Post-surgical hypoparathyroidism is a well-recognized complication after thyroid surgery or operations in other parts of the neck. It results from inadvertent or unavoidable damage to the parathyroid glands and/or their blood supply during surgery [1,2]. Biochemically, hypoparathyroidism is characterized by hypocalcemia, hyperphosphatemia, and increased fractional excretion of calcium in the urine, and the extent of these biochemical derangements can vary depending on the degree of damage to the parathyroid glands [1]. Although some patients with postsurgical hypoparathyroidism exhibit undetectable parathyroid hormone (PTH) levels due to the total removal of the four glands, most other patients have remnant or autotransplanted parathyroid tissue that secretes PTH to a certain extent, but not enough to maintain normal serum calcium levels [2]. The conventional treatment for hypoparathyroidism is high-dose calcium and vitamin D supplementation. This regimen can correct hypocalcemia, but increases the risk of renal insufficiency, kidney stones, and ectopic calcification [3]. To address these issues, replacement therapy with PTH analogues has been developed and is currently under investigation, as detailed in the previous issue of Endocrinology and Metabolism [4].
A new drug being investigated with a different mechanism from PTH analogues is encaleret, a calcium-sensing receptor (CaSR) antagonist or calcilytic that binds to CaSR on parathyroid cells and antagonizes the inhibitory signal of PTH secretion, consequently leading to the hypersecretion of PTH [5]. Encaleret is currently being studied in clinical research targeting autosomal dominant hypocalcemia type 1 (ADH1). In a phase 2 clinical trial involving adults with ADH1, encaleret corrected hypocalcemia, reduced hypercalciuria, and increased PTH secretion [6]. We previously reported that AXT914, another calcilytic, increased PTH secretion and corrected abnormal calcium and phosphorus homeostasis in rats with post-surgical hypoparathyroidism [7]. Furthermore, the biochemical improvements induced by AXT914 treatment persisted even after the drug was discontinued, which implies that AXT914 might have facilitated the recovery of parathyroid tissue autotransplanted into the sternocleidomastoid (SCM) muscle, although the precise mechanism remains unclear [7].
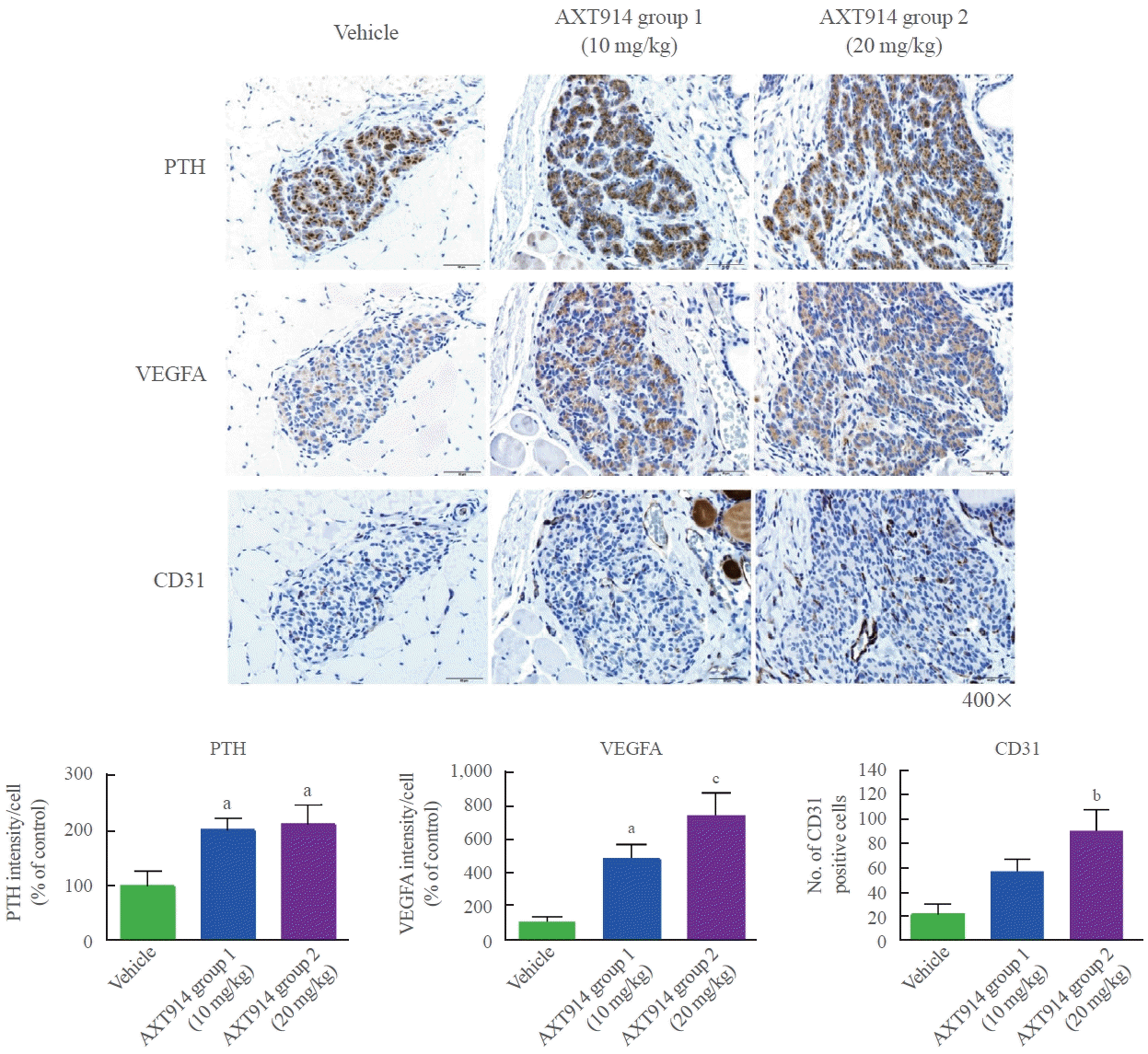
In a follow-up study, we further investigated the effect of AXT914 on autotransplanted parathyroid tissue, with a focus on histological analyses. The study protocol was approved by the Animal Institutional Review Board at Dongguk University (AIRB no. 2019-07188). A total of 33 nine-week-old female Wistar rats were used to create a model of post-surgical hypoparathyroidism resulting from total parathyroidectomy and autotransplantation, as previously described [7]. Both parathyroid glands of each rat were removed, minced into small pieces, and put into a small pocket made in the SCM muscle. The rats were divided into three groups: the vehicle group (n=11), AXT914 group 1 (10 mg/kg, n=11), and AXT914 group 2 (20 mg/kg, n=11). The vehicle or AXT914 (10 or 20 mg/kg) was orally administered once daily for 5 days a week for a 3-week period using a Zonde needle. After the rats were sacrificed, their SCM muscles, where the removed parathyroid tissue had been autotransplanted, were collected for histological examination. Hematoxylin and eosin staining showed that the area of autotransplanted parathyroid tissue was larger in the AXT914 groups than in the vehicle group. AXT914 group 1 showed a 35.7% increase in area, and AXT914 group 2 showed an increase of 201.6% (Fig. 1). Immunohistochemical analyses showed that PTH expression increased by 101.8% and 111.4% in AXT914 group 1 and 2 compared to that in the vehicle group (Fig. 2). The expression of vascular endothelial growth factor (VEGF) A also increased by 387.4% and 646.5% in AXT914 group 1 and 2, respectively (Fig. 2). The number of CD31 positive cells was approximately 3-fold higher in AXT914 group 1 and about 4.5-fold higher in AXT914 group 2 (Fig. 2). However, the expression of Ki67, which is a marker of cell proliferation, was not significantly different between the groups. These results suggest that the administration of AXT914 significantly restores autotransplanted parathyroid tissue by promoting angiogenesis, with results that are observable both functionally and histologically. The mechanism through which AXT914 enhanced angiogenesis is unclear. A previous ex vivo study showed that PTH induced angiogenesis in isolated rat microvessels embedded in a threedimensional collagen I matrix, for which VEGF expression was essential [8]. In a clinical study, serum VEGF levels increased 12 months after the initiation of PTH therapy in patients with hypoparathyroidism [9]. The possibility that calcilytics exert a direct effect on blood vessels can also be considered, as CaSR was found to be expressed in smooth muscle and endothelial cells in human arteries [10].
In conclusion, our findings suggest that calcilytics have potential as a new therapeutic agent to restore damaged or autotransplanted parathyroid tissue, while avoiding adverse effects such as renal complications and ectopic calcification in postsurgical hypoparathyroidism.
REFERENCES
1. Mannstadt M, Bilezikian JP, Thakker RV, Hannan FM, Clarke BL, Rejnmark L, et al. Hypoparathyroidism. Nat Rev Dis Primers. 2017; 3:17055.

2. Lorente-Poch L, Sancho JJ, Munoz-Nova JL, Sanchez-Velazquez P, Sitges-Serra A. Defining the syndromes of parathyroid failure after total thyroidectomy. Gland Surg. 2015; 4:82–90.
3. Bilezikian JP, Brandi ML, Cusano NE, Mannstadt M, Rejnmark L, Rizzoli R, et al. Management of hypoparathyroidism: present and future. J Clin Endocrinol Metab. 2016; 101:2313–24.

4. Rejnmark L. Treatment of hypoparathyroidism by re-establishing the effects of parathyroid hormone. Endocrinol Metab (Seoul). 2024; 39:262–6.

5. Widler L. Calcilytics: antagonists of the calcium-sensing receptor for the treatment of osteoporosis. Future Med Chem. 2011; 3:535–47.

6. Gafni RI, Hartley IR, Roszko KL, Nemeth EF, Pozo KA, Lombardi E, et al. Efficacy and safety of encaleret in autosomal dominant hypocalcemia type 1. N Engl J Med. 2023; 389:1245–7.

7. Lim YS, You BH, Kim HB, Lim SH, Song JG, Bae MG, et al. A new therapeutic approach using a calcilytic (AXT914) for postsurgical hypoparathyroidism in female rats. Endocrinology. 2020; 161:bqaa145.

8. Carter WB, Uy K, Ward MD, Hoying JB. Parathyroid-induced angiogenesis is VEGF-dependent. Surgery. 2000; 128:458–64.

Fig. 1.
Hematoxylin and eosin staining in autotransplanted parathyroid tissue in the sternocleidomastoid muscle. The data are presented as means±standard error of the mean. aP<0.05 vs. the vehicle group.
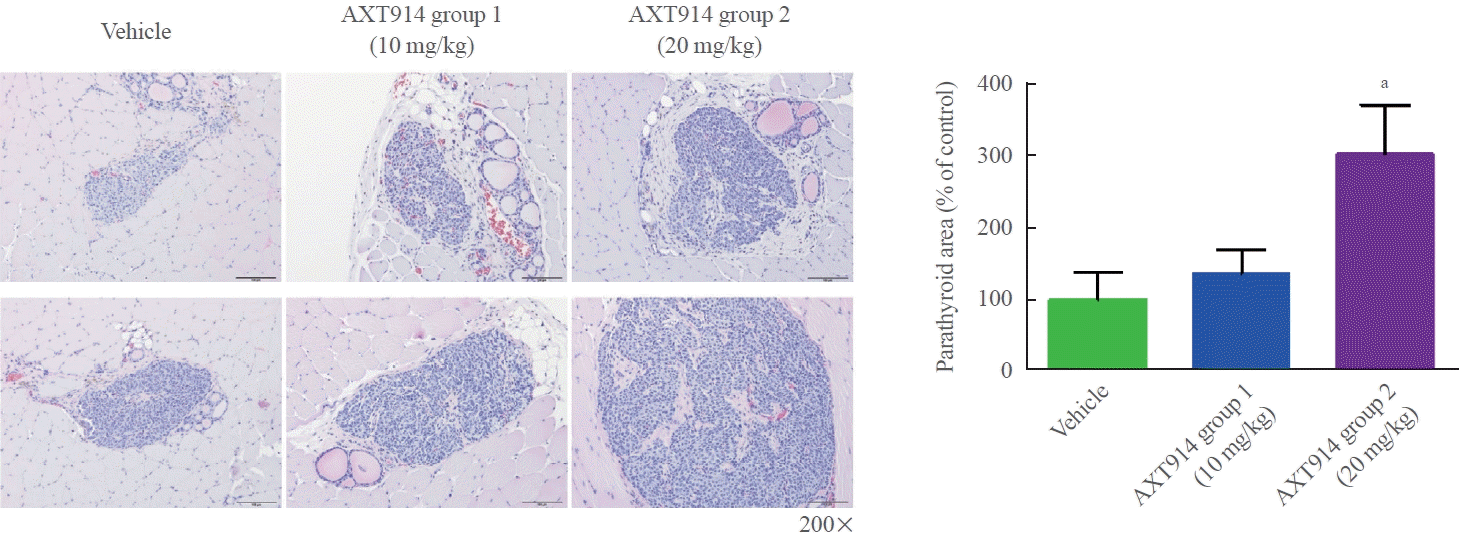
Fig. 2.
Immunohistochemical staining for parathyroid hormone (PTH), vascular endothelial growth factor A (VEGFA), and cluster of differentiation 31 (CD31) in autotransplanted parathyroid tissue in the sternocleidomastoid muscle. The data are presented as means±standard error of the mean. aP<0.05, bP<0.01, cP<0.001 vs. the vehicle group.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download