II. Brief Communication
A 24-year-old Korean female with a lump on her gums was referred to Seoul National University Dental Hospital under suspicion of a tumor between teeth #43 and #44. The patient reported that the lump had been present for many years but had started growing one year prior. Upon intraoral examination, a 1.5-cm-diameter solid round mass expanded buccolingually in the area of teeth #43-#44, and tooth #43 was displaced mesiobuccally.(
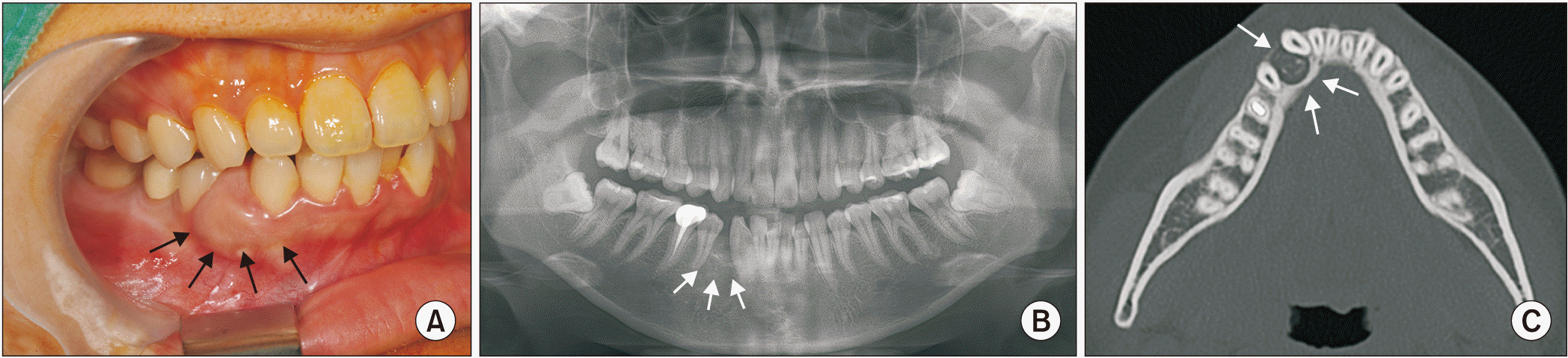
Fig. 1. A) Radiographs revealed a round, soft tissue attenuated lesion with a rough and thick corticated margin in the alveolar bone between the roots of teeth #43 and #44, with #43 displaced to the mesial buccal side. High attenuated foci were scattered inside. The buccal cortical bone was thinning, and the tendency of expansion was not significant.(
Fig. 1. B, 1. C) Based on the radiographs and clinical findings, differential diagnosis was ossifying fibroma, calcifying epithelial odontogenic tumor, and AOT.
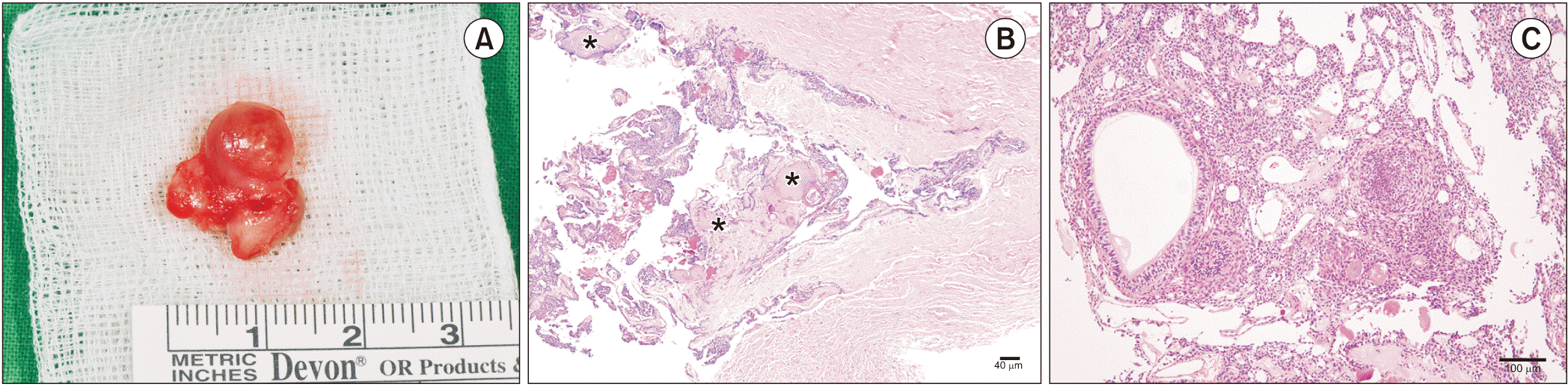
After obtaining informed consent, surgery was performed under sedation and local anesthesia using lidocaine 2% with epinephrine 1:100,000. A crevicular incision was made from tooth #42 to #45 using a No. 12 blade, and the flap was elevated using a molt curette. The main mass was identified, the base of which was attached to the lingual side of the alveolar bone between teeth #43 and #44. The mass was enucleated, followed by curettage. The retrieved mass was a 1.5-cm-diameter round mass with calcified particles inside.(
Fig. 2. A) Finally, the wound was sutured using 4-0 Polyglactin 910 Vicryl (Johnson & Johnson Co.).
Histopathology of the 0.7 cm×1.2 cm×0.6 cm excised mass revealed a circumscribed tumor containing spindle cells surrounded by a thick fibrous capsule that contained small amounts of calcification.(
Fig. 2. B) Rosette-like structures with sheets of spindle-shaped tumor cells showing whorled areas or duct-like and tubular structures were observed.(
Fig. 2. C) A diagnosis of extrafollicular AOT was finally confirmed. Clinical and radiographic follow-up appointments were set at one month, two months, four months, six months, and nine months after surgery to check the healing process and to monitor recurrence.
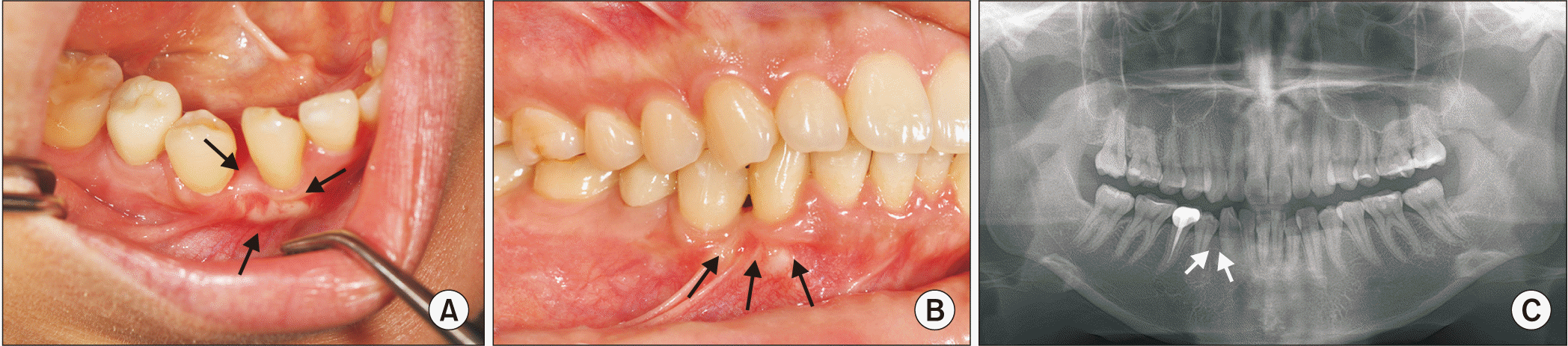
At the two-month follow-up, the diastema between displaced teeth #43 and #44 reduced (
Fig. 3. A); at the five-month follow-up, the distal part of tooth #43 was in contact with the mesial part of #44. At the nine-month follow-up, there were no signs of AOT recurrence and displaced tooth #43 was in the correct position of mandibular arch alignment.(
Fig. 3. B, 3. C) Follow-up was continued at one year to check for recurrence.
III. Discussion
The true nature of AOT as neoplasm, hamartoma, or cyst remains unclear due to its presentation, behavior, and histogenesis. Thakur et al.
3 hypothesized that AOT, which is derived from reduced enamel epithelium lining or hyperplastic dental follicle, shows a cystic appearance but may not be a true cyst. Meanwhile, if a mass arises from epithelial remnants in the gubernacular dentis, it will appear as a solid type and is designated as neoplastic. AOT has also been described as a hamartoma due to its minimal growth potential, lack of recurrence, and histopathology of enamel resemblance.
Radiographically, extrafollicular AOT appears as a well-defined unilocular radiolucency with a corticated border and has no association with the tooth crown
1. Philipsen et al.
4 subdivided the extrafollicular variant into four types (E
1-E
4): E
1 has no relation to tooth structures; E
2, interradicular, demonstrates tumor expansion causing apical divergence of adjacent roots; E
3 is superimposed on the root apex, mimicking a radicular cyst; and E
4 is superimposed at the midroot level. Based on this classification, our case involves an extrafollicular variant, subtype E
2 AOT.
Histologically, the most prominent pattern is a rosette-like structure with minimal stromal connective tissue from the formation of various-sized spindle cells, solid nodules, or cuboidal epithelial cells with the presence of eosinophilic material in the center. Moreover, duct-like structures may or may not be present in the lesion
2. Repeated recurrence of AOT was reported by Lang et al.
5 due to incomplete mass removal in the first surgery. They emphasized the importance of enucleation to guarantee a successful outcome without recurrence. However, whether the removed lesion was AOT is dubious as recurrence of AOT is rare but possible, which justifies the conservative management as the preferred therapy
6,7. Of 1,300 cases worldwide, one unequivocal AOT recurrence was reported by Chuan-Xiang and Yan
8, in which the AOT recurred twice, 12 years following primary AOT removal and 88 months after recurred AOT removal
6. Furthermore, if left untreated, AOT may result in facial asymmetry and distortion
9. In addition, tumor expansion may lead to compression of vital anatomical structures, such as the inferior alveolar nerve, potentially resulting in extreme discomfort for the patient. In our case, we performed enucleation and curettage and observed a successful outcome, without recurrence at nine months.
Vitkus and Meltzer
10 suggested a guided bone regeneration (GBR) procedure following AOT enucleation to promote rapid bone restoration as granulation tissue, which forms following enucleation, can persist and damage the tooth if the defect remains unfilled. However, Chiapasco et al.
11 reported uneventful spontaneous bony healing without GBR through adequate blood clot formation on 27 mandibles following large cyst removal. Ettl et al.
12 also found that grafts do not have a significant advantage and added that periosteum has a large capacity for bone regeneration, which should be preserved upon the mass excision. Also, allogeneic grafting can prolong the bone healing process as the graft must be resorbed and replaced by new bone; postoperative complication such as infection has also been reported
11. A 3-cm defect can be completely regenerated in 12 months following enucleation without GBR, whereas it will take up to 24 months for larger defects
11,12. In our case, we did not fill the defect. At the nine-month follow-up, the bone was completely healed without complications. Spontaneous bone healing will simplify the surgical procedure and reduce treatment cost.
In the context of extrafollicular AOT, the potential for tooth displacement is a multi-faceted concern. Tumor growth exerts pressure on adjacent bones and tooth roots, possibly resulting in migration and displacement of the associated teeth
13,14. Concurrently, the inflammatory response and bone resorption associated with the tumor can disrupt normal bone architecture, further influencing tooth position
15. Precise diagnosis of the extent of AOT involvement and its relationship with the adjacent tooth, facilitated by computed tomography, is paramount for effective treatment planning. During AOT removal, meticulous enucleation and curettage techniques are employed, with careful attention to separating the tumor from the tooth surface and surrounding bone. Contemporary surgical methods prioritize minimal trauma to preserve a tooth with periodontal ligament integrity and minimize the potential for extraction.
Tooth repositioning following enucleation without orthodontic intervention has not been reported previously. This observation can be explained through a mechanism of physiologic drift of the tooth. Reduced bone thickness may induce physiologic drifting of the tooth
16. Furthermore, transseptal fibers play an important role in physiologic drift through self-repair and reconnecting adjacent teeth following periodontal ligament destruction. Moreover, soft tissues and the pressure of lip and cheek muscles, especially in the canine area that has the highest muscle pressure, can induce distal movement of a canine to the gap area
17. Thus, it can be hypothesized that diastema closure following enucleation occurred owing to (1) development of E
2 AOT between the first premolar and canine with cortical bone destruction, decreasing bone thickness and facilitating mesial movement of the premolar and distal movement of the canine after enucleation, (2) transseptal fibers reconnecting the canine and first premolar, and (3) the activity of lip and cheek muscles during mastication, speaking, and facial expressions, inducing canine distalization.
In conclusion, AOT should be managed via enucleation and curettage to obtain successful outcomes without recurrence. Radiographic and histologic examinations are very important to differentiate AOT from other lesions. Spontaneous bone regeneration following enucleation can be achieved without GBR. Also, diastema closure and repositioning of displaced teeth can occur without orthodontic interventions through physiologic drift.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download