Abstract
Peripheral ameloblastoma (PA) is believed to be the rarest variant of ameloblastoma and only has been described in isolated case reports. PA is usually confined to the soft tissues surrounding the supporting tissues of the teeth. Although it manifests nonaggressive behavior and can be treated with complete removal by local surgical excision, long term follow up is mandatory to prevent future recurrence and possible malignant transformation.
Peripheral ameloblastoma (PA) is a rare benign odontogenic tumor that constitutes 1% to 5% of all ameloblastomas1. PA is confined to the soft tissues surrounding the supporting tissues of the teeth2; however, PA is an invasive lesion and is recurrent, able to undergo a malignant transformation and even cause metastasis3. Few consecutive case series studies on clinical profile and outcome of PA are available, and this disease was described only in isolated case reports in the literature4.
Clinically, PA appears as a painless, exophytic, sessile growth of the gingiva. The tumors usually have a firm consistency, with a smooth surface and a range of color from pink to dark red. Radiologically, PAs are silent because they rarely invade the bone. Biopsy is usually performed for diagnosis of this disease. The best treatment for ameloblastoma is surgical removal. Despite the non-aggressive course and low recurrence rate, long-term follow-up is necessary.
Herein, we report an unusual case of PA in the maxillary gingiva.
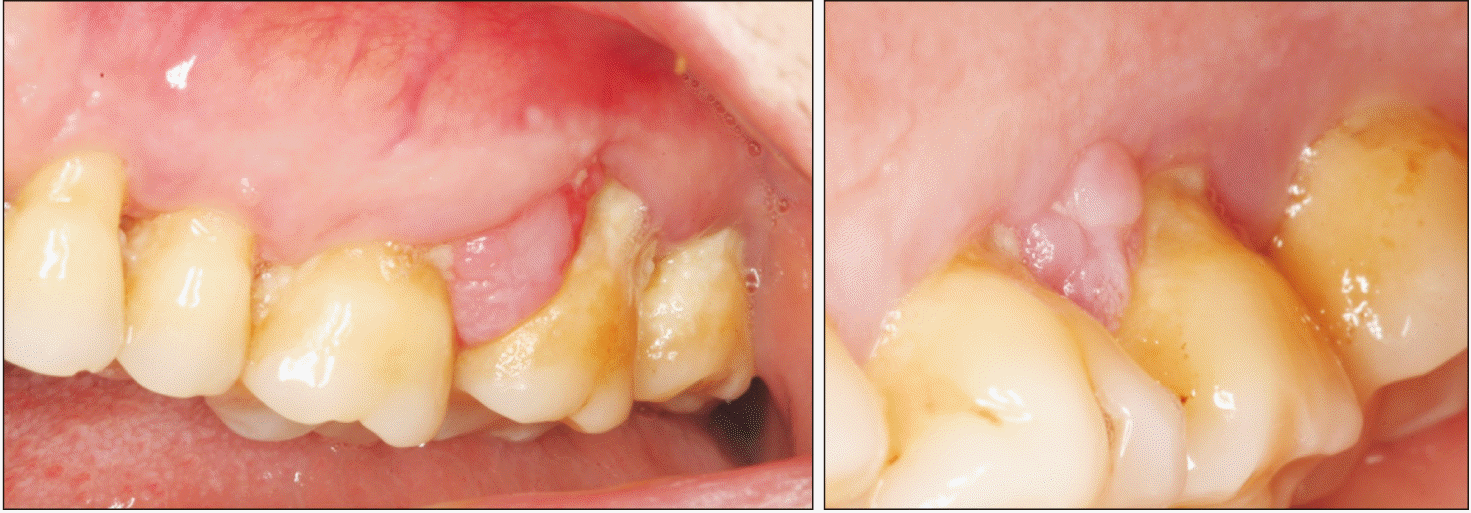
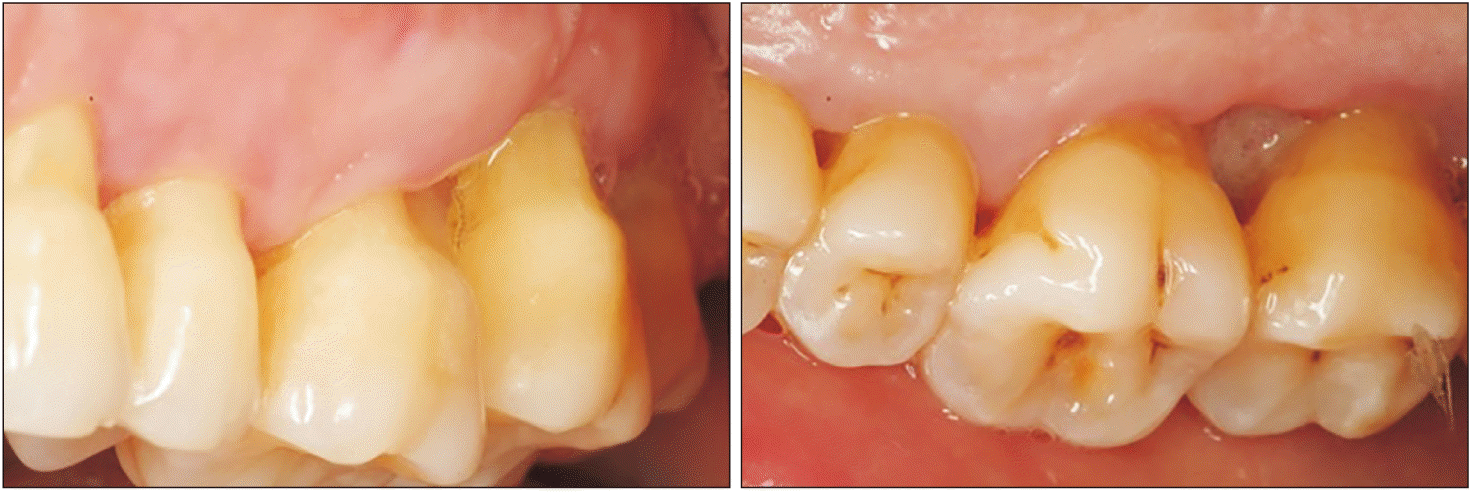
An 80-year-old Caucasian female was referred by her dental practitioner for 12-month presence of a painless exophytic lesion in the interdental papilla between the first and second upper left molars noted in a routine exploration. The patient had no history of trauma or infection. The patient was a nonsmoker and under treatment for hypertension (beta blocker, Atenolol 50 mg; Laboratorios Cinfa). Periodontal therapy combined with antiseptic therapy with chlorhexidine 0.2% (Bexident Post; Isdin) was performed without remission of the lesion. Intraoral examination showed exophytic lesions with a fibroelastic consistency when palpated at the level of the interdental papilla; a 9 mm×7 mm lesion was found on the buccal side, and a 5 mm×5 mm lesion was on the palatal side. The color of the tissue was the same as that of the adjacent mucosa.(Fig. 1) Bleeding did not occur on probing and the involved teeth were viable. Radiologic examination showed horizontal bone loss likely due to periodontal disease. Significant radiographic changes were not observed between 2018 and 2021.(Fig. 2)
Clinical diagnosis was pyogenic granuloma. As PA typically is confined to the soft tissue surrounding teeth and rarely invades the bone, excisional biopsy is recommended to resect visible tumor lesions, followed by curettage of the bony region to support complete extirpation. An excision biopsy was performed under local anesthesia with proper antiseptic measures for resecting tumor lesions. The two excised lesions (buccal and palatal) were immersed in 10% formalin solution and were submitted to histopathological examination.
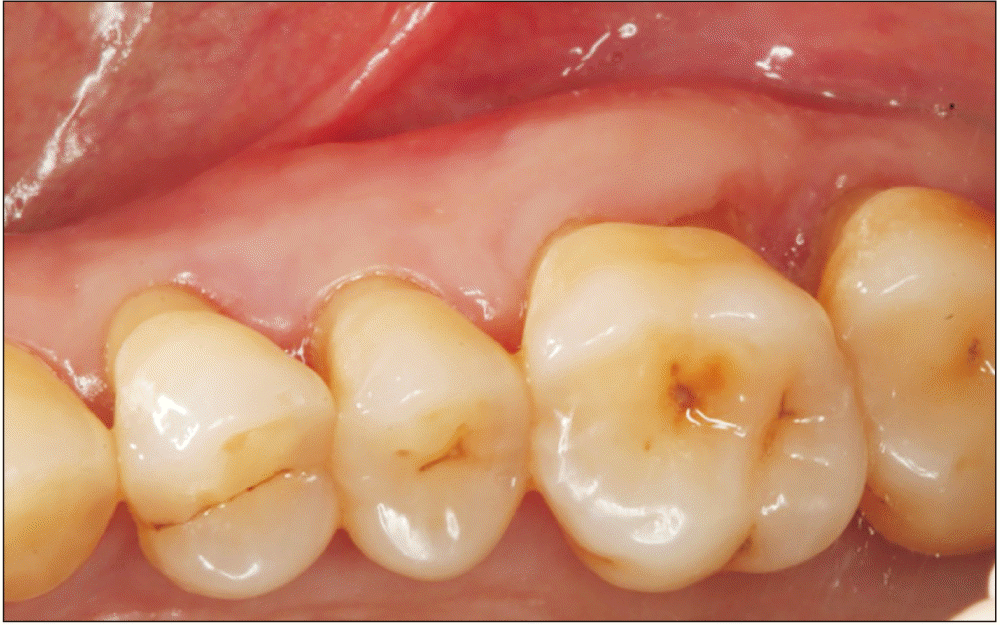
The patient was examined 7 days postoperatively, and proper healing was observed.(Fig. 3)
Microscopic examination showed partially ulcerated sections of gingival mucosa, with tumor origination in the basal cell layer of the ridges of the lining epithelium and extensive growth, preserving the epithelial basement membrane (no invasive epithelial infiltration). The peripheral tumor cells exhibited hyperchromatic, columnar nuclei with palisaded arrangement and areas of reverse nuclear polarity. The tumor did not present atypia or abnormal mitoses. In addition, intense chronic inflammation, collagen degeneration, calcium salt deposits in the chorion, and neo angiogenesis without a characteristic pyogenic granuloma-like arrangement were observed, which had not been considered for the diagnosis.(Fig. 4, 5) Based on the clinical, radiographic, and histopathological examinations, PA was diagnosed.
Clinical evidence of recurrence was not observed 24 months after surgery.(Fig. 6)
When the clinical presentation is not pathognomonic, PA is rarely the initial preoperative diagnosis. Clinically, PAs present as firm, painless, exophytic growths usually ranging in diameter from 1 to 2 centimeters5. The surface of the tumor is generally smooth, but a granular or pebbly surface has been described in several cases1,6.
PAs should be differentiated from peripheral reactive lesions such as epulis, pyogenic granuloma, fibroma, peripheral giant-cell granuloma, peripheral-ossifying fibroma, and basal cell carcinoma1. Although rare, peripheral variants of other odontogenic tumors, such as squamous odontogenic tumor, odontogenic fibroma, calcifying epithelial odontogenic tumor, and calcifying cyst odontogenic tumor, should be included in the differential diagnosis7.
PA has a male predominance with a male to female ratio of 1.9:1 and can occur at all ages but most frequently is diagnosed in middle-aged or older adults1. The mean age at the time of diagnosis varies in the range of 48.6 to 60.4 years4,8-10.
A greater predilection (2:1) for dentate regions, close to interdental papilla, has been reported11. The most common location is the mandible, specifically in the gingival area of the canine/premolar area, affecting both the lingual and vestibular areas, although the lingual area is more common9. The anterior region is the second most frequently affected location in the mandible1,6.
In most cases, PA does not involve bone and is not evident on radiography. However, there may be a small depression of the underlying bone surface associated with the tumor, known as “cupping” or “saucerization”6,9. This superficial erosion or depression of the bone is thought to be due to pressure resorption in contrast to resorption caused by neoplastic invasion1.
Microscopically, PAs are characterized by odontogenic epithelium embedded in a stroma of mature fibrous connective tissue1. In the present case, the histopathological presentation followed the pattern of the traditional PA with palisading peripheral cells. An inflammatory component was also observed subjacent to the epithelium12.
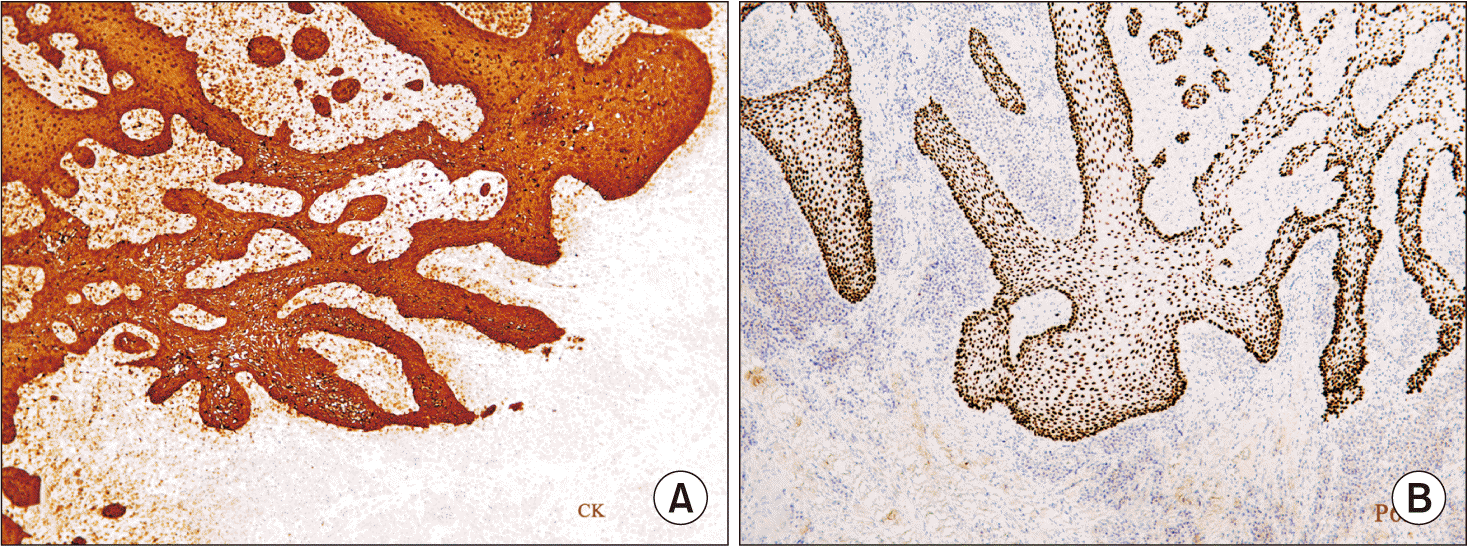
Immunohistochemically, PAs show positive reactivity for cytokeratins AE1/AE3, KL1, 34, and E12, as observed in human enamel13. The cellular proliferation rate of ameloblastoma is generally low. PAs generally manifest benign behavior with an average growth rate lower than those of other ameloblastoma subtypes13. The Ki67 protein is positive in PA cells, with the present case showing a Ki-67 cell proliferation index <2%, which is a good prognostic parameter and an indication of a lower rate of recurrence6.
The recommended treatment is conservative supraperiosteal surgical excision with adequate disease-free margins and thorough microscopic evaluation of the margins for any odontogenic islands that may contribute to recurrence14. Recurrence is rare (16%-19%) and considered a sign of incomplete removal rather than aggressiveness9. In cases of recurrence, the treatment choices are the same as for the primary tumor, surgical removal and anatomopathological study. An annual follow-up is recommended because progression to malignancy and recurrence in the form of severe epithelial dysplasia has been reported15.
In conclusion, PA is an uncommon odontogenic neoplasm. The clinical appearance of this tumor is not pathognomonic, and the diagnosis can only be determined based on histological examination. The biopsy result emphasizes the importance of a histopathological study of surgically removed lesions to establish the correct diagnosis.
Notes
Authors’ Contributions
C.V.R., R.M.A.O., and M.I.S.J. participated in performing the clinical treatment, and data collection. R.M.A.O. and J.C.B.B. participated in writing the manuscript. N.Q.L. helped to draft the manuscript. All authors read and approved the final manuscript.
Ethics Approval and Consent to Participate
The Committee for Ethics in research at Universidad Alfonso X el Sabio, Madrid, Spain (No. 2023_03/192) approved this case report, which followed the ethical guidelines established in the Declaration of Helsinki by the World Medical Association.
References
1. Philipsen HP, Reichart PA, Nikai H, Takata T, Kudo Y. 2001; Peripheral ameloblastoma: biological profile based on 160 cases from the literature. Oral Oncol. 37:17–27. https://doi.org/10.1016/s1368-8375(00)00064-6. DOI: 10.1016/S1368-8375(00)00064-6. PMID: 11120479.

2. Gardner DG. 1984; A pathologistʼs approach to the treatment of ameloblastoma. J Oral Maxillofac Surg. 42:161–6. https://doi.org/10.1016/s0278-2391(84)80026-9. DOI: 10.1016/S0278-2391(84)80026-9. PMID: 6583361.

3. Baden E, Doyle JL, Petriella V. 1993; Malignant transformation of peripheral ameloblastoma. Oral Surg Oral Med Oral Pathol. 75:214–9. https://doi.org/10.1016/0030-4220(93)90096-m. DOI: 10.1016/0030-4220(93)90096-M. PMID: 8426721.

4. Zhang X, Tian X, Hu Y, Zhang C, Wei C, Yang X. 2018; Oral peripheral ameloblastoma: a retrospective series study of 25 cases. Med Oral Patol Oral Cir Bucal. 23:e277–81. https://doi.org/10.4317/medoral.22225. DOI: 10.4317/medoral.22225. PMID: 29680843. PMCID: PMC5945233.

5. El-Mofty SK, Gerard NO, Farish SE, Rodu B. 1991; Peripheral ameloblastoma: a clinical and histologic study of 11 cases. J Oral Maxillofac Surg. 49:970–4. discussion 974–5. https://doi.org/10.1016/0278-2391(91)90061-p. DOI: 10.1016/0278-2391(91)90061-P. PMID: 1886025.

6. Kishino M, Murakami S, Yuki M, Iida S, Ogawa Y, Kogo M, et al. 2007; A immunohistochemical study of the peripheral ameloblastoma. Oral Dis. 13:575–80. https://doi.org/10.1111/j.1601-0825.2006.01340.x. DOI: 10.1111/j.1601-0825.2006.01340.x. PMID: 17944675.

7. Nonaka CF, de Oliveira PT, de Medeiros AM, de Souza LB, Freitas RA. 2013; Peripheral ameloblastoma in the maxillary gingiva: a case report. N Y State Dent J. 79:37–40.
8. Ochsenius G, Ortega A, Godoy L, Peñafiel C, Escobar E. 2002; Odontogenic tumors in Chile: a study of 362 cases. J Oral Pathol Med. 31:415–20. https://doi.org/10.1034/j.1600-0714.2002.00073.x. DOI: 10.1034/j.1600-0714.2002.00073.x. PMID: 12165060.

9. Buchner A, Merrell PW, Carpenter WM. 2006; Relative frequency of peripheral odontogenic tumors: a study of 45 new cases and comparison with studies from the literature. J Oral Pathol Med. 35:385–91. https://doi.org/10.1111/j.1600-0714.2006.00437.x. DOI: 10.1111/j.1600-0714.2006.00437.x. PMID: 16827840.

10. Moskow BS, Baden E. 1982; The peripheral ameloblastoma of the gingiva. Case report and literature review. J Periodontol. 53:736–42. https://doi.org/10.1902/jop.1982.53.12.736. DOI: 10.1902/jop.1982.53.12.736. PMID: 6961202.

11. Mintz S, Anavi Y, Sabes WR. 1990; Peripheral ameloblastoma of the gingiva. A case report. J Periodontol. 61:649–52. https://doi.org/10.1902/jop.1990.61.10.649. DOI: 10.1902/jop.1990.61.10.649. PMID: 2231232.

12. Chae MP, Smoll NR, Hunter-Smith DJ, Rozen WM. 2015; Establishing the natural history and growth rate of ameloblastoma with implications for management: systematic review and meta-analysis. PLoS One. 10:e0117241. https://doi.org/10.1371/journal.pone.0117241. DOI: 10.1371/journal.pone.0117241. PMID: 25706407. PMCID: PMC4338260.

13. Bologna-Molina R, Mosqueda-Taylor A, Lopez-Corella E, Almeida OP, Carrasco-Daza D, Garcia-Vazquez F, et al. 2008; Syndecan-1 (CD138) and Ki-67 expression in different subtypes of ameloblastomas. Oral Oncol. 44:805–11. https://doi.org/10.1016/j.oraloncology.2007.10.007. DOI: 10.1016/j.oraloncology.2007.10.007. PMID: 18207448.

14. Reichart PA, Philipsen HP, Sonner S. 1995; Ameloblastoma: biological profile of 3677 cases. Eur J Cancer B Oral Oncol. 31B:86–99. https://doi.org/10.1016/0964-1955(94)00037-5. DOI: 10.1016/0964-1955(94)00037-5. PMID: 7633291.

15. Wettan HL, Patella PA, Freedman PD. 2001; Peripheral ameloblastoma: review of the literature and report of recurrence as severe dysplasia. J Oral Maxillofac Surg. 59:811–5. https://doi.org/10.1053/joms.2001.24302. DOI: 10.1053/joms.2001.24302. PMID: 11429748.

Fig. 2
Clinical image of the lesion. Gingival swelling between first and second upper-left molars affecting buccal and palatal.
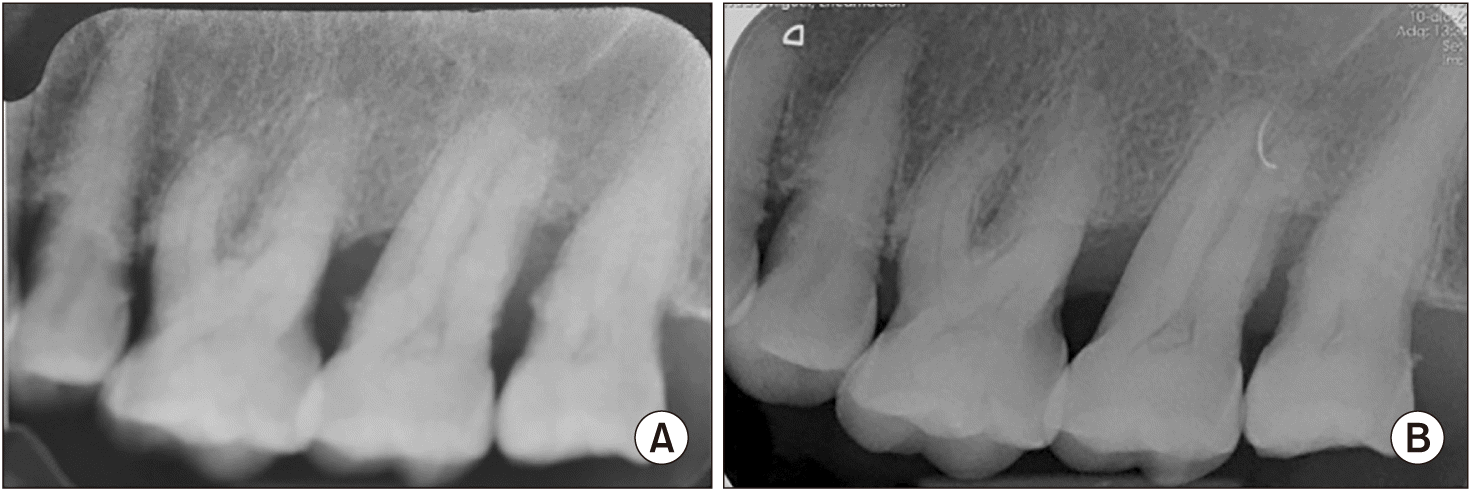
Fig. 4
Histological image of Masson’s trichrome stain lesion. A. Origin: masticatory mucosa with marked ridge hyperplasia. Masson’s trichrome staining, ×120. B. The cells in the peripheral zone adopt a palisade distribution with well-polarized nuclei and present the appearance of a lax reticulum at the central level. Masson’s trichrome staining, ×240.
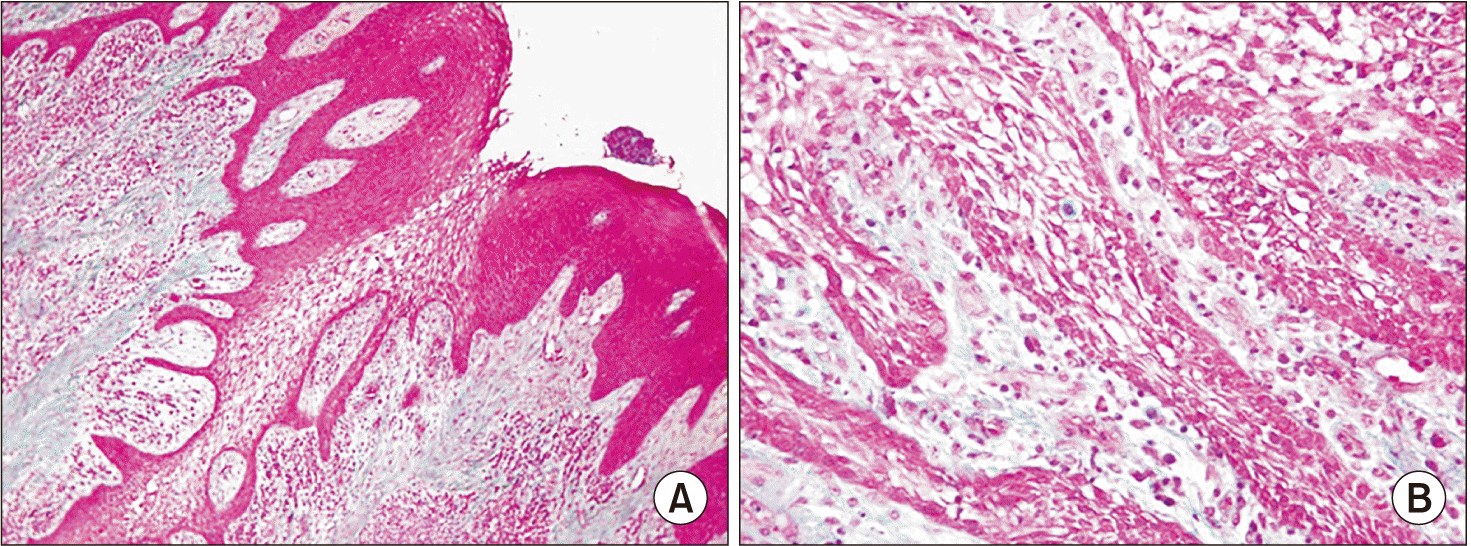
Fig. 5
Inmunohistochemistry study. Peroxidase antiperoxidase stain (PAP). A. Immunohistochemistry for cytokeratin demonstrates the intense positivity of the epithelial ridges. PAP cytokeratins (CKs) AE1/AE3 staining, ×120. B. The nuclear protein p63, which identifies odontogenic epithelial proliferations among others, is positive in this lesion but is not specific for ameloblastoma. PAP p63 1/800 staining, ×120.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download