Osteoporosis is a skeletal disorder characterized by decreased bone density and quality, which is gaining increasing clinical significance in an aging society. The ultimate aim of studies focusing on bone metabolism related to osteoporosis is to prevent fragility fractures, which occur frequently among older individuals. Indeed, hip fractures, the most severe form of fractures caused by low trauma, are associated with a mortality rate of up to 16% within 1 year [1]. Furthermore, even after fractures heal, many individuals are unable to lead independent lives, underscoring the importance of fracture prevention for a healthy old age. However, despite the development and clinical use of numerous medications intended to treat osteoporosis, the incidence of fractures has paradoxically been on the rise [1]. Although various factors require consideration, a critical oversight has been a predominant research focus on bone, neglecting the role of muscle, which directly contributes to the increased risk of falls and subsequent fractures [2,3]. Therefore, achieving the goal of fracture prevention necessitates comprehensive studies on both bone and muscle, including their interrelationship.
In non-obese individuals, skeletal muscles and bones constitute the largest tissues within the human body, forming the musculoskeletal system. This system is intricately designed to facilitate mobility and protect internal organs [4]. Within the context of natural selection, the synergistic operation between muscles and bones is imperative. Illustratively, robust bones paired with weak muscles will compromise an animal’s ability to elude predators. Conversely, powerful muscles coupled with frail bones may lead to an increased susceptibility to fractures as a result of sudden stress exerted by muscular contractions on adjacent bony structures. Consequently, it is inferred that adaptations in muscular and skeletal composition are synchronously regulated throughout an individual’s lifespan. The interplay between bone and muscle tissues embodies a multifaceted system, necessitating an integrative approach for a holistic understanding of their cooperative functionalities [5]. The dialogue between these tissues is underpinned by both biochemical interactions and mechanical forces [6]. This bidirectional communication is mediated through factors secreted from bones and skeletal muscles, namely osteokines and myokines, respectively, highlighting a profound interdependence [7]. Furthermore, as skeletal muscles exert forces on bones, the latter reciprocally offers structural support and anchorage for the muscles [8]. Hence, the intricacy of the bone-muscle interface is attributed to the intertwined biochemical and mechanical influences that pervade this dynamic interaction.
In alignment with the foundational understanding of bonemuscle crosstalk, a plethora of epidemiological research has elucidated that the concomitant loss of muscle and bone mass and strength is a prevalent phenomenon, particularly among older populations [9]. Hirschfeld et al. [10] introduced the term “osteosarcopenia” to describe the concurrent onset of osteoporosis and sarcopenia, identifying the condition as a distinct geriatric syndrome. Osteosarcopenia arises from a multifaceted etiology that transcends a single causative factor. Several common risk factors detrimentally influence both bone and muscle health, including adipose infiltration within musculoskeletal tissues, sedentarism, inadequate intake of protein and calcium, vitamin D deficiency, corticosteroid use, and aging-associated hormonal alterations, such as decreased levels of testosterone, estrogen, and insulin-like growth factor 1 [9,11]. These factors are believed to exert their effects either independently or in concert, contributing to the pathophysiology of osteosarcopenia. In addition to these genetic, mechanical, and endocrine influences, the reciprocal relationship between bones and muscles, as previously defined, may offer additional insights into the concurrent deterioration of these tissues [11,12].
In older individuals, the simultaneous decline in the integrity of skeletal muscles and bones results in significantly more severe adverse outcomes compared to those in the isolated presence of osteoporosis or sarcopenia. Individuals diagnosed with osteosarcopenia face a heightened risk of falls and subsequent fractures [3,10]. These potentially fatal events precipitate a fear of falling and injury, which in turn undermines physical confidence. Such a decline is rapidly succeeded by social withdrawal, diminished mobility and autonomy, malnutrition, and a poor quality of life [13]. Consequently, osteosarcopenia can be regarded as a preliminary step into a debilitating cycle characterized by increased susceptibility to fractures, frailty, disability, institutional care, and ultimately, mortality among older individuals. Thus, therapeutic approaches focusing solely on either osteoporosis or sarcopenia are inadequate for fostering independent and healthy aging. Devising interventions that simultaneously improve both bone and muscle health is imperative.
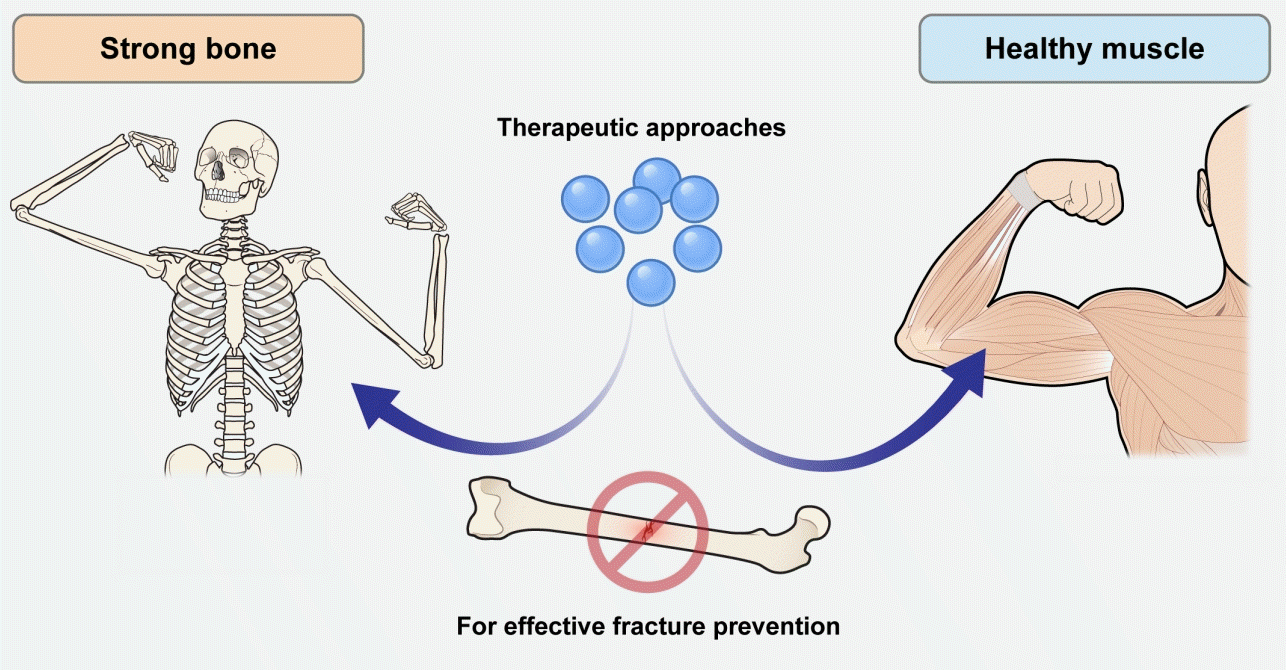
Despite the recognized necessity to address osteosarcopenia, concurrently improving musculoskeletal health presents significant challenges. Nutritional strategies, including protein-rich diets, coupled with resistance and balance exercises, are broadly advocated [14,15]. Nonetheless, such lifestyle modifications often yield limited efficacy among frail older individuals. Additionally, the absence of United States Food and Drug Administration approval for pharmacological treatments specifically targeting sarcopenia, let alone osteosarcopenia, compounds the problem. Even though preliminary evidence suggests potential benefits of certain osteoporosis medications, such as denosumab, in increasing muscle strength [16,17], these findings have yet to be substantiated through rigorous randomized controlled trials. Consequently, a pressing need exists to pivot towards the development of therapeutic interventions that can concurrently improve both muscle and bone strength, surpassing the current focus on improving muscle or bone health individually (Fig. 1).
In the field of geriatric health and longevity, the emphasis on expanding research perspectives to include osteosarcopenia for the prevention of fractures and the promotion of independent, healthy aging cannot be overstated. However, the lack of a global consensus on the diagnosis of osteosarcopenia presents a significant impediment to research progress in this field [3]. Moreover, the need for studies to identify predictive biomarkers for osteosarcopenia before its manifestation is important [18]. From a researcher’s perspective, the numerous unresolved questions surrounding osteosarcopenia offer an exciting frontier for exploration. The collective endeavors and focus of the scientific community on this field are eagerly awaited, with the hope of paving the way for older individuals to enjoy a lifestyle free from the constraints of fractures.
REFERENCES
1. Ahn SH, Park SM, Park SY, Yoo JI, Jung HS, Nho JH, et al. Osteoporosis and osteoporotic fracture fact sheet in Korea. J Bone Metab. 2020; 27:281–90.

2. Ji S, Jung HW, Baek JY, Jang IY, Lee E. Sarcopenia as the mobility phenotype of aging: clinical implications. J Bone Metab. 2024; 31:1–12.

3. Chen S, Xu X, Gong H, Chen R, Guan L, Yan X, et al. Global epidemiological features and impact of osteosarcopenia: a comprehensive meta-analysis and systematic review. J Cachexia Sarcopenia Muscle. 2024; 15:8–20.

4. Tagliaferri C, Wittrant Y, Davicco MJ, Walrand S, Coxam V. Muscle and bone, two interconnected tissues. Ageing Res Rev. 2015; 21:55–70.

5. Cariati I, Bonanni R, Onorato F, Mastrogregori A, Rossi D, Iundusi R, et al. Role of physical activity in bone-muscle crosstalk: biological aspects and clinical implications. J Funct Morphol Kinesiol. 2021; 6:55.

6. Bettis T, Kim BJ, Hamrick MW. Impact of muscle atrophy on bone metabolism and bone strength: implications for muscle-bone crosstalk with aging and disuse. Osteoporos Int. 2018; 29:1713–20.

7. Kim BJ. Effects of muscles on bone metabolism-with a focus on myokines. Ann Geriatr Med Res. 2022; 26:63–71.

8. Battafarano G, Rossi M, Marampon F, Minisola S, Del Fattore A. Bone control of muscle function. Int J Mol Sci. 2020; 21:1178.

9. Kirk B, Zanker J, Duque G. Osteosarcopenia: epidemiology, diagnosis, and treatment-facts and numbers. J Cachexia Sarcopenia Muscle. 2020; 11:609–18.

10. Hirschfeld HP, Kinsella R, Duque G. Osteosarcopenia: where bone, muscle, and fat collide. Osteoporos Int. 2017; 28:2781–90.

11. Gielen E, Dupont J, Dejaeger M, Laurent MR. Sarcopenia, osteoporosis and frailty. Metabolism. 2023; 145:155638.

12. Kirk B, Miller S, Zanker J, Duque G. A clinical guide to the pathophysiology, diagnosis and treatment of osteosarcopenia. Maturitas. 2020; 140:27–33.

13. Yoo JI, Kim H, Ha YC, Kwon HB, Koo KH. Osteosarcopenia in patients with hip fracture is related with high mortality. J Korean Med Sci. 2018; 33:e27.

14. Bae S, Lee S, Park H, Ju Y, Min SK, Cho J, et al. Position statement: exercise guidelines for osteoporosis management and fall prevention in osteoporosis patients. J Bone Metab. 2023; 30:149–65.

15. Polito A, Barnaba L, Ciarapica D, Azzini E. Osteosarcopenia: a narrative review on clinical studies. Int J Mol Sci. 2022; 23:5591.

16. Bonnet N, Bourgoin L, Biver E, Douni E, Ferrari S. RANKL inhibition improves muscle strength and insulin sensitivity and restores bone mass. J Clin Invest. 2019; 129:3214–23.





 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download