INTRODUCTION
METHODS
Search strategy and data sources
Inclusion criteria and outcomes of interest
Interventions
Outcomes
Study design
Selection of studies
Data extraction
Quality assessment
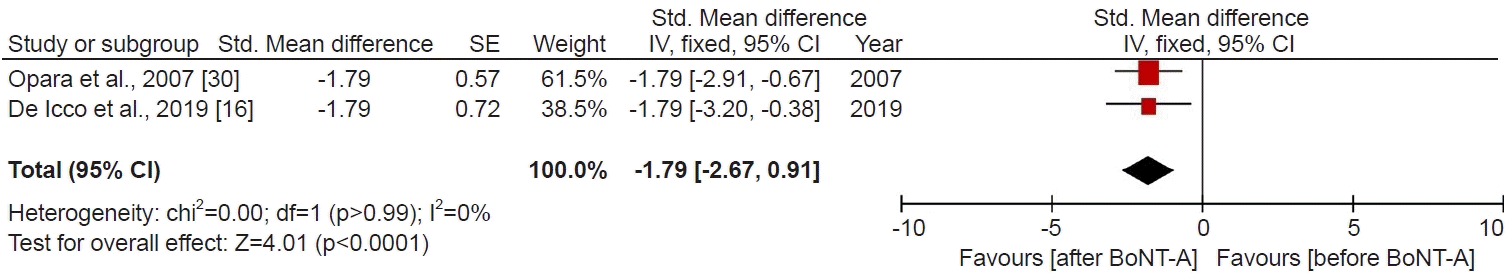
Data analysis
RESULTS
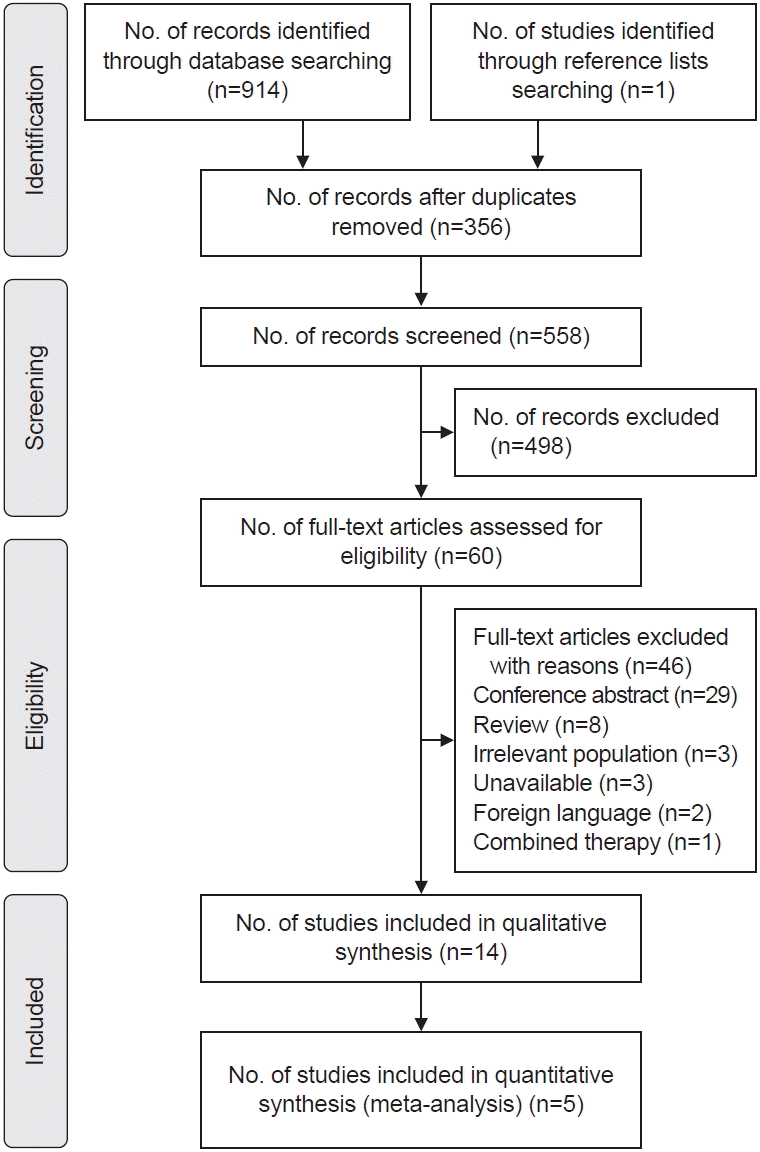
Search strategy
Characteristics of included studies
Table 1.
Table 2.
| Reference (country) | Study design | Participant | Inclusion criteria | Experimental group | Control group | Outcome measure |
|---|---|---|---|---|---|---|
| Yan et al., 2018 [15] (China) | Parallel-group | Total: n=224 (66 male, 158 female) | 1. Duration since injury: more than 6 months | BoNT-A 500 IU | Control group 1: locomotor training and intensive task-specific training for 6 weeks | MAS, DAS, BI |
| Control group 1: n=112 (35.47±2.21 yr) | 2. ≤2 MAS | Control group 2: 5 mg of baclofen 3 times/day for 1 week, 10 mg of baclofen | ||||
| Control group 2: n=112 (36.55±3.42 yr) | 3 times/day for 1 week in the second week, 15 mg of baclofen 3 times/day for 1 week in the third week, and 20 mg of baclofen 3 times/day for 1 week in the 4th week | |||||
| BoNT-A group: n=112 (36.95±7.12 yr) | ||||||
| Richardson et al., 2000 [27] (UK) | Parallel-group | Total: n=6a) | 1. Duration since injury: more than 6 months | BoNT-A 100 IU | Placebo | AS, RMA, BI, GAS |
| Placebo group: n=2a) | 2. Moderate to severe spasticity | |||||
| BoNT group: n=4a) |
Age presented as mean±standard deviation.
Control group 1=physical therapy; control group 2=baclofen.
BoNT-A, botulinum toxin-A; MAS, modified Ashworth scale; DAS, disability assessment scale; BI, Barthel index; AS, Ashworth scale; RMA, Rivermead motor assessment; GAS, goal attainment scale.
a)Etiology specific data not reported.
Table 3.
| Reference (country) | Population | ASIA impairment scale | N | Mean age (range), yr | Male (%) | Intervention | Outcome | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Opara et al., 2007 [30] (Poland) | SCI patients with lower limb paresis and spasticity confirmed by MAS | NR | 8 | 40.25 (23–62) | 100 | BoNT-A, 100–300 IU | MAS, VAS, MRMI | ||||||||||
| Bernuz et al., 2012 [28] (France) | SCI patients with lower limb spasticity and spasticity confirmed by Ely test as well as MAS | D | 15 | 43 (28–58) | 93 | BoNT-A, 200 IU | MTS | ||||||||||
| Béseler et al., 2012 [29] (Spain) | SCI patients with lower limb spasticity and spasticity confirmed by MAS | NR | 2 | NR (mean) (>18) | 50 | BoNT-A, 100–300 IU | MAS | ||||||||||
| Spiegl et al., 2014 [31] (Germany) | SCI patients with lower limb spasticity and spasticity confirmed by MAS | A, C | 9 | 40 (24–56) | 100 | BoNT-A, 100 IU | MAS | ||||||||||
| De Icco et al., 2019 [16] (Italy) | SCI patients with lower limb spasticity and spasticity confirmed by MAS as well as MRC | NR | 5 | 39.5 (30.3–48.7) | 100 | BoNT-A, 50–200 IU | MAS, BI, FIM, NRS |
ASIA, American Spinal Injury Association; SCI, spinal cord injury; MAS, modified Ashworth scale; NR, not reported; BoNT-A, botulinum toxin-A; VAS, visual analogue scale; MRMI, modified Rivermead mobility index; MTS, modified Tardieu scale; BI, Barthel index; FIM, Functional Independence Measure; NRS: numerical rating scale.
Table 4.
| Reference (country) | Study population | Case definition | Intervention (dose) | Outcome | Author conclusion | ||
|---|---|---|---|---|---|---|---|
| Age (yr) | Sex | n | |||||
| Richardson et al., 1997 [37] (UK) | 23 | Male | 1 | Incomplete C5/6 SCI with flaccid paralysis of all four limbs. Mild spastic paraparesis in the right leg, and mild hyperreflexia and clumsiness in the left arm and weakness in the right arm, most noticeable in the wrist, finger and thumb extensors | BoNT-A (210 IU) | RMA, VAS, AS | BoNT-A produced a temporary effect on muscle strength but may have a prolonged effect on function |
| Al-Khodairy et al., 1998 [32] (Switzerland) | 50 | Male | 1 | Incomplete T12 paraplegia (ASIA impairment scale=C) patient. presenting spasticity in his lower limbs with frequent painful spasms predominantly at night and with cold humid weather | BoNT-A (100–400 IU) | MAS, SFS, VAS | BoNT-A has its place in the treatment of spasticity in SCI. Although high doses of the product are well tolerated, the quantity should be tailored to the patient’s needs |
| Tang et al., 2009 [38] (USA) | 60 | Male | 1 | C3-C4 SCI with right upper and lower limbs painful spasms. On the physical exam, he had right upper and lower limb hyperspasticity, hemiparesis, and poor positional and vibrational sense | BoNT-A (50–100 IU) | AS, NRS | BoNT-A can relief pain to biceps, flexor digitorum superficilais, brachialis, and pronator teres muscles |
| Naicker et al., 2009 [36] (Malaysia) | 56 | Male | 1 | C-5 SCI with spasticity in hips and knees. Patient had generalized pain in his legs, which was movement-related, dull, aching, and increased during spastic cramps | BoNT-A (NR) | MAS, BI, VAS | BoNT-A reduced spasticity and improved functional outcomes |
| Gross et al., 2012 [34] (France) | 54 | Male | 1 | The traumatic SCI pateint, AIS grade C spastic tetraplegia with right motor level C8, left motor level C5, right sensory level C6, and left sensory level C4. The initial ASIA upper limb motor score was 32 and the lower limb motor score 30 | BoNT-A (100 IU) | MAS | The spastic activity of the rectus femoris and the abnormal knee motion totally reversed after BoNT-A injection |
| Htwe et al., 2016 [35] (Malaysia) | 22 | Female | 1 | T5 AIS A paraplegic patient, who presented with severe lower limb spasticity with unstable hips | BoNT-A (25–75 IU) | MAS, MTS | BoNT-A effective in a case of severe spasticity with unstable hips in SCI patient |
| Frost et al., 2021 [33] (Canada) | 64 | Female | 1 | The traumatic SCI patient, C6 AIS D with hip adductor spasticity that affecting her gait | BoNT-A (200 IU total) | MTS, pain | BoNT-A minimizing postoperative increase in spasticity, reduce pain, and no longer required a gait aid |
C, cervical; SCI, spinal cord injury; ASIA, American Spinal Injury Association; AIS, ASIA impairment scale; T, thoracic; BoNT-A, botulinum toxin-A; NR, not reported; RMA, Rivermead motor assessment; VAS, visual analogue scale; AS, Ashworth scale; MAS, modified Ashworth scale; SFS, spasm frequency score; NRS, numerical rating scale; BI, Barthel index; MTS, modified Tardieu scale.




 PDF
PDF Citation
Citation Print
Print



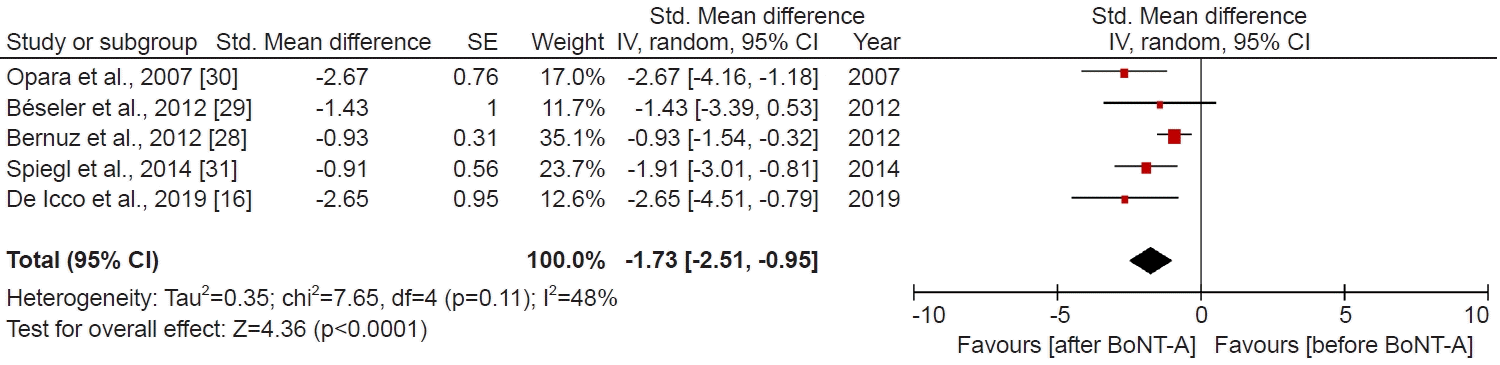
 XML Download
XML Download