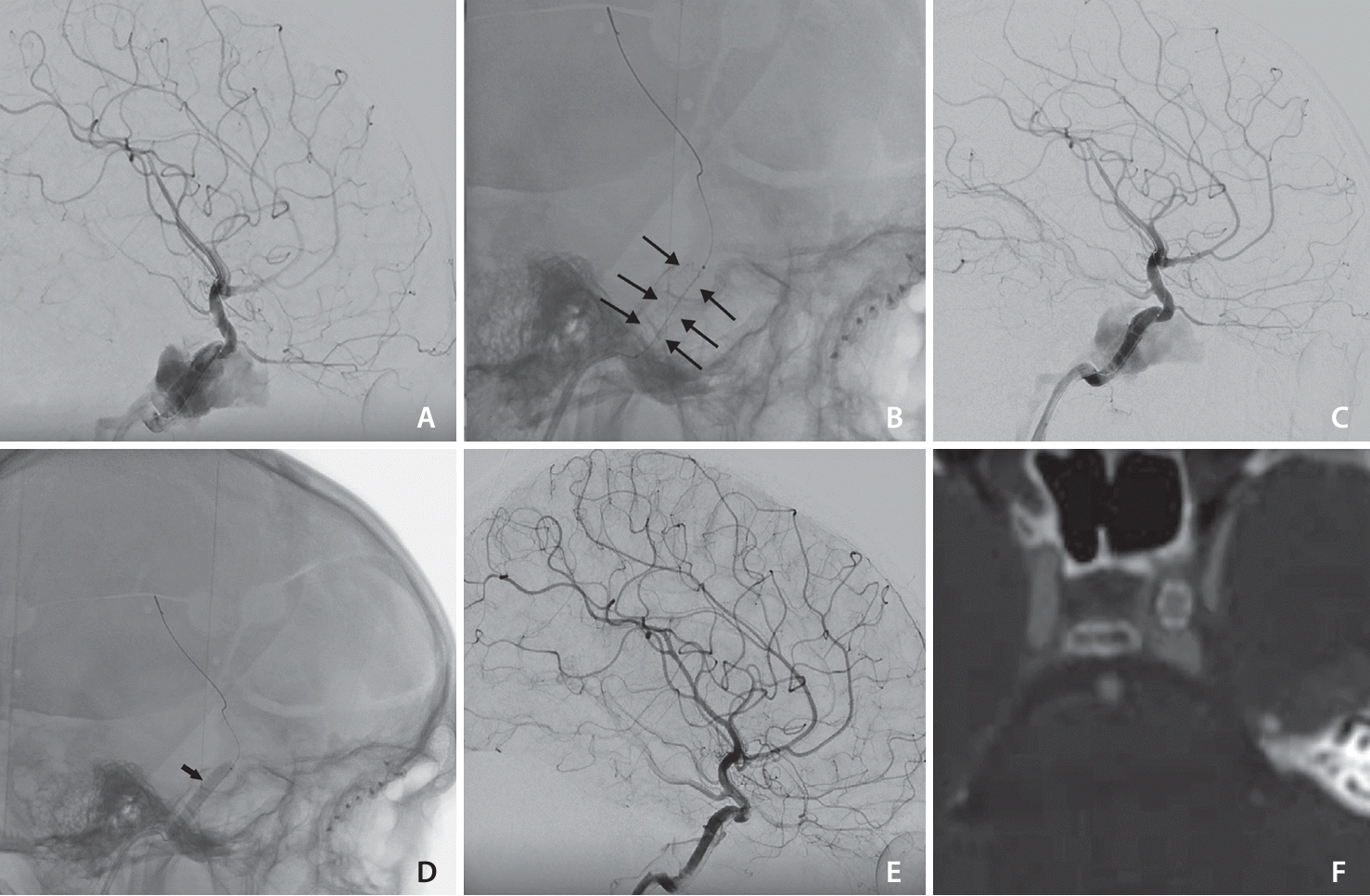
In all 4 patients, the BeGraft-covered stent was deployed in the cavernous part of the ICA across the fistulous hole covering the fistulae without any complications. With stent deployment, the fistula was completely obliterated in 1 patient immediately. Three patients needed re-dilatation using 1 or more balloon inflations because of endoleak after stent deployment. The re-dilatation stopped endoleak in 2 patients with complete occlusion of the dCCF (
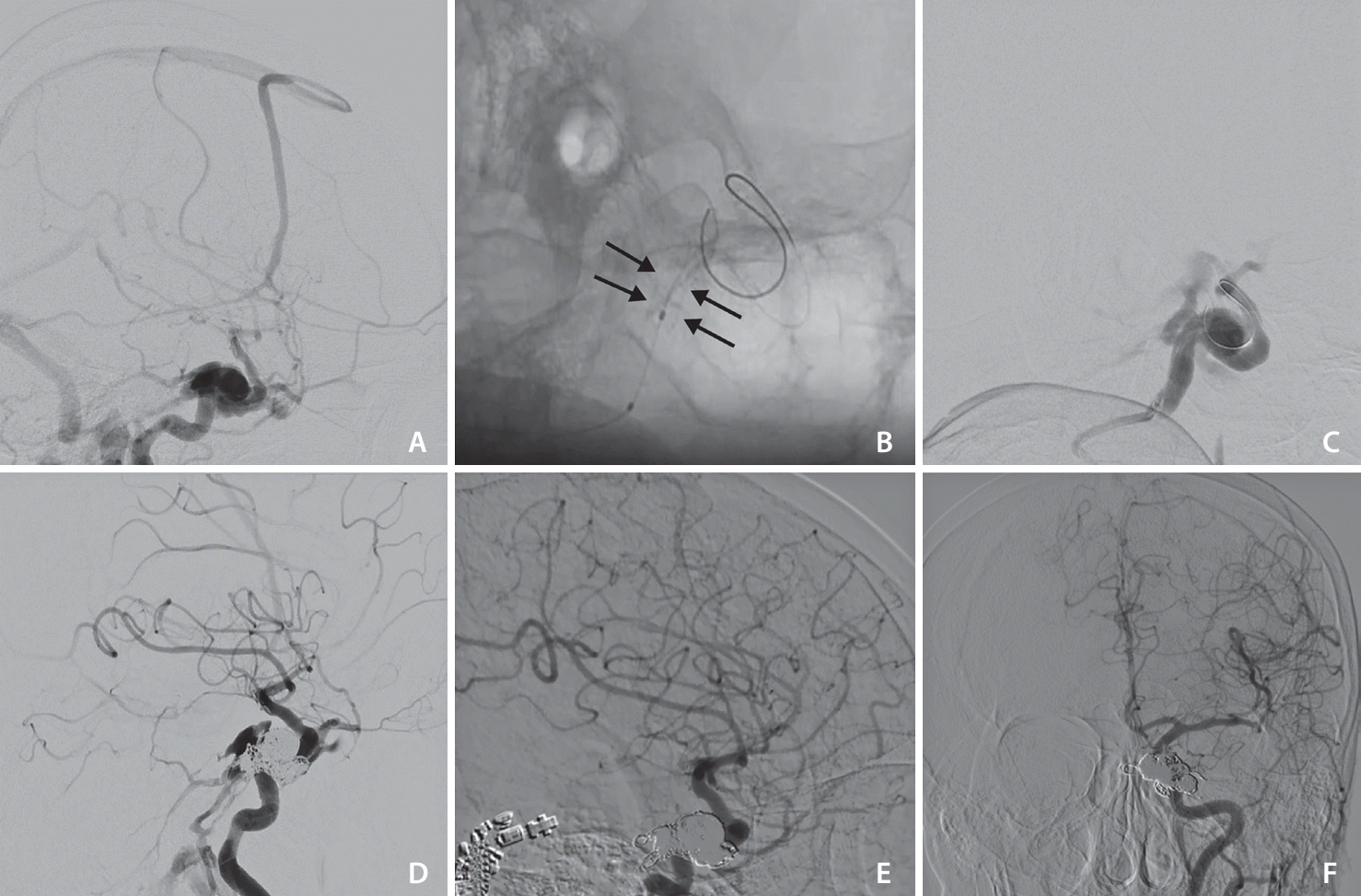
Fig. 1). In 1 patient, the endoleak persisted despite multiple balloon inflation. Then, the venous pouch was catheterized through a transvenous approach and the venous pouch was closed by coiling. At the end of the procedure, the fistula was completely obliterated (
Fig. 2). There were no thromboembolic complications during or after the operation.
All patients’ ocular symptoms were completely improved within 1 to 2 weeks after the operation. Patients were clinically followed for a median of 21 months, with a range between 4 and 30 months. One patient died after 4 months because of myocardiac infarction. During follow-up, the patients were stable without any sign of ocular symptom recurrence or any thromboembolic events. Three patients had radiological follow-ups, 1 with DSA and 2 others with computed tomography angiographies 18 to 24 months after the procedure. All showed complete exclusion of CCF with resolution of the large venous pouch and prominent superior ophthalmic veins. There was no stenosis in the stent or ICA in follow-up imaging.
DISCUSSION
To our knowledge, this is the first study to focus on treating patients with dCCF using a BeGraft-covered stent, a single-layer polytetrafluoroethylene (PTFE) covered stent. We presented 4 cases successfully treated with the BeGraft-covered stent as a primary. The immediate occlusion was 75%. One patient required transvenous coiling to secure complete occlusion of the fistula. There were no complications during or after treatment. Follow-up imaging confirmed complete occlusion of dCCFs with preservation of the ICA.
With successful application in cardiac intervention, several neurointerventionists have utilized covered stents for the treatment of different intracranial vascular diseases, such as pseudoaneurysm, blister aneurysms, dissection, and CCF [
5]. During the last 2 decades, several authors applied a covered stent for occlusion of dCCFs. The immediate occlusion rate of dCCFs has been reported to be between 50% and 91% after deployment of a covered stent without additional devices, such as coils. In follow-up, the occlusion rate of dCCFs increased to 70–100% [
6-
13]. Ocular symptoms were improved in 100% of patients with a full recovery rate of 86–100% [
6-
9,
12]. While covered stents could achieve occlusion rates comparable to other endovascular techniques, such as detachable balloons [
6], coiling, and liquid embolic agents, they may have several advantages over other approaches. Through reconstruction of the ICA and closure of the fistulous site, covered stents could provide a proper anatomical cure of dCCFs without risk of coil protrusion into the ICA and ICA closure and without risk of mass effect on cranial nerves in the cavernous sinus [
12]. The use of a covered stent and covering branches of the ICA are considered safe in its cavernous part. However, the intradural use of covered stents is limited by several factors including the difficulty of navigation, the limited size of stents, side branch occlusion, and their thrombogenicity [
14].
Different covered stents have been used for diverse intracranial lesions, such as pseudoaneurysms, aneurysms, and dCCFs, during past decades (
Table 1) [
7,
11,
12,
15-
18]. They are different according to their design (sandwich vs. single-layer) and composition of the covered membrane (PTFE vs. polyurethane [PU]). The Graftmaster (Jostent; Abbott Vascular) and Symbiot stent (Boston Scientific/Scimed) were among the earliest covered stents used for the treatment of dCCFs. The Graftmaster was a sandwich-type covered stent, including 2 stainless steel balloon-expandable stents enveloping the membrane layer of PTFE [
4]. The stiffness of this sandwich-type covered stent resulted in navigation failure in some of the patients [
8,
10,
13]. In their study, Gomez et al. [
10] failed to navigate the Graftmaster stent in the carotid siphon in 1 of their 7 patients because of the rigidity of the system. However, Jeong et al. [
17] and Li et al. [
11] have both reported successful placement of this covered stent in retrospective series of 10 and 19 patients, respectively.
To solve the rigidity of sandwich-type covered stents, single-layer covered stents have been developed to improve their navigation ability [
12,
18]. Single-layer covered stents made of a thin membrane sleeve (PTFE or PU) cover either the interior surface or the outer surface of a 3D bare scaffold stent [
4]. Among them, there are the Willis-covered stent (MicroPort), PK papyrus-covered stent (Biotronik Inc.), and BeGraft stent [
12,
18]. The Willis-covered stent, a cobalt-chromium stent with an external expandable PTFE layer, approved in China in 2013, was the first marketed covered stent designed for intracranial use [
4]. Several studies reported the treatment of dCCFs using Willis-covered stents with a 90–100% successful rate of deployment [
7,
12]. Wang et al. [
12] showed 100% successful implantation of 44 Willis-covered stents for the treatment of 27 dCCFs. In a recent study of 10 patients with dCCFs, Liu et al. [
7] could not implant the Willis-covered stent in 1 of these patients because of the tortuous ICA. As the Willis-covered stent was not available in the USA and European markets, other single-layer covered stents have been used for the treatment of dCCFs [
18]. The PK papyrus is an Food and Drug Administration-approved balloon mounted coronary covered stent with PU membrane covering a cobalt-chromium stent [
19]. The reports by Wroe et al. [
18] on 2 patients with dCCFs showed 100% successful deployment, 50% immediate complete occlusion, and full recovery of clinical symptoms without any complications. In our study, we could successfully deploy all 4 BeGraft stents for the treatment of 4 patients with dCCFs. The BeGraft stent is a single-layer covered stent, made of a cobalt-chromium, open-cell platform covered with a single-layer of a PTFE membrane (thickness of 89±25 micrometers), which is clamped at the proximal and distal site of the stent. The single-layer design of BeGraft results in a crossing profile between 1.1 and 1.4 mm with guiding catheter compatibility of 5 Fr for all sizes of its coronary stents [
19].
In addition to technical and navigation issues, there are several concerns about complications following covered stents, including endoleak, in-stent acute and late thrombosis, ICA occlusion, and in-stent stenosis. Following deployment of a covered stent, endoleak, defined as remaining fistulous flow, was reported in 20% to 50% of cases by several studies [
6,
12,
13,
18]. It is usually seen after stent deployment during operation. The main reason for endoleak could be the mismatch between the stent size and ICA diameter [
12]. Different strategies were described for managing endoleak. Commonly, the re-dilatation of the stent by balloon inflation is enough to close the gap between the stent and ICA in most of cases [
12,
13]. Another strategy is the implantation of another covered stent in the proximal or distal part of the first stent [
12,
18]. Some authors used transvenous or transarterial coiling of the venous pouch for stopping endoleak and complete closure of the fistula [
12,
17]. If endoleak is insignificant, it could be managed by observation and usually resolved spontaneously [
6]. Endoleak rarely occurs in the days following the operation. The resolution of the spasm after the procedure may lead to a gap between the stent and ICA resulting in endoleak [
12,
17]. Late endoleak can be revealed by the recurrence of ocular symptoms and can be treated by the same strategies as procedural endoleak [
12,
17].
Thromboembolic events are another important complication of intracranial-covered stent implantation, which could happen during or after the operation. Acute thrombosis has been infrequently reported among patients with dCCF treated by covered stents [
12,
17]. In a series of 15 patients, Jeong et al. [
17] reported 2 acute thromboembolic events after deployment of covered stents. Wang et al. [
12] also had 1 case of acute in-stent thrombosis following delivery of the 2nd stent for treatment of endoleak leading to asymptomatic ICA occlusion. The risk of thrombosis can increase by using multiple overlapping stents and covering long segments of the ICA [
12]. To prevent thromboembolic events, DAPT was recommended to start 4 to 7 days before the procedure and continue at least for 3 months after the procedure. Several studies reported 0–14% incidence of ICA occlusion in the long-term follow-up of patients with dCCF treated by covered stents that were asymptomatic in all cases [
9-
12]. In-stent stenosis was rarely reported [
9,
10,
12] and usually resolved within 12 months with continuing DAPT [
12]. In our series, we encountered neither late ICA occlusion nor in-stent stenosis in the long-term follow-up. The rate of late stent stenosis following the BeGraft stent was reported less than previously covered stents in coronary interventions [
20].




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download