Abstract
Notes
Ethics Statement
Ethical approval was granted in accordance with national requirements, and the need for written informed consent was waived (Institutional Review Board of Jeju National University Hospital). We anonymized patient information, such as sex and age, in the case section to prevent the identification of individuals.
Conflicts of Interest
YS has been the assistant editor of Neurointervention since 2018. However, he/she has not been involved in the peer reviewer selection, evaluation, or decision process of this article. No potential conflict of interest relevant to this article was reported. No other authors have any conflicts of interest to disclose.
REFERENCES
Fig. 1.
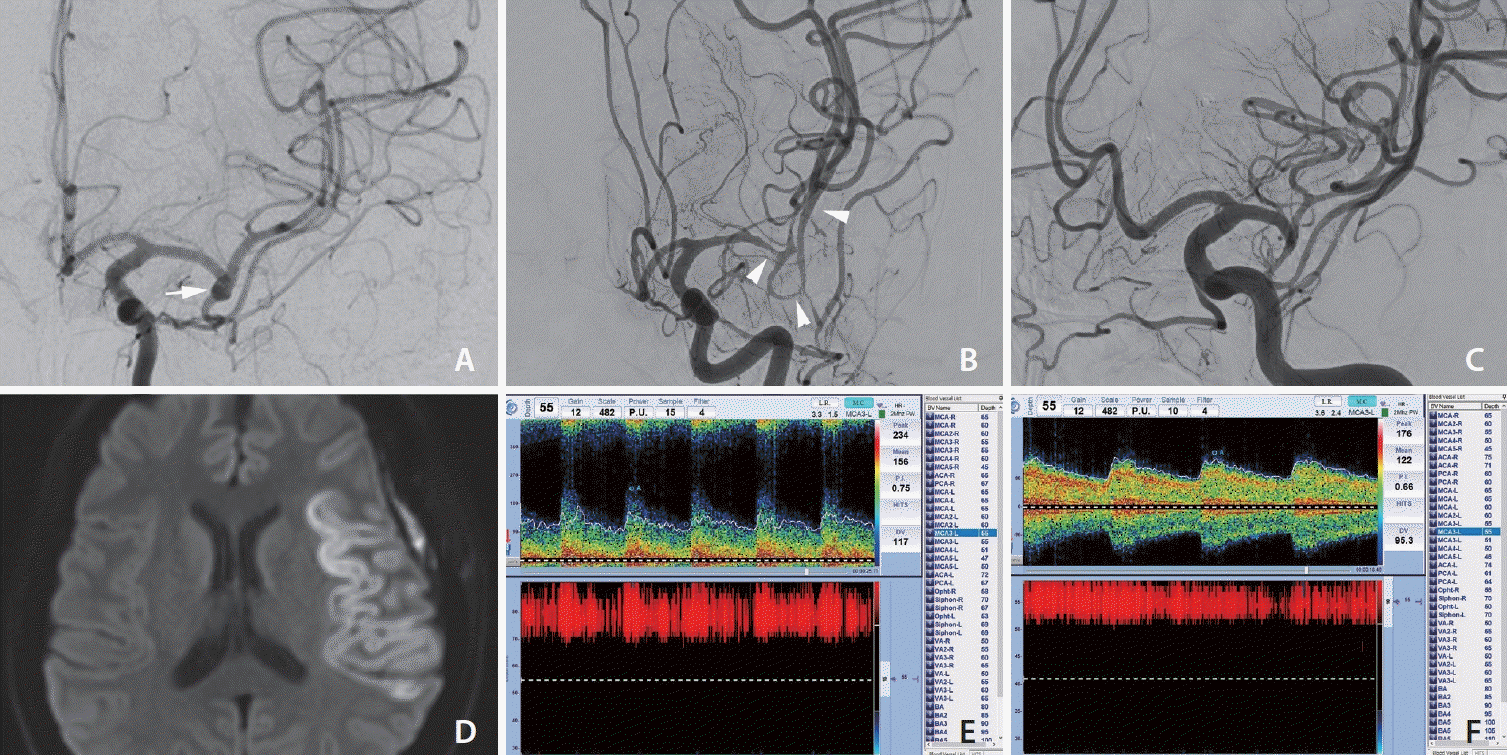
Fig. 2.
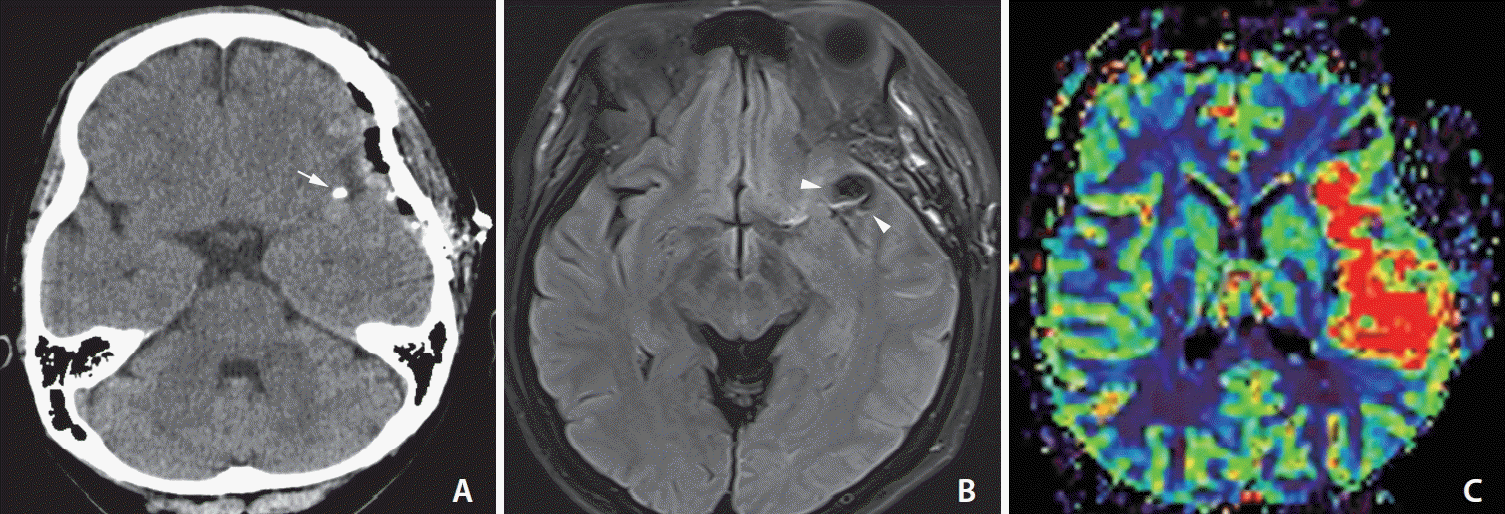
Table 1.
| Study (y) | Location headache | Size | Postop | Symptom | POD | Postop neuroimaging findings | Management | Remained deficit |
|---|---|---|---|---|---|---|---|---|
| Bloomfield and Sonntag [16] (1985) | Right MCA bifurcation | 7 | ND | Left hemiparesis | 9 | Dexamethasone | Left hemiparesis | |
| Gutiérrez [11] (2001) | Left opthalmic | 5 | ND | Aphasia, right hemiparesis | 1 | Intraarterial papaverine | Right hemiparesis | |
| Kitazawa [12] (2005) | Left paraclinoid | 4 | ND | Aphasia | 12 | Intraarterial angioplasty, triple H | None | |
| Kitazawa [12] (2005) | Left paraclinoid | 4 | ND | Aphasia, right hemiparesis | 9 | SAH | Triple H | None |
| Kitazawa [12] (2005) | Left paraclinoid | 5 | ND | Left hemiparesis | 5 | Small EDH | Intraarterial papaverine, triple H | None |
| Paolini [8] (2005) | Right MCA bifurcation | 8 | ND | Aphasia, right hemiparesis | 28 | Small ICH | Hydration antithrombotics | None |
| Yang [18] (2015) | Left ICA terminal | 5 | ND | Aphasia | 28 | Hydration antithrombotics | Dysphasia | |
| Yang [18] (2015) | Left MCA bifurcation | 6 | ND | Aphasia | 10 | Intraarterial papaverine, hydration antithrombotics | Dysphasia | |
| Tsyben [13] (2016) | Left MCA | 5 | ND | Right hemiparesis | 2 | Intraarterial verapamil | Dysphasia | |
| Tsyben [13] (2016) | AcomA, left MCA | ND | Right hemiparesis | 2 | Intraarterial verapamil, nimodipine | None | ||
| Hashimoto [15] (2016) | Left PcomA | 5 | Moderate headache | Aphasia, right hemiparesis | 11 | Hypervolemia, antithrombotics | Acalculia, paraphasia | |
| Campe [7] (2019) | Right MCA bifurcation | ND | Left hemiparesis | 12 | Oral nimodipine, antithrombotics | None | ||
| Cuoco [19] (2020) | Left MCA bifurcation | 5 | ND | Right hemiparesis | 13,26 | Intraarterial nimodipine | Right hemiparesis | |
| Ceraudo [17] (2020) | Left MCA bifurcation | 5 | ND | Aphasia, right hemiparesis | 6 | Intraarterial nimodipine | Dysphasia | |
| Knight [20] (2020) | AcomA | 5 | ND | Aphasia | 5 | Intraarterial nicardipine, triple H | Short-term memory deficit | |
| Vachata [10] (2020) | Left MCA | 10 | ND | Aphasia | 5 | Triple H, milrinon, nimodipine | None | |
| Vachata [10] (2020) | Left MCA | 7 | ND | Aphasia | 6 | Triple H, milrinon, nimodipine | Fatigue, depression deterioration | |
| Kim [14] (2020) | AcomA | 5 | ND | Motor dysphasia | 14 | Intraarterial nimodipine | None | |
| Kim [14] (2020) | Left PcomA | ND | Motor dysphasia, right hemiparesis | 12 | Intraarterial nimodipine | None | ||
| Peterson [9] (2020) | Right MCA bifurcation | 8 | ND | Left hemiparesis | 29 | Intraarterial verapamil, triple H, aspirin | Left hemiparesis | |
| Present study | Left MCA bifurcation | 3.7 | Severe headache | Aphasia, right hemiparesis | 7 | Minimal EDH | Intraarterial nimodipine | Dysphasia |
postop, postoperation; POD, postoperative day; MCA, middle cerebral artery; ND, no described; ICA, internal carotid artery; Triple H, hypertension, hypervolemia, and hemodilution; SAH, subarachnoid hemorrhage; EDH, epidural hemorrhage; ICH, intracerebral hemorrhage; AcomA, anterior communicating artery; PcomA, posterior communicating artery.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download