Abstract
Neuro-Behçet's disease (NBD) represents a significant complication of Behçet's syndrome, potentially leading to elevated mortality and disability rates. The standard treatment for parenchymal NBD typically entails administering high-dose corticosteroids to prompt rapid-onset effects, coupled with immunosuppressants to prevent subsequent relapses. A 48-year-old male with NBD presented with progressively worsening dysarthria over 9 months. This patient experienced increased intraocular pressure while using glucocorticoids, which worsened his pre-existing glaucoma. The patient had a prior diagnosis of NBD and presented with progressive dysarthria over a period of nine months, leading to a brain magnetic resonance imaging (MRI) scan. The brain MRI revealed multifocal punctate high signal intensities in the left frontoparietal area, insula, and basal ganglia. Instead of the standard steroid pulse therapy, the patient received adalimumab-cyclophosphamide combination as an alternative induction therapy. Subsequent serial brain MRI scans exhibited no emergence of new lesions, and the patient remained devoid of clinical relapses even after 17 months from the commencement of induction treatment. Adalimumab-cyclophosphamide combination could be used as a corticosteroid-free induction strategy for NBD. Further investigations are warranted to establish the most suitable combination regimen.
Neuro-Behçet’s disease (NBD) is observed in approximately 9% of patients with Behçet’s syndrome (BS) and has a high mortality (25%~35%) and disability rates (65%) [1]. The treatment for parenchymal NBD includes high-dose corticosteroids to induce rapid-onset effects and immunosuppressants, such as azathioprine, methotrexate, or cyclophosphamide, to prevent further relapse [2-4]. Recently, anti-tumor necrosis factor (TNF)-α agents have been recommended in BS patients who fail to respond to conventional treatments or those with poor prognostic factors [5]. However, there has been no discussion on the treatment of patients with NBD who have an intolerance or a contraindication to high-dose corticosteroids during the induction treatment period. Herein, we present the case of a BS patient who was treated with corticosteroid-free adalimumab-cyclophosphamide combination therapy for parenchymal NBD due to steroid-induced acute intraocular hypertension.
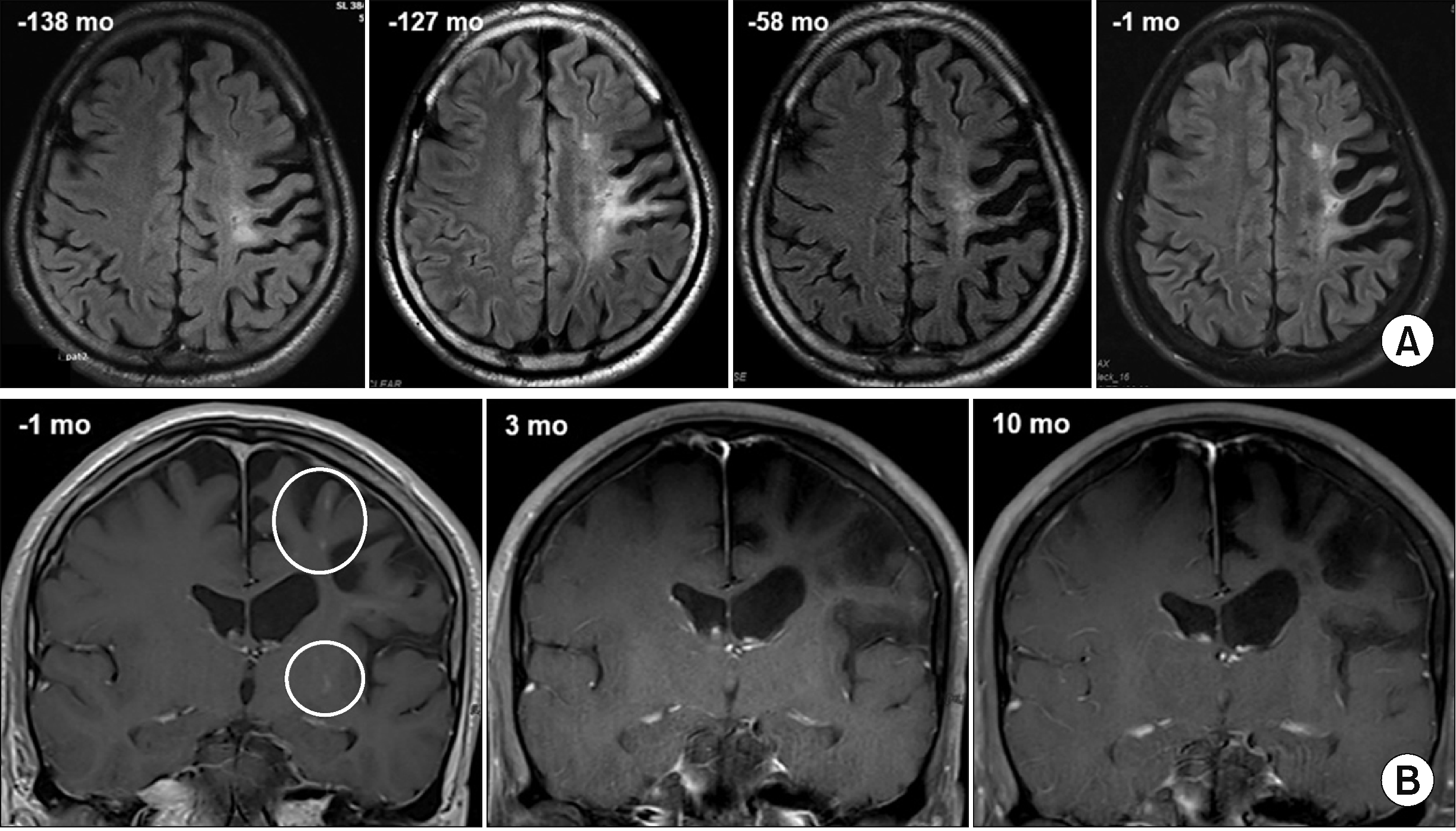
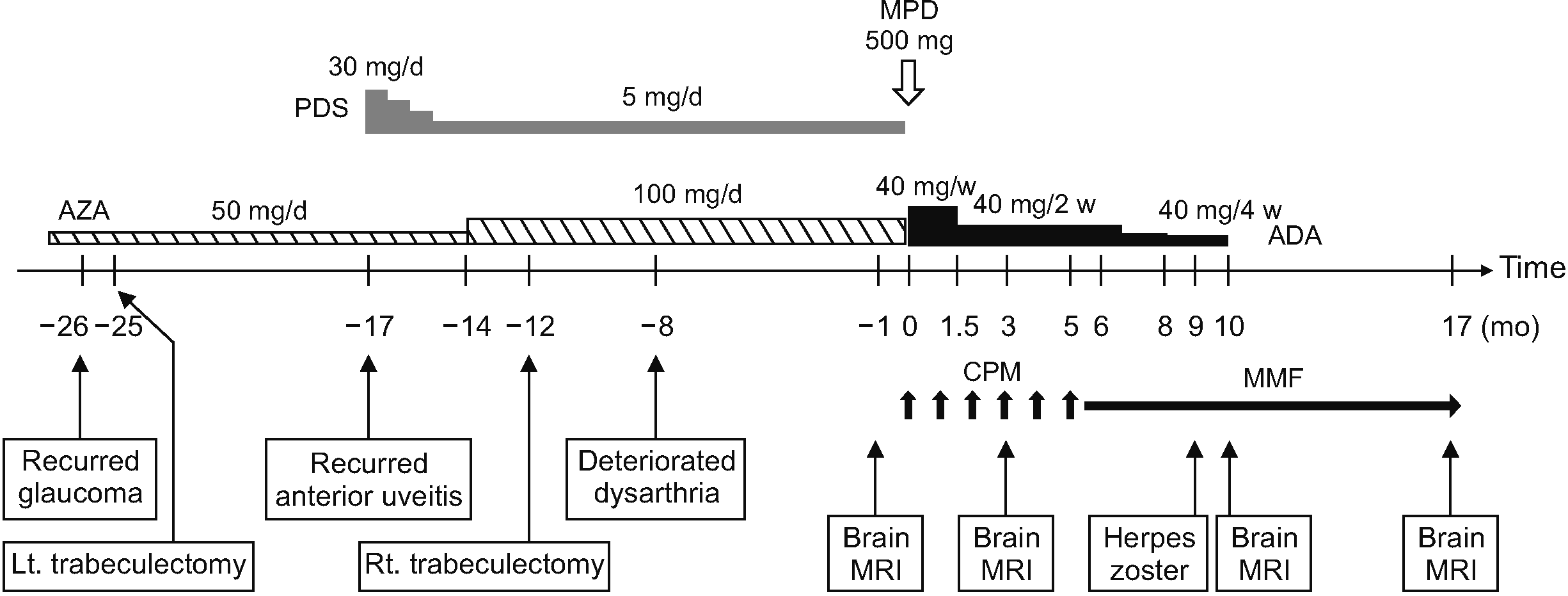
A 48-year-old male with NBD presented with progressively worsening dysarthria over 9 months. Twelve years ago, the patient received a diagnosis of NBD based on a constellation of symptoms, including recurrent oral ulcers, anterior uveitis, retinal vasculitis, and neurological manifestations. There is no pertinent family history of relevant medical conditions. The Human Leukocyte Antigen-B51 test showed positive results. Initial complete blood count profiles and acute phase reactants were within normal limits. Liver function tests, blood urea nitrogen, and creatinine levels were also within the normal range. His neurological symptoms included weakness and motor clumsiness in the right extremities, tonic-clonic seizures, and dysarthria. Brain magnetic resonance imaging (MRI) showed multifocal high signal intensities in the left parietal lobe and pre- and post-central gyri (Figure 1A). Despite a good response to corticosteroids and pan-retinal photocoagulation, the patient relapsed when the corticosteroids were tapered. After undergoing a left vitrectomy, his BS was well-controlled with azathioprine (100 mg/day), anticonvulsants, and anti-glaucoma eye drops for 10 years. Five years later, azathioprine was reduced to 50 mg/day because of the absence of disease activity and new lesions on follow-up brain MRIs.
Twenty-six months before presentation, the patient had undergone left trabeculectomy and received intraocular bevacizumab and 5-fluorouracil injections for neovascular glaucoma (Figure 2). Because of the presence of cells in the anterior chamber and retinal vascular leakage on fluorescein angiography, prednisolone was restarted at 30 mg/day, and the dose of azathioprine was increased to 100 mg/day 17 months before presentation. However, his intraocular pressure (IOP) was not adequately controlled, and a right trabeculectomy was performed 6 months later. Moreover, his dysarthria gradually progressed, and follow-up brain MRI revealed new lesions (Figures 1A and 2).
The patient experienced decreased visual acuity and severe ocular pain after receiving a single dose of methylprednisolone (500 mg/day). Because of the steroid-induced IOP elevation, the adalimumab-cyclophosphamide combination was used as an alternative induction therapy after a thorough discussion with the patient. Adalimumab is approved for the treatment of BS uveitis in Korea, and corticosteroids-adalimumab-cyclophosphamide triple therapy in an NBD case has been reported [6]. The patient received adalimumab 240 mg over 6 weeks based on the loading dose, followed by maintenance doses of 40 mg every 2 weeks [7]. Since cyclophosphamide pulse therapy (900 mg/month) was performed for 6 months, mycophenolate mofetil (MMF) (2 g/day) was used as maintenance therapy and adalimumab was tapered out.
Two weeks after the adalimumab-cyclophosphamide combination therapy, the patient reported an improvement in neurological symptoms. Follow-up brain MRIs showed rapid resolution of high signal intensity lesions 3 and 10 months after treatment initiation (Figure 1B), although adalimumab was tapered and switched to mycophenolate. The patient had a localized herpes zoster infection in the chest wall 9 months after the treatment initiation. Bevacizumab was discontinued after 10 months. Serial brain MRIs revealed no new lesions, and the patient remained free of clinical relapse at 17 months after treatment initiation.
The current case is unique because acute NBD symptoms were treated with a combination of adalimumab and cyclophosphamide as induction therapy instead of high-dose corticosteroids. Corticosteroids play a crucial role in the acute management of various immunological conditions, including NBD. However, because many adverse effects are unavoidable with high-dose or long-term corticosteroid use, corticosteroid-free protocols have been proposed for organ transplantation and autoimmune diseases [8,9]. To our knowledge, this is the first reported case of a corticosteroid-free induction regimen for NBD.
It is recommended to start immunosuppressants while tapering corticosteroids over several months to reduce the risk of NBD relapse [2-4]. Azathioprine is considered the first-line treatment for major organs involved in BS. Recently, both infliximab and adalimumab have been listed as second-line agents for the treatment of refractory NBD [2,3]. Desbois et al. [10] reported that, although an overall improvement was observed in 94% of patients, the complete response rate of anti-TNF-α therapies was only 29%, and the median time to remission was 3 months [10]. Additionally, the efficacy of anti-TNF-α monotherapy was comparable to that of anti-TNF-α combination therapy with azathioprine or methotrexate in NBD. Cyclophosphamide may be viewed as a first-line option in handicapped patients with NBD or patients with vascular BS [2,4]. Korkmaz et al. [6] described a corticosteroid-refractory NBD patient treated with the concurrent administration of cyclophosphamide and adalimumab.
Cyclophosphamide exerts its therapeutic effects through multi-target mechanisms, similar to glucocorticoids. On the other hand, anti-TNF-α monoclonal antibodies including adalimumab have a single-targeted mechanism of action. Additionally, the TNF-α blocking agents do not cross the blood-brain barrier whereas cyclophosphamide and methylprednisolone can reach therapeutic levels in the central nervous system (CNS) [11]. Moreover, the addition of anti-TNF-α antibodies does not appear to heighten the risk of cyclophosphamide-induced bone marrow suppression, a critical concern associated with cyclophosphamide administration. Considering differences in their mechanisms of action, CNS levels, and potential adverse effects, we utilized both adalimumab and cyclophosphamide for the induction therapy in the present case.
In our therapeutic strategy, we incorporated MMF for maintenance therapy because MMF is considered an alternative option for NBD and non-infectious uveitis [5,12,13]. This decision was also based on MMF’s favorable safety profile compared to cyclophosphamide [14]. Additionally, anti-TNF-α agents are not reimbursed for patients with NBD in Korea.
While the case received adalimumab and cyclophosphamide, the primary concern regarding adverse effects was opportunistic infections. Given that Korkmaz et al.’s cases [6] involved tuberculous pleurisy and our case was diagnosed with herpes zoster, infection risk should be carefully considered during adalimumab-cyclophosphamide combination treatment.
We report the case of a patient with NBD who achieved remission with corticosteroid-free adalimumab-cyclophosphamide combination therapy. This report provides a new option for patients with NBD who are intolerant or have contraindications to corticosteroids. Further studies are required to establish the most suitable combination regimen as a corticosteroid-free induction strategy for NBD.
Notes
FUNDING
This study was partially supported by a fund from Seoul National University Bundang Hospital (No. 21-2023-0079).
AUTHOR CONTRIBUTIONS
J.H.K. contributed to manuscript writing (original draft preparation). Y.J.L. contributed to conceptualization. Y.J.L. contributed to visualization. J.H.K. contributed to investigation. S.W.J. contributed to manuscript writing (reviewing and editing); all authors have read and approved the final manuscript.
REFERENCES
1. Borhani-Haghighi A, Kardeh B, Banerjee S, Yadollahikhales G, Safari A, Sahraian MA, et al. 2020; Neuro-Behcet's disease: an update on diagnosis, differential diagnoses, and treatment. Mult Scler Relat Disord. 39:101906. DOI: 10.1016/j.msard.2019.101906. PMID: 31887565.

2. Hatemi G, Christensen R, Bang D, Bodaghi B, Celik AF, Fortune F, et al. 2018; 2018 update of the EULAR recommendations for the management of Behçet's syndrome. Ann Rheum Dis. 77:808–18. DOI: 10.1136/annrheumdis-2018-213225. PMID: 29625968.

3. Hirohata S, Kikuchi H, Sawada T, Okada M, Takeno M, Kuwana M, et al. 2020; Recommendations for the management of neuro-Behçet's disease by the Japanese National Research Committee for Behçet's Disease. Intern Med. 59:2359–67. DOI: 10.2169/internalmedicine.4705-20. PMID: 32611961. PMCID: PMC7644487.

4. Kone-Paut I, Barete S, Bodaghi B, Deiva K, Desbois AC, Galeotti C, et al. 2021; French recommendations for the management of Behçet's disease. Orphanet J Rare Dis. 16(Suppl 1):352. DOI: 10.1186/s13023-020-01620-4. PMID: 33622338. PMCID: PMC7903591.

5. Alpsoy E, Leccese P, Emmi G, Ohno S. 2021; Treatment of Behçet's disease: an algorithmic multidisciplinary approach. Front Med (Lausanne). 8:624795. DOI: 10.3389/fmed.2021.624795. PMID: 33996847. PMCID: PMC8115406.

6. Korkmaz FN, Ozen G, Ünal AU, Kahraman Koytak P, Tuncer N, Direskeneli H. 2016; Severe neuro-Behcet's disease treated with a combination of immunosuppressives and a TNF-inhibitor. Acta Reumatol Port. 41:367–71.
7. Tanida S, Inoue N, Kobayashi K, Naganuma M, Hirai F, Iizuka B, et al. 2015; Adalimumab for the treatment of Japanese patients with intestinal Behçet's disease. Clin Gastroenterol Hepatol. 13:940–8.e3. DOI: 10.1016/j.cgh.2014.08.042. PMID: 25245624.

8. Luo G, Falta EM, Elster EA. 2005; Steroid-free immunosuppression in organ transplantation. Curr Diab Rep. 5:305–10. DOI: 10.1007/s11892-005-0028-x. PMID: 16033684.

9. Fischer-Betz R, Chehab G, Sander O, Vordenbäumen S, Voiculescu A, Brinks R, et al. 2012; Renal outcome in patients with lupus nephritis using a steroid-free regimen of monthly intravenous cyclophosphamide: a prospective observational study. J Rheumatol. 39:2111–7. DOI: 10.3899/jrheum.120537. PMID: 22984276.

10. Desbois AC, Addimanda O, Bertrand A, Deroux A, Pérard L, Depaz R, et al. 2016; Efficacy of anti-TNFα in severe and refractory neuro-Behcet disease: an observational study. Medicine (Baltimore). 95:e3550. DOI: 10.1097/MD.0000000000003550. PMID: 27281066. PMCID: PMC4907644.
11. Pardridge WM. 2010; Biologic TNFα-inhibitors that cross the human blood-brain barrier. Bioeng Bugs. 1:231–4. DOI: 10.4161/bbug.1.4.12105. PMID: 21327054. PMCID: PMC3026461.

12. Sota J, Capuano A, Emmi G, Iannone F, Cantarini L, Hatemi G, et al. 2023; Therapeutic approach to central nervous system involvement of Behçet's disease. Semin Arthritis Rheum. 61:152206. DOI: 10.1016/j.semarthrit.2023.152206. PMID: 37172497.

13. Corbitt K, Nowatzky J. 2023; Inflammatory eye disease for rheumatologists. Curr Opin Rheumatol. 35:201–12. DOI: 10.1097/BOR.0000000000000933. PMID: 36943695. PMCID: PMC10461883.

14. Mak A, Cheak AA, Tan JY, Su HC, Ho RC, Lau CS. 2009; Mycophenolate mofetil is as efficacious as, but safer than, cyclophosphamide in the treatment of proliferative lupus nephritis: a meta-analysis and meta-regression. Rheumatology (Oxford). 48:944–52. DOI: 10.1093/rheumatology/kep120. PMID: 19494179.

Figure 1
Serial brain magnetic resonance imaging. (A) T2-weighted, fluid-attenuated inversion recovery (FLAIR) images show temporal changes in high signal intensities in the left pre- and post-central gyrus and parietal lobe. (B) Pre-treatment (at 1 month) T1-weighted fat-suppressed contrast-enhanced coronal images show multifocal punctate hyperintense lesions in the left frontoparietal and basal ganglia regions. The intensities disappeared after the adalimumab-cyclophosphamide combination therapy (at 3 and 10 months).




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download