Abstract
Notes
Authors’ Contributions
G.S. participated in original draft preparation and writing the manuscript. H.K. and H.J.K. participated in conceptualization and reviewing and editing the manuscript. M.G., S.Y.H., and E.S.C. participated in data curation and investigation. All authors read and approved the final manuscript.
References
Fig. 1
Fig. 2
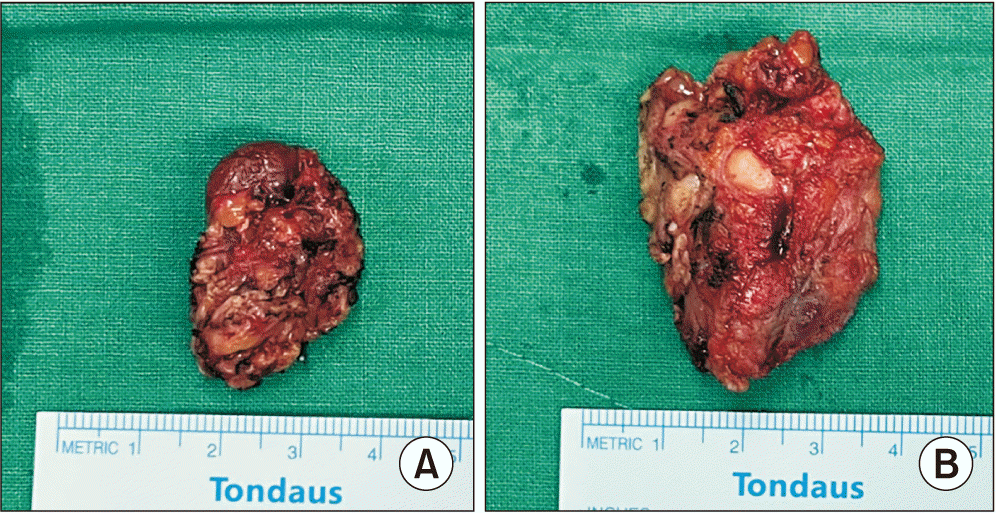
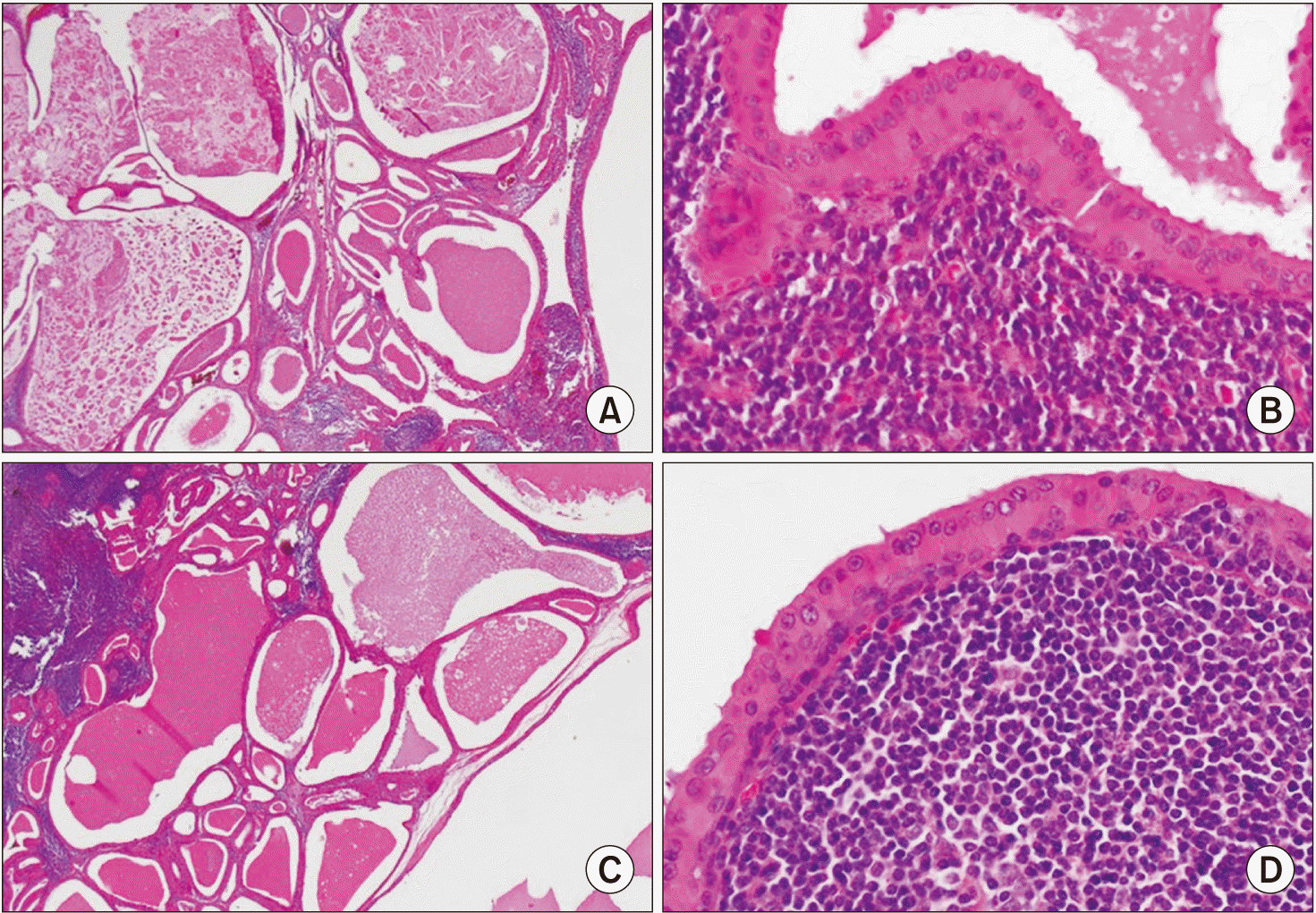
Table 1
| Study | Age (yr)/sex | Initial symptom | Site of SCC |
Site of WT (ipsilateral/contralateral/bilateral) |
Alcohol history | Smoking history | Other metastases | TNM stage | Treatment | Survival |
|---|---|---|---|---|---|---|---|---|---|---|
| Sato et al.7 (1998) | 72/M | Sore throat | RMT, oropharynx | Parotid, ipsilateral cervical lymph node, ipsilateral | NR | Yes (2 packs/day, 50 years) | None | T2N0M0 |
Neoadjuvant RTx SND (level I-III) Partial parotidectomy |
DFS 7 years |
| 60/M | Ulcer | Buccal mucosa | Parotid, ipsilateral cervical lymph node, ipsilateral | NR | Yes (0.5 packs/day, 35 years) | None | NR |
Wide excision Lymph node excision Partial parotidectomy |
DFS 1.5 years | |
| Maiorano et al.6 (2002) | NR | NR | Larynx | Parotid, ipsilateral | NR | NR | NR | NR |
Wide excision Partial parotidectomy |
NR |
| NR | NR | Larynx | Parotid, ipsilateral | NR | NR | NR | NR |
Wide excision Partial parotidectomy |
NR | |
| Sheahan et al.5 (2005) | 55/M | NR | RMT | Cervical lymph node, ipsilateral | NR | NR | None | TxN1M0 |
Wide excision mRND |
DFS 2 years |
| Schwarz et al.8 (2009) | 42/M | Tongue pain | Tongue | Cervical lymph node, ipsilateral | NR | Yes | None | T2N0M0 |
Wide excision SND (level I-III, bilateral) |
NR |
| Enomoto et al.9 (2011) | 67/M | Cheek swelling | Mandible | Parotid, ipsilateral | NR | NR | None | T4aN0M0 |
Wide excision SND (level I-III) |
NR |
| Iwai et al.10 (2012) | 77/F | Tongue pain | Tongue | Paraparotid node, ipsilateral | NR | Yes | None | T2N0M0 |
Wide excision Partial parotidectomy |
NR |
| Bhatlawande et al.4 (2020) | 51/M | Gingival pain | Lower gingiva | Cervical lymph node, ipsilateral | Yes (20 years) | Yes (5-6 pack/day, 20 years) | None | TxN0M0 |
Wide excision SND (level I-IV) |
NR |
| Sato et al.11 (2020) | 56/M | Gingival pain | Buccal mucosa, upper gingiva | Cervical lymph node, ipsilateral | NR | Yes (1.5 pack/day, 23 years) | None | T4aN0M0 |
Neoadjuvant CCRTx RND |
DFS 8 years |
| Kumar et al.12 (2020) | 52/M | Voice problem | Larynx, bilateral | Submandibular gland, unilateral | Yes (200 mL/day for the last 30 years) | Yes (2 pack/day, 30 years) | None | T3N0M0 |
Wide excision SND (level I-IV), bilateral |
NR |
| Yang et al.13 (2021) | 65/M | Mouth floor ulcer | FOM | Submandibular gland, ipsilateral | NR | Yes (30 years) | None | T2N1M0 |
Wide excision mRND & SND (level I-III) |
DFS 3 years |
| Goh et al.14 (2022) | 63/F | Cheek mass | Buccal mucosa | Parotid, ipsilateral | NR | Yes | None | T2N1M0 |
Wide excision mRND |
DFS 4 months |
| 69/M | Tongue ulcer | Tongue | Parotid, ipsilateral | NR | Yes (40 years) | None | T1N0M0 |
Wide excision SND (level I-III) Partial parotidectomy |
DFS 7 years | |
| Gontarz et al.15 (2022) | 86/F | NR | Buccal mucosa | Cervical lymph node, ipsilateral | NR | NR | None | T3N2bM0 |
Wide excision SND (level I-III) |
DFS 1.5 years |
| 67/F | Tongue | Cervical lymph node, ipsilateral | None | T2N0M0 |
Wide excision mRND |
DFS 2 years 11 months |
||||
| 65/F | Lower gingiva | Cervical lymph node, ipsilateral | None | T2N0M0 |
Wide excision SND (level I-III) |
DFS 2 years 4 months |
||||
| 54/M | FOM | Cervical lymph node, ipsilateral | None | T3N1M0 |
Wide excision SND (level I-III) |
DFS 3 years 10 months |
||||
| Present case | 64/M | FOM ulcer | FOM | Parotid, unilateral | Yes (40 years) | Yes (1 pack/day, 40 years) | None | T2N0M0 |
Wide excision SND (level I-III), bilateral Partial parotidectomy |
DFS 8 months |
(SCC: squamous cell carcinoma, WT: Warthin’s tumor, M: male, F: female, RMT: retromolar triagone, NR: not reported, RTx: radiotherapy, SND: selective neck dissection, DFS: disease-free survival, CCRTx: concurrent chemo-radiotherapy, RND: radical neck dissection, FOM: floor of mouth, mRND: modified radical neck dissection)




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download