Abstract
Objectives
This study identifies factors for differential diagnosis among lesions by retrospectively comparing panoramic and cone-beam computed tomography images and analyzing the characteristics of lesions associated with impacted mandibular third molars (IMTs).
Materials and Methods
A retrospective cohort study was conducted in patients who simultaneously underwent IMT extraction surgery and related benign tumor resection or cyst enucleation at our institution from 2017 to 2021. To compare the characteristics of each group, two comparative analyses were conducted. The first comparison considered the most frequently observed lesions associated with IMTs dentigerous cysts, odontogenic keratocysts (OKCs), and ameloblastoma. The second comparison involved placing dentigerous cysts, which have a relatively low recurrence rate, into group A and placing OKC, ameloblastoma, and odontogenic myxoma, which have high recurrence rates, into group B.
Results
Significant differences in the size of the lesion were found in the order of ameloblastoma, OKC, and dentigerous cyst (P<0.05). The buccolingual width of ameloblastoma differed significantly from that of the other groups, with no significant difference observed between the OKCs and dentigerous cysts (P=0.083).
Conclusion
Patient age and lesion size differed significantly among lesion types associated with IMTs, with younger age and larger lesions for OKCs and odontogenic tumors. OKCs are likely to have a larger mesiodistal width than dentigerous cysts. The buccolingual width of ameloblastomas was larger than those of dentigerous cysts and OKCs.
Various mandibular lesions are related to an impacted mandibular third molar (IMT), including dentigerous cysts, odontogenic keratocysts (OKCs), ameloblastomas, ameloblastic fibromas, calcifying epithelial odontogenic tumors, and adenomatoid odontogenic tumors1-5. The prevalence of cysts or tumors associated with IMTs has been reported to range from 2.8% to 6.2%, and Mello et al.6 reported cysts and tumors in 5.3% of extracted IMTs4,6-8. Despite their relatively low prevalence, these lesions can proliferate and cause complications that make surgical treatment inevitable, such as fracture of the mandible9.
Odontogenic cysts and tumors related to IMTs are diagnosed by radiographic examination and treated by surgical cyst enucleation and excision2,10-12. Some odontogenic tumors and cysts, such as ameloblastoma and odontogenic myxoma, are known to have a high recurrence rate13-17. Treatments for such lesions might need to include additional treatment modalities such as Carnoy’s solution or cryosurgery, along with extensive excision and additional patient education10,18-20. If necessary, histologic diagnosis by incisional biopsy can be used to formulate a treatment plan, but it is difficult to make a differential diagnosis in cases involving an impacted third molar with only an intraosseous lesion. It is also difficult to differentiate an odontogenic tumor from a dentigerous cyst radiologically if the mass is unilocular and well demarcated.
According to the literature, radiographic findings such as cortical bone perforation, bulging, and root resorption of adjacent teeth are used to differentiate odontogenic tumors from odontogenic cysts11,21-23. However, few studies have compared features to differentiate lesions associated with IMTs. The purpose of this paper is to retrospectively compare panoramic and cone-beam computed tomography (CBCT) images of lesions occurring around an IMT to identify features that can be used for differential diagnosis and compare the features of lesions with high and low recurrence rates.
Patients who underwent tooth extraction surgery under general anesthesia at our institution to remove an IMT and resect an associated benign tumor or excise a cyst at the same time between 2017 and 2021 were included in this study. The following cases were excluded from the study group:
• Patients younger than 18 years
• Patients who did not have an adequate radiographic study
• Patients who were not diagnosed with a tumor or cyst based on histologic findings such as inflammation
Initially, 390 patients were enrolled based on their clinical, radiologic, and histologic data. Of them, 26 patients were younger than 18 years, 43 were not diagnosed with tumors or cysts due to histologic findings of simple papules, inflammation, etc., and 12 patients did not have adequate radiological examinations, leaving 309 patients for analysis.
In total, the 309 patients had 317 cases of IMT-related tumors or cysts, as shown in Table 1 organized according to their histologic findings. Among the 309 patients, 212 patients (68.6%) had histologic confirmation of a dentigerous cyst, with 8 patients having bilateral occurrence, for a total of 220 cases. OKC was the second most common diagnosis, with 43 patients (13.9%), and ameloblastoma was the fifth most common diagnosis, with 6 patients (1.9%). Odontogenic myxoma and hemangioma each occurred in 1 patient (0.3%).
Overall, 317 cases from 309 patients were analyzed by a single investigator who used panoramic and CBCT images to compare the following variables: patient sex and age, left-right location of the lesion, lesion position, cortical bone penetration, thinning, expansion, locularity, inferior alveolar nerve (IAN) displacement, bilateral occurrence, 2nd molar displacement, 2nd molar root resorption, 2nd molar vitality, preoperative symptoms, preoperative numbness, 3rd molar root contact of the lesion, range of 3rd molar root contact, size of the lesion, ratio of the mesiodistal width of the lesion to the buccolingual width of the lesion (MD:BL), ratio of the vertical height to the BL width of the lesion (height:BL), ratio of the vertical height of the lesion to its MD width (height:MD), and ratio of the size of the lesion to the patient’s age (size:age). The location of the lesion was defined as central if the distance from the center of the lesion to the tooth axis of the impacted third molar on the panoramic view was less than 3 mm and as lateral if the distance was greater than 3 mm.(Fig. 1) A single investigator measured the BL width, MD width, and vertical height of each lesion on the CBCT images, and the maximum value was defined as the size of the lesion. To determine the morphology of the lesion, the MD:BL ratio, height:BL ratio, and height:MD ratio were measured. Because developmental cysts and odontogenic tumors are generally asymptomatic, making the onset of the lesion unknown, the size:age ratio was measured as a reference for the lesion’s rate of size increase.
Penetration, thinning, or bulging of the cortical bone, displacement of the IAN, contact of the lesion with the third molar, bilaterality, displacement of the erupted second molar, and root resorption were confirmed by examining the panoramic and CBCT images.
This retrospective study used patient medical records and radiographic data and was approved by the Institutional Review Board (IRB) of Pusan National University Dental Hospital (IRB No. PNUDH-2023-04-001). The written informed consent was waived by the IRB due to the retrospective nature of the study.
To delineate the characteristics of each lesion, the lesions most commonly associated with IMTs (dentigerous cysts, OKCs, and ameloblastomas) were compared. Furthermore, dentigerous cysts, which have a relatively low recurrence rate (group A), were compared with OKCs, ameloblastomas, and odontogenic myxoma, which have a high recurrence rate (group B). Epithelial cysts were included in that analysis as dentigerous cysts. Fisher’s exact test, the Mann–Whitney U test, one-way ANOVA, and Pearson’s chi-square test were used in IBM SPSS Statistics (ver. 26.0; IBM Corp.) to analyze significance.
A comparative analysis of lesions associated with IMTs is presented in Tables 2 and 3. The mean age of the patients was 43.9 years. The mean size of the lesions was 22.7 mm, with a mean BL width of 12.2 mm, a mean MD width of 18.5 mm, and a mean vertical height of 21.8 mm.
When dentigerous cysts, OKCs, and ameloblastomas associated with IMTs were analyzed, no difference in patient sex was observed (P=0.202), and no left-right difference in location was observed (P=0.638).(Table 2) Patient age was significantly lower in ameloblastoma, but no significant difference was observed between OKCs and dentigerous cysts. Dentigerous cysts were more often unilateral than bilateral (P<0.05), located more central than lateral (P<0.05), and did not generally perforate the cortical bone (P<0.05). Root resorption was more common in ameloblastoma than in the other two lesion types (P<0.05). No significant differences among the types were observed in mandibular canal displacement (P=0.115), preoperative symptoms (P=0.271), or preoperative numbness (P=0.170).
Significant differences in lesion size were found in the order of ameloblastoma, OKC, and dentigerous cyst (P<0.05).(Fig. 2) The BL width was significantly larger in ameloblastoma than in the other lesion types, but no significant difference was observed between OKCs and dentigerous cysts (P=0.083). The MD width was significantly smaller in dentigerous cysts than in the other lesion types, but no significant difference was observed between OKCs and ameloblastomas (P=0.295). Significant differences in the height of the lesion were found in the order of ameloblastoma, OKC, and dentigerous cyst.
When the lesions associated with IMTs were analyzed according to their recurrence propensity, the patients in group A were more likely to be male than female (P<0.05) and older than those in group B (P<0.05); the group A lesions were more likely to have a central location than a lateral one (P<0.05).(Table 3) The lesions in group B were more likely than the lesions in group A to involve cortical bone perforation, bulging, and thinning (P<0.05); root resorption of the second molar (P<0.05); and multifocality (P<0.05). In group B, there was more root contact of the third molar than in group A (P<0.05).
The lesions in group B were significantly larger than those in group A (P<0.05).(Fig. 3. A) The BL width didn’t differ significantly between the groups (P=0.102), but group B had significantly larger MD width (P<0.05) and height (P<0.05).
In terms of the lesion size ratios, group B had a higher MD:BL ratio (P<0.05) and Height:BL ratio than group A (P<0.05).(Fig. 3. B) No significant difference between groups was observed in the Height:MD ratio (P=0.409). The size:age ratio was significantly higher in group B (P<0.05), and a significant difference was also observed (P<0.05).(Fig. 3. B)
The third molar is the most frequently impacted tooth, and any cyst or tumor associated with an IMT requires surgical treatment24. Previous radiologic studies have reported that the prevalence of dentigerous cysts associated with IMTs ranges from 0.8% to 4.6%25,26, and in a systematic review and meta-analysis, Mello et al.6 reported that cysts and tumors were observed in 5.3% of extracted IMTs, with dentigerous cysts as the most common (2.1%) and odontogenic cysts as the second most common (0.5%). However, few studies have compared the characteristics of pericoronal lesions associated with IMTs.
In this study, lesions associated with IMTs were divided into high and low recurrence groups based on histologic examination, and radiologic and clinical features that could differentiate the two groups were investigated. The goal of this analysis was to determine the relationship between clinical and radiographic features, which are not difficult to measure, and histopathologic findings, which cannot be known before surgery.
In a retrospective analysis of 280 cases, Caruso et al.27 reported that the size of a lesion associated with an IMT, with a 2 cm cutoff, was an independent predictor for distinguishing a dentigerous cyst from other pathologic conditions; age was also a predictor. In this study, we observed significant differences in 15 factors: size, age, sex, lesion location, perforation, bulging, thinning, locularity, root resorption in the second molar, root contact in the third molar, BL width, MD width, vertical height of the lesion, MD:BL ratio, height:BL ratio, and size:age ratio.
Few studies have analyzed the sizes and shapes of lesions associated with IMTs. Wali et al.28 compared dentigerous cysts and normal germ tooth by measuring the size of the follicular space on panoramic images based on the angle of the tooth axis, and Terauchi et al.29 conducted a clinical study of dentigerous cysts associated with mandibular third molars, presenting the size of the lesion as a percentage of the size of the crown. In this study, no difference in BL width was observed between dentigerous cysts and OKCs, but it was significantly larger in ameloblastomas. Thus, BL width might be useful in the differential diagnosis of ameloblastoma.(Table 2) Dentigerous cysts and OKCs differed in MD width, but ameloblastoma and OKCs did not. The radiographic features of OKCs are that the lesion grows along the inner surface of the mandible with no obvious cortical bone bulge21. Therefore, OKCs with no obvious BL expansion might be the result of anteroposterior growth, and it might be clinically useful to compare the MD width to differentiate between an odontogenic cyst and an OKC.
Larger lesions are more likely to be tumors than cysts, but it is difficult to diagnose them based on size alone. If there is a correlation between recurrence and growth rate, the differential diagnosis would be easier. Withholding surgery for odontogenic cysts or tumors below a certain size could increase the size of the cyst or tumor and increase postoperative complications. However, it is difficult to measure the growth rate through observation in clinical practice. In this study, we compared and analyzed the size:age ratio as a reference value for the growth rate of the lesion. Because most odontogenic cysts and tumors start as developmental lesions regardless of inflammation30, dividing the size of the lesion by the patient’s age could be helpful for differential diagnosis. In group B, which had a high recurrence rate, the proportion of patients with a size:age ratio of 1 or more was high.(Table 3) In other words, a 45 mm lesion in patients younger than 45 years might have a higher recurrence rate than the same lesion in patients older than 45 years. However, future studies will be needed to test that hypothesis.
In this study, a higher proportion of multifocal lesions and second molar root resorption was observed in group B, which contained OKCs and ameloblastomas with high recurrence rates.(Table 3) This finding might be related to the higher rate of root resorption observed in ameloblastoma31,32. For third molar root contact, the 68.0% observed in group B was higher than the 9.7% found in group A (P<0.05). Most cysts of an impacted third molar crown are developmental and occur in association with the epithelial tissue of the crown. Because of their slow growth rate, such cysts are unlikely to migrate in the direction of the root or involve the root. In the case of odontogenic tumors, on the other hand, even if their development begins in the crown, they might not be limited to the crown because of their fast growth rate and uneven tissue proliferation direction. Cases with multiple lesions are reportedly associated with a higher recurrence rate than similar lesions that occur alone33. Clinically, therefore, a multifocal lesion, a lesion that is not confined to the crown of an IMT, or a lesion that shows root resorption in the adjacent second molar should be treated more like an OKC or odontogenic tumor than a dentigerous cyst in terms of surgical preparation and patient education.
None of the comparative analyses in this study found any significant differences in preoperative clinical symptoms or preoperative sensory loss.(Tables 2, 3) Odontogenic cysts usually grow slowly and present as a painless swelling of the mandible, with pain or symptoms occurring secondary to infection22. Therefore, in cases involving an IMT, early radiographic recognition and diagnosis can minimize resorption or destruction of the mandible, especially in the absence of clinical infection12.
OKCs, along with dentigerous cysts, are currently classified as developmental cysts in the World Health Organization (WHO) classification34. Clinically, however, it is not advisable to approach OKCs in the same way as odontogenic cysts. The recurrence rate of OKCs has been reported to vary from 2.5% to 62.5%, and the recurrence rate is not related to the size of the lesion or patient sex, but rather to resorption of adjacent teeth, removal of the associated teeth, and surgical technique10,18,19,35-38. OKCs were classified as odontogenic tumors by WHO in 2005 because of their high recurrence rate, aggressive nature, association with basal cell nevus syndrome, and mutations in the PTCH gene, but they were reclassified in 2017 because they are clinically marsupializable, and mutations in the PTCH gene are also found in nonneoplastic lesions34,39. Slusarenko da Silva et al.40 reviewed the expression of p53 protein in OKCs and concluded that they behave more like odontogenic tumors than odontogenic cysts. In this study, OKCs were separated and compared with dentigerous cysts in Table 3. They were also analyzed separately from ameloblastoma in Table 2 for differentiation.
The most common odontogenic tumor in this study was ameloblastoma (Table 1), a benign tumor that occurs with a frequency of 1% among all tumors arising in the mandible and 10% among odontogenic tumors41. The recurrence rate of ameloblastoma depends on the surgical approach: cystectomy alone has been reported to have a recurrence rate of 30.5%, surgery with Carnoy’s solution has been reported to have a recurrence rate of 16%, and surgery with osteotomy has been reported to have a recurrence rate of 3.6%15. Of the recurrences that do occur, 5% occur within 5 years16. Ghazi et al.42 found that stromal expression of tenascin was higher in ameloblastomas than in dentigerous cyst and OKCs and suggested that that could explain the immaturity of its stroma and aggressiveness, compared with other studied lesions. When treating odontogenic tumors, it is sometimes necessary to sacrifice adherent surrounding anatomical structures to reduce the recurrence rate. Tabrizi et al.43 reported in a retrospective study that sacrificing the IAN in the treatment of an ameloblastoma adjacent to it did not decrease the lesional recurrence rate. The IMT is anatomically adjacent to the IAN, and if the involved lesion is suspected to be an ameloblastoma, a modification of the surgical approach might be necessary, but sacrificing the IAN should be considered further.
This study allowed us to identify comparable features for each lesion. However, a limitation of this study is that due to the difference in prevalence, the 6 cases of ameloblastoma differed significantly from the 257 cases of dentigerous cyst, making direct comparisons difficult. Further studies are needed, such as a prospective cohort study with a large number of cases or a systematic review that includes a comparison of lesion sizes.
Lesions associated with IMTs were compared and analyzed, and the following conclusions are drawn.
(1) Patient age and lesion size differed significantly among the different types of lesions associated with IMTs.
(2) OKCs and odontogenic tumors, which have a higher recurrence rate than dentigerous cysts, are younger and larger than dentigerous cysts.
(3) Compared with dentigerous cysts, OKCs have a larger MD width.
(4) The BL width of an ameloblastoma is larger than that of a dentigerous cyst or OKC.
(5) Even if histological test results cannot be known in advance, knowing the characteristics of the lesions presented above and performing surgery accordingly will likely reduce the recurrence rate or unnecessary sacrifice of adjacent tissue.
Comparing the morphologic differences and sizes of intraosseous lesions associated with IMTs has allowed us to recognize differences among the lesions and might be applicable to clinical practice. However, due to the low prevalence of odontogenic tumors, including ameloblastoma, a direct comparison was not possible. Future studies should include a large number of cases in a prospective cohort study or a systematic review of the literature.
Notes
Authors’ Contributions
D.M.L., J.R., and H.K. participated in data collection. D.M.L. designed the study, performed statistical analysis, and wrote the manuscript. J.R. and J.Y.L. helped to design the study and draft the manuscript. All authors have reviewed and approved the final manuscript.
References
1. Sarica I, Derindag G, Kurtuldu E, Naralan ME, Caglayan F. 2019; A retrospective study: do all impacted teeth cause pathology? Niger J Clin Pract. 22:527–33. https://doi.org/10.4103/njcp.njcp_563_18. DOI: 10.4103/njcp.njcp_563_18. PMID: 30975958.

2. Bilodeau EA, Collins BM. 2017; Odontogenic cysts and neoplasms. Surg Pathol Clin. 10:177–222. https://doi.org/10.1016/j.path.2016.10.006. DOI: 10.1016/j.path.2016.10.006. PMID: 28153133.

3. Toller P. 1967; Origin and growth of cysts of the jaws. Ann R Coll Surg Engl. 40:306–36.
4. Güven O, Keskin A, Akal UK. 2000; The incidence of cysts and tumors around impacted third molars. Int J Oral Maxillofac Surg. 29:131–5. DOI: 10.1016/S0901-5027(00)80011-9. PMID: 10833151.

5. Sato D, Matsuzaka K, Yama M, Kakizawa T, Inoue T. 2004; Adenomatoid odontogenic tumor arising from the mandibular molar region: a case report and review of the literature. Bull Tokyo Dent Coll. 45:223–7. https://doi.org/10.2209/tdcpublication.45.223. DOI: 10.2209/tdcpublication.45.223. PMID: 15960159.

6. Mello FW, Melo G, Kammer PV, Speight PM, Rivero ERC. 2019; Prevalence of odontogenic cysts and tumors associated with impacted third molars: a systematic review and meta-analysis. J Craniomaxillofac Surg. 47:996–1002. https://doi.org/10.1016/j.jcms.2019.03.026. DOI: 10.1016/j.jcms.2019.03.026. PMID: 31005378.

7. Stathopoulos P, Mezitis M, Kappatos C, Titsinides S, Stylogianni E. 2011; Cysts and tumors associated with impacted third molars: is prophylactic removal justified? J Oral Maxillofac Surg. 69:405–8. https://doi.org/10.1016/j.joms.2010.05.025. DOI: 10.1016/j.joms.2010.05.025. PMID: 21050646.

8. Al-Khateeb TH, Bataineh AB. 2006; Pathology associated with impacted mandibular third molars in a group of Jordanians. J Oral Maxillofac Surg. 64:1598–602. https://doi.org/10.1016/j.joms.2005.11.102. DOI: 10.1016/j.joms.2005.11.102. PMID: 17052585.

9. Kouhsoltani M, Mesgarzadeh AH, Moradzadeh Khiavi M. 2015; Mandibular fracture associated with a dentigerous cyst: report of a case and literature review. J Dent Res Dent Clin Dent Prospects. 9:193–8. https://doi.org/10.15171/joddd.2015.035. DOI: 10.15171/joddd.2015.035. PMID: 26697153. PMCID: PMC4682017.

10. Voorsmit RA, Stoelinga PJ, van Haelst UJ. 1981; The management of keratocysts. J Maxillofac Surg. 9:228–36. https://doi.org/10.1016/s0301-0503(81)80049-5. DOI: 10.1016/S0301-0503(81)80049-5. PMID: 6172530.

11. Rioux-Forker D, Deziel AC, Williams LS, Muzaffar AR. 2019; Odontogenic cysts and tumors. Ann Plast Surg. 82:469–77. https://doi.org/10.1097/sap.0000000000001738. DOI: 10.1097/SAP.0000000000001738. PMID: 30856625.

12. Kang YH, Kim YD, Kim UK, Kim CH, Song JM, Shin SH, et al. 2023. Textbook of oral and maxillofacial surgery. 4th ed. Koonja;Paju:
13. Leiser Y, Abu-El-Naaj I, Peled M. 2009; Odontogenic myxoma--a case series and review of the surgical management. J Craniomaxillofac Surg. 37:206–9. https://doi.org/10.1016/j.jcms.2008.10.001. DOI: 10.1016/j.jcms.2008.10.001. PMID: 19027311.

14. Saalim M, Sansare K, Karjodkar FR, Farman AG, Goyal SN, Sharma SR. 2019; Recurrence rate of odontogenic myxoma after different treatments: a systematic review. Br J Oral Maxillofac Surg. 57:985–91. https://doi.org/10.1016/j.bjoms.2019.09.005. DOI: 10.1016/j.bjoms.2019.09.005. PMID: 31551163.

15. Lau SL, Samman N. 2006; Recurrence related to treatment modalities of unicystic ameloblastoma: a systematic review. Int J Oral Maxillofac Surg. 35:681–90. https://doi.org/10.1016/j.ijom.2006.02.016. DOI: 10.1016/j.ijom.2006.02.016. PMID: 16782308.

16. Reichart PA, Philipsen HP, Sonner S. 1995; Ameloblastoma: biological profile of 3677 cases. Eur J Cancer B Oral Oncol. 31B:86–99. https://doi.org/10.1016/0964-1955(94)00037-5. DOI: 10.1016/0964-1955(94)00037-5. PMID: 7633291.

17. Laborde A, Nicot R, Wojcik T, Ferri J, Raoul G. 2017; Ameloblastoma of the jaws: management and recurrence rate. Eur Ann Otorhinolaryngol Head Neck Dis. 134:7–11. https://doi.org/10.1016/j.anorl.2016.09.004. DOI: 10.1016/j.anorl.2016.09.004. PMID: 27793625.

18. Titinchi F, Nortje CJ. 2012; Keratocystic odontogenic tumor: a recurrence analysis of clinical and radiographic parameters. Oral Surg Oral Med Oral Pathol Oral Radiol. 114:136–42. https://doi.org/10.1016/j.oooo.2012.01.032. DOI: 10.1016/j.oooo.2012.01.032. PMID: 22727103.

19. Thanarojvanich S, Chaiyasamut T. 2022; Factors affecting odontogenic keratocyst recurrence: a retrospective chart review. Mahidol Dent J. 42:209–22.
20. Tay ZW, Sue WL, Leeson RMA. 2021; Chemical adjuncts and cryotherapy in the management of odontogenic keratocysts: a systematic review. Adv Oral Maxillofac Surg. 3:100116. https://doi.org/10.1016/j.adoms.2021.100116. DOI: 10.1016/j.adoms.2021.100116.

21. Korean Academy of Oral and Maxillofacial Radiology Faculty Council. 2015. Oral and maxillofacial radiology. 5th ed. Narae Books.
22. Rajendra Santosh AB. 2020; Odontogenic cysts. Dent Clin North Am. 64:105–19. https://doi.org/10.1016/j.cden.2019.08.002. DOI: 10.1016/j.cden.2019.08.002. PMID: 31735221.

23. Waldron CA. 1964; The differential diagnosis of radiolucent areas in the jaws. Dent Clin N Am. 8:299–314. https://doi.org/10.1016/S0011-8532(22)01970-X. DOI: 10.1016/S0011-8532(22)01970-X.

24. NIH consensus development conference for removal of third molars. 1980; NIH consensus development conference for removal of third molars. J Oral Surg. 38:235–6.
25. Brickley M, Kay E, Shepherd JP, Armstrong RA. 1995; Decision analysis for lower-third-molar surgery. Med Decis Making. 15:143–51. https://doi.org/10.1177/0272989x9501500207. DOI: 10.1177/0272989X9501500207. PMID: 7783575.

26. Eliasson S, Heimdahl A, Nordenram A. 1989; Pathological changes related to long-term impaction of third molars. A radiographic study. Int J Oral Maxillofac Surg. 18:210–2. https://doi.org/10.1016/s0901-5027(89)80055-4. DOI: 10.1016/S0901-5027(89)80055-4. PMID: 2507670.

27. Caruso DP, Lee CC, Peacock ZS. 2022; What factors differentiate dentigerous cysts from other pericoronal lesions? Oral Surg Oral Med Oral Pathol Oral Radiol. 133:8–14. https://doi.org/10.1016/j.oooo.2021.05.003. DOI: 10.1016/j.oooo.2021.05.003. PMID: 34511358.

28. Wali GG, Sridhar V, Shyla HN. 2012; A study on dentigerous cystic changes with radiographically normal impacted mandibular third molars. J Maxillofac Oral Surg. 11:458–65. https://doi.org/10.1007/s12663-011-0252-7. DOI: 10.1007/s12663-011-0252-7. PMID: 24293941. PMCID: PMC3485464.

29. Terauchi M, Akiya S, Kumagai J, Ohyama Y, Yamaguchi S. 2019; An analysis of dentigerous cysts developed around a mandibular third molar by panoramic radiographs. Dent J (Basel). 7:13. https://doi.org/10.3390/dj7010013. DOI: 10.3390/dj7010013. PMID: 30720707. PMCID: PMC6473924.

30. Kaplan I, Hirshberg A. 2004; The correlation between epithelial cell proliferation and inflammation in odontogenic keratocyst. Oral Oncol. 40:985–91. https://doi.org/10.1016/j.oraloncology.2004.04.017. DOI: 10.1016/j.oraloncology.2004.04.017. PMID: 15509489.

31. Struthers P, Shear M. 1976; Root resorption by ameloblastomas and cysts of the jaws. Int J Oral Surg. 5:128–32. https://doi.org/10.1016/s0300-9785(76)80061-0. DOI: 10.1016/S0300-9785(76)80061-0. PMID: 820661.

32. Kitisubkanchana J, Reduwan NH, Poomsawat S, Pornprasertsuk-Damrongsri S, Wongchuensoontorn C. 2021; Odontogenic keratocyst and ameloblastoma: radiographic evaluation. Oral Radiol. 37:55–65. https://doi.org/10.1007/s11282-020-00425-2. DOI: 10.1007/s11282-020-00425-2. PMID: 32030659.

33. Chirapathomsakul D, Sastravaha P, Jansisyanont P. 2006; A review of odontogenic keratocysts and the behavior of recurrences. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 101:5–9. discussion 10. https://doi.org/10.1016/j.tripleo.2005.03.023. DOI: 10.1016/j.tripleo.2005.03.023. PMID: 16360602.

34. Sarradin V, Siegfried A, Uro-Coste E, Delord JP. 2018; [WHO classification of head and neck tumours 2017: main novelties and update of diagnostic methods]. Bull Cancer. 105:596–602. French. https://doi.org/10.1016/j.bulcan.2018.04.004. DOI: 10.1016/j.bulcan.2018.04.004. PMID: 29759330.

35. Seong HS, Lee JM, Hwang DS, Kim YD, Kim U, Kim JR, et al. 2009; Clinical study of odontogenic keratocyst. J Korean Oral Maxillofac Surg. 35:89–93.
36. Borges LBO, Almeida RS, Da Silva RA, Sato FRL. 2021; Retrospective study of therapeutic approaches, recurrence and prevalence of cases of odontogenic keratocysts at a general hospital. Adv Oral Maxillofac Surg. 2:100047. https://doi.org/10.1016/j.adoms.2021.100047. DOI: 10.1016/j.adoms.2021.100047.

37. Sapp JP, Eversole LR, Wysocki GP. 1997. Contemporary oral and maxillofacial pathology. Mosby;DOI: 10.1097/00008505-199700630-00027.
38. Bataineh AB, Al Qudah M. 1998; Treatment of mandibular odontogenic keratocysts. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 86:42–7. https://doi.org/10.1016/s1079-2104(98)90148-2. DOI: 10.1016/S1079-2104(98)90148-2. PMID: 9690244.

39. Barnes L, Eveson JW, Reichart P, Sidransky D. 2005. Pathology and genetics of head and neck tumours. 3rd ed. World Health Organization.
40. Slusarenko da Silva Y, Stoelinga PJW, Grillo R, da Graça Naclério-Homem M. 2021; Cyst or tumor? A systematic review and meta-analysis on the expression of p53 marker in odontogenic keratocysts. J Craniomaxillofac Surg. 49:1101–6. https://doi.org/10.1016/j.jcms.2021.09.015. DOI: 10.1016/j.jcms.2021.09.015. PMID: 34620539.

41. Vallicioni J, Loum B, Dassonville O, Poissonnet G, Ettore F, Demard F. 2007; [Ameloblastomas]. Ann Otolaryngol Chir Cervicofac. 124:166–71. French. https://doi.org/10.1016/j.aorl.2006.08.006. DOI: 10.1016/j.aorl.2006.08.006. PMID: 17673157.

42. Ghazi N, Saghravanian N, Mirhashemi M, Ardakani AA. 2023; Evaluation of tenascin expression in ameloblastoma, odontogenic keratocyst, and dentigerous cyst by immunohistochemistry. Adv Biomed Res. 12:66. https://doi.org/10.4103/abr.abr_131_21. DOI: 10.4103/abr.abr_131_21. PMID: 37200755. PMCID: PMC10186055.

43. Tabrizi R, Alam M, Amoular E, Malekigorji M. 2023; Does preservation of the inferior alveolar nerve in the close margin of the mandibular ameloblastoma increase the risk of recurrence? J Oral Maxillofac Surg. 81:101–6. https://doi.org/10.1016/j.joms.2022.09.009. DOI: 10.1016/j.joms.2022.09.009. PMID: 36257340.

Fig. 1
Position of the lesions. A. Central. B. Lateral. When the distance between the axis of the impacted third molar and the center of the lesion was less than 3 mm in the panoramic image, the case was defined as central, and when it was more than 3 mm, it was defined as lateral.
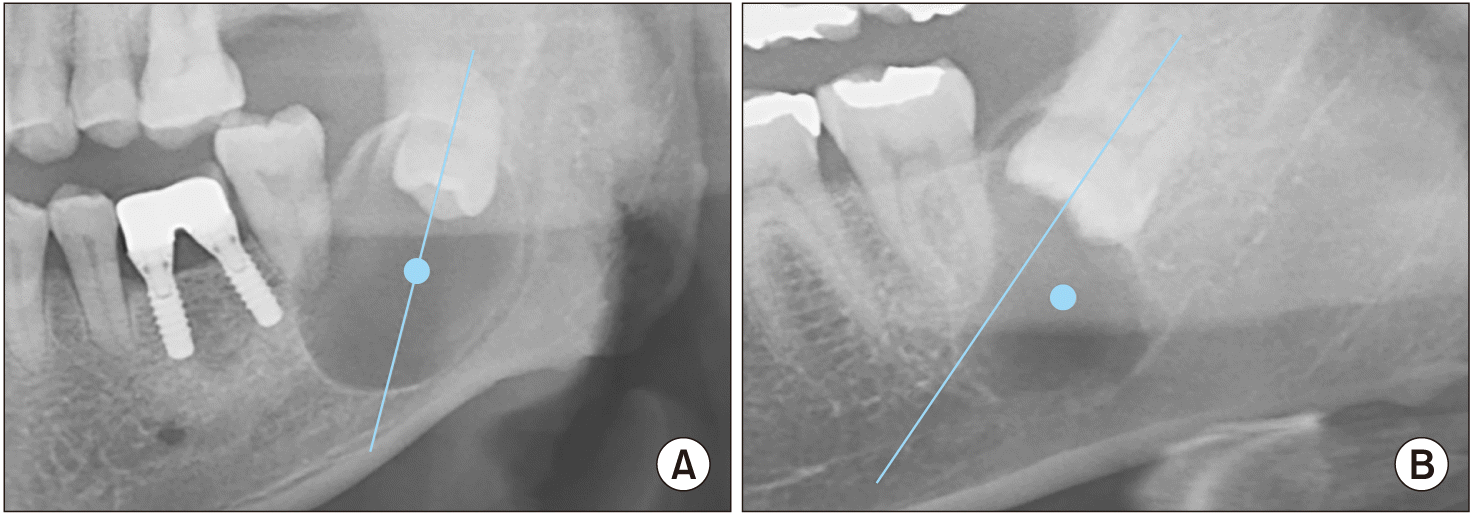
Fig. 2
A. Size comparison between group A and group B. B. Ratio comparison between group A and group B. Group A: dentigerous cyst, Group B: odontogenic keratocyst, ameloblastoma, and odontogenic myxoma. *P<0.05. (BL: buccolingual, MD: mesiodistal)
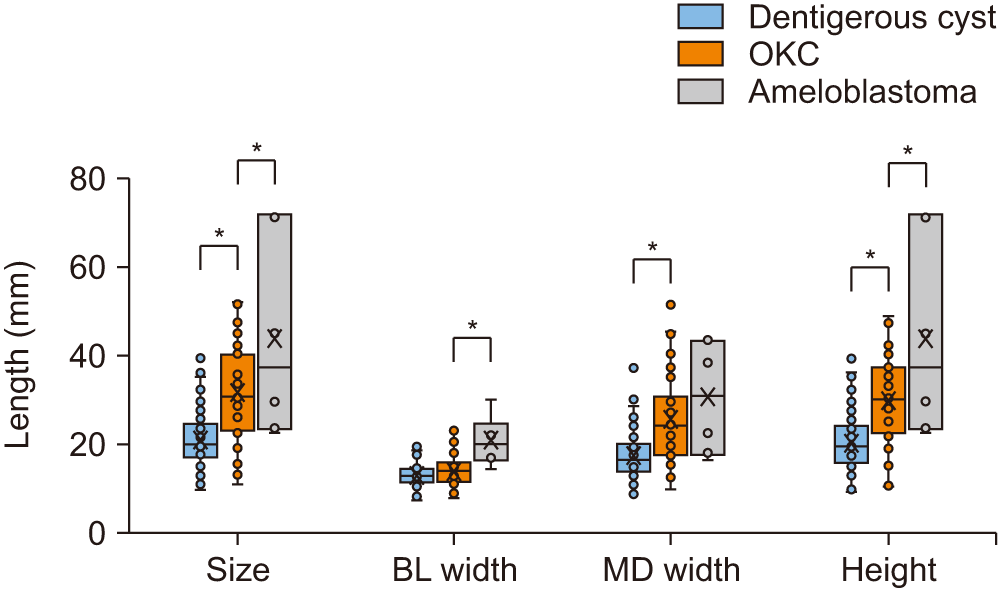
Fig. 3
Comparison of characteristics by lesion size. *P<0.05. (OKC: odontogenic keratocyst, BL: buccolingual, MD: mesiodistal)
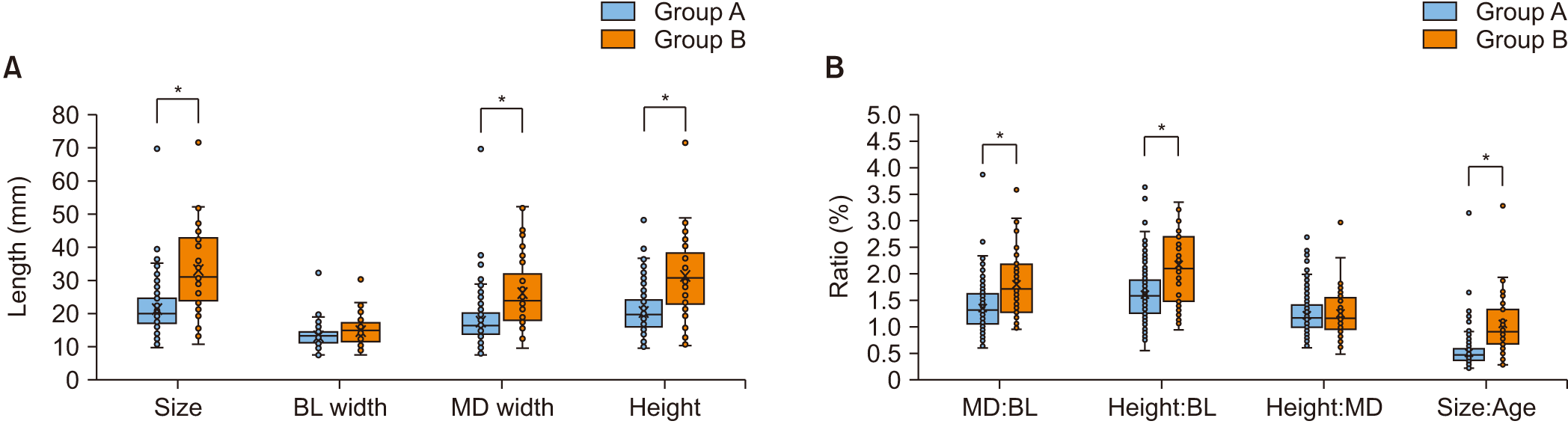
Table 1
Histological findings of lesions associated with impacted mandibular third molars
| Dentigerous cyst | OKC | Epithelial cyst | Radicular cyst | Ameloblastoma | Odontogenic myxoma | Hemangioma | |
|---|---|---|---|---|---|---|---|
| No. of patients | 212 | 43 | 37 | 9 | 6 | 1 | 1 |
| Bilateral occurrence | 8 | 0 | 0 | 0 | 0 | 0 | 0 |
| No. of cases | 220 | 43 | 37 | 9 | 6 | 1 | 1 |
Table 2
Clinical and radiographical comparison of characteristics by lesion
| Dentigerous cyst (n=257) | OKC (n=43) | Ameloblastoma (n=6) | P-value | |
|---|---|---|---|---|
| Sex | ||||
| Male | 198 (77.0) | 30 (69.8) | 3 (50.0) | 0.202 |
| Female | 59 (23.0) | 13 (30.2) | 3 (50.0) | |
| Age (yr) | 44.8±12.0 | 38.1±13.7 | 22.5±4.0 | <0.001* |
| Location | ||||
| Left | 127 (49.4) | 23 (53.5) | 4 (66.7) | 0.638 |
| Right | 130 (50.6) | 20 (46.5) | 2 (33.3) | |
| Position | ||||
| Central | 163 (63.4) | 11 (25.6) | 2 (33.3) | <0.001* |
| Lateral | 94 (36.6) | 32 (74.4) | 4 (66.7) | |
| Penetration | ||||
| Present | 45 (17.5) | 20 (46.5) | 2 (33.3) | <0.001* |
| Absent | 212 (82.5) | 23 (53.5) | 4 (66.7) | |
| Expansion | ||||
| Present | 109 (42.4) | 28 (65.1) | 5 (83.3) | 0.004* |
| Absent | 148 (57.6) | 15 (34.9) | 1 (16.7) | |
| Locularity | ||||
| Monocular | 250 (97.3) | 33 (76.7) | 4 (66.7) | <0.001* |
| Multilocular | 7 (2.7) | 10 (23.3) | 2 (33.3) | |
| IAN displacement | ||||
| Present | 157 (61.1) | 33 (76.7) | 3 (50.0) | 0.115 |
| Absent | 100 (38.9) | 10 (23.3) | 3 (50.0) | |
| 2nd molar displacement | ||||
| Present | 2 (0.8)1 | 1 (2.4)2 | 1 (16.7) | <0.001* |
| Absent | 250 (99.2)1 | 41 (97.6)2 | 5 (83.3) | |
| 2nd molar root resorption | ||||
| Present | 10 (4.0)1 | 5 (11.9)2 | 4 (66.7) | 0.027* |
| Absent | 242 (96.0)1 | 37 (88.1)2 | 2 (33.3) | |
| Preop. symptoms3 | ||||
| Present | 58 (22.6) | 9 (20.9) | 3 (50.0) | 0.271 |
| Absent | 199 (77.4) | 34 (79.1) | 3 (50.0) | |
| Preop. numbness3 | ||||
| Present | 8 (3.1) | 1 (2.3) | 1 (16.7) | 0.170 |
| Absent | 249 (96.9) | 42 (97.7) | 5 (83.3) | |
| 3rd molar root contact | ||||
| Present | 25 (9.7) | 31 (72.1) | 3 (50.0) | <0.001* |
| Absent | 232 (90.3) | 12 (27.9) | 3 (50.0) | |
| Range of 3rd molar root contact | ||||
| 1/2 or more | 12 (48.0) | 22 (71.0) | 2 (66.7) | 0.211 |
| Less than 1/2 | 13 (52.0) | 9 (29.0) | 1 (33.3) | |
Table 3
Clinical and radiographical comparison between group A and group B
|
Group A (n=257) |
Group B (n=50) |
P-value | |
|---|---|---|---|
| Sex | |||
| Male | 198 (77.0) | 17 (34.0) | <0.001* |
| Female | 59 (23.0) | 33 (66.0) | |
| Age (yr) | 44.8±12.0 (18-78) | 35.8±13.9 (18-65) | 0.001* |
| Location | |||
| Left | 127 (49.4) | 29 (58.0) | 0.283 |
| Right | 130 (50.6) | 21 (42.0) | |
| Position | |||
| Central | 163 (63.4) | 13 (26.0) | <0.001* |
| Lateral | 94 (36.6) | 37 (74.0) | |
| Penetration | |||
| Present | 45 (17.5) | 22 (44.0) | <0.001* |
| Absent | 212 (82.5) | 28 (56.0) | |
| Expansion | |||
| Present | 109 (42.4) | 33 (66.0) | 0.003* |
| Absent | 148 (57.6) | 17 (34.0) | |
| Thinning | |||
| Present | 202 (78.6) | 47 (94.0) | 0.010* |
| Absent | 55 (21.4) | 3 (6.0) | |
| Locularity | |||
| Monocular | 250 (97.3) | 38 (76.0) | <0.001* |
| Multilocular | 7 (2.7) | 12 (24.0) | |
| IAN displacement | |||
| Present | 157 (61.1) | 37 (74.0) | 0.108 |
| Absent | 100 (38.9) | 13 (26.0) | |
| Bilateral occurrence | |||
| Present | 8 (3.2)1 | 0 (0) | 0.360 |
| Absent | 241 (96.8)1 | 50 (100) | |
| 2nd molar displacement | |||
| Present | 2 (0.8)2 | 2 (4.1)3 | 0.125 |
| Absent | 250 (99.2)2 | 47 (95.9)3 | |
| 2nd molar root resorption | |||
| Present | 10 (4.0)2 | 9 (18.4)3 | 0.001* |
| Absent | 242 (96.0)2 | 40 (81.6)3 | |
| 2nd molar vitality loss4 | |||
| Present | 12 (25.0) | 5 (45.5) | 0.267 |
| Absent | 36 (75.0) | 6 (54.5) | |
| Preop. symptoms4 | |||
| Present | 58 (22.6) | 12 (24.0) | 0.854 |
| Absent | 199 (77.4) | 38 (76.0) | |
| Preop. numbness4 | |||
| Present | 8 (3.1) | 2 (4.0) | 0.669 |
| Absent | 249 (96.9) | 48 (96.0) | |
| 3rd molar root contact | |||
| Present | 25 (9.7) | 34 (68.0) | <0.001* |
| Absent | 232 (90.3) | 16 (32.0) | |
| Range of 3rd molar root contact | |||
| 1/2 or more | 12 (48.0) | 24 (70.6) | 0.107 |
| Less than 1/2 | 13 (52.0) | 10 (29.4) | |
| Size:age | |||
| 1 or more | 8 (3.1) | 19 (38.0) | <0.001* |
| Less than 1 | 249 (96.9) | 31 (62.0) | |




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download