Abstract
Apart from basic roles such as supporting the body, protecting internal organs, and storing calcium, the skeletal system also performs hormonal functions. In recent years, several reports have been published on proteins secreted by bones and their impact on the homeostasis of the entire body. These proteins include fibroblast growth factor 23, sclerostin, lipocalin 2, and osteocalcin. Osteocalcin, the most abundant non-collagenous protein in bone tissue, is routinely measured as a clinical marker for diagnosing bone metabolism disorders. Its molecule undergoes numerous transformations, with decarboxylation being the critical process. Decarboxylation occurs in the acidic environment typical of bone resorption, facilitating the release of the molecule into the bloodstream and enabling its hormonal action. Decarboxylated osteocalcin promotes insulin secretion and stimulates the proliferation of pancreatic islet β-cells. It also plays a role in reducing the accumulation of visceral fat and decreasing fat storage in the liver. Furthermore, decarboxylated osteocalcin levels are inversely correlated with fasting serum glucose levels, total body fat, visceral fat area, and body mass index. Apart from its role in energy metabolism, osteocalcin affects testosterone production and the synthesis of glucagon-like peptide-1. It is also actively involved in muscle-bone crosstalk and influences cognitive function.
Our understanding of the skeleton and its biological functions has undergone a dramatic transformation over the past few decades. Previously, the emphasis on the skeleton’s features was primarily on its mechanical properties, which facilitate movement, protect internal organs, and support muscular structures. It was also recognised as the primary reservoir for calcium and other minerals in the human body. However, the discovery of proteins secreted by bone tissue into the bloodstream, which influence the regulation of homeostasis throughout the body, has led to the recognition of the skeletal system as a hormonally active entity.
Fibroblast growth factor 23 (FGF23) and osteocalcin (OC) are currently considered to be novel hormones secreted by the skeleton. The role of FGF23, which is produced mainly by osteocytes and osteoblasts, has been well documented in the literature [1,2]. It is mainly involved in the regulation of phosphate metabolism and calcitriol synthesis in the kidneys, and its overactivity can lead to the development of rare forms of rickets and hypophosphatemic osteomalacia, such as X-chromosome-associated hypophosphatemia and tumour-induced osteomalacia [3,4]. Additionally, there are reports of the endocrine functions of sclerostin, which may regulate the body’s energy metabolism through its paracrine effects. Lipocalin 2 is also implicated in the regulation of glucose metabolism [5-8].
This paper focuses on the role of OC in the regulation of the body’s homeostasis, a topic that continues to be debated among researchers [9]. It presents a review of studies conducted in both mouse models and the human population, enabling a more thorough understanding of OC function and the identification of its potential clinical implications.
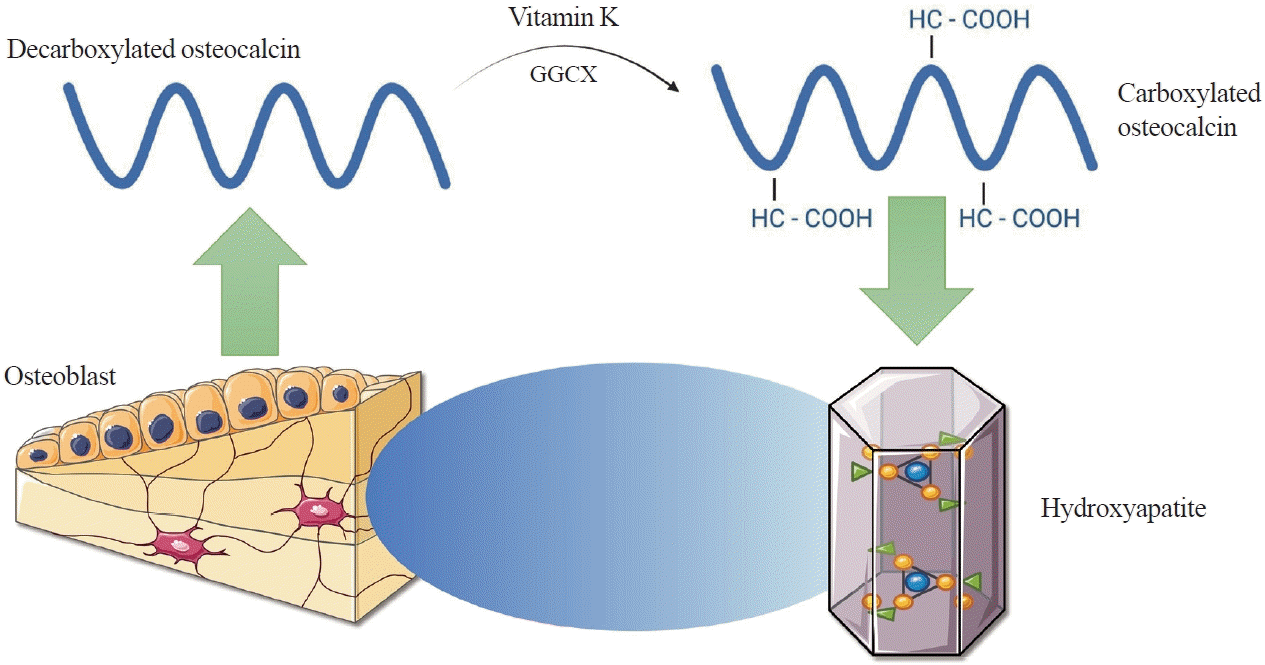
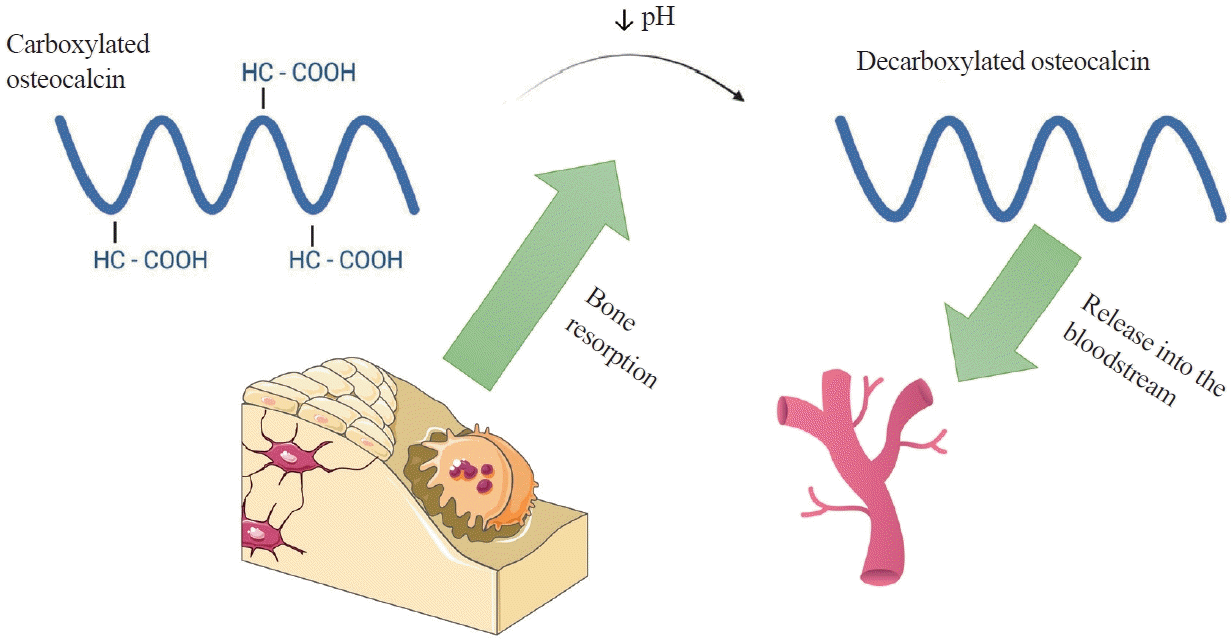
OC, also known as bone gamma-carboxyglutamic acid (Gla) protein (BGP), is the most common non-collagenous protein in bone tissue and is produced by osteoblasts during bone formation. It is an important clinical marker used in the diagnosis of bone metabolic diseases such as osteoporosis, Paget’s disease, and bone tumours [10,11]. Human OC is encoded by the bone gamma-carboxyglutamate protein (BGLAP) gene and composed of 49 amino acids. The molecule undergoes numerous transformations, the key one being the enzymatic carboxylation of the glutamine residues at positions 17, 21, and 24 using gamma-glutamylcarboxylase (GGCX) and vitamin K as a cofactor [12]. These residues have a high affinity for hydroxyapatite, allowing OC to be easily incorporated into the extracellular matrix (ECM) of bone tissue (Fig. 1) [13]. The reverse process, decarboxylation of OC, occurs in the acidic environment produced by osteoclasts during bone reabsorption. Decarboxylated OC (GluOC) is released into the bloodstream and functions as a hormone in the body (Fig. 2) [6].
Works aimed at identifying the function of GluOC began with the creation of genetically modified OC-deficient (Osc-/Osc-) mice by Desbois et al. [14] in 1996. In rodents, OC is encoded by two genes, Bglap and Bglap2, that are mainly expressed in osteoblasts. Osc-/Osc- mice, generated through embryonic stem cell technology, lacked the above genes and were characterised by increased glucose levels, reduced insulin production, greater visceral fat accumulation, reduced muscle mass, and, in the case of male mice, smaller gonads and lower blood testosterone levels than wild-type (WT) mice [15]. Due to this phenotype indicative of impaired glucose-fat and endocrine metabolism, it has been postulated that GluOC affects the regulation of energy metabolism, male fertility, and muscle tissue development.
Further evidence of a relationship between GluOC secretion and the regulation of energy metabolism was provided by an analysis of studies on genetically modified mice, published in 2007 by Lee et al. [16]. These mice lacked the Esp, also known as protein tyrosine phosphatase receptor type V (Ptprv) gene encoding osteo-testicular protein tyrosine phosphatase (OST-PTP), which is a receptor-like negative regulator for OC. Their phenotype mirrored that of Osc-/Osc- mice—namely, they had high serum GluOC levels, hypoglycaemia, increased beta-cell proliferation in the pancreas, increased insulin secretion, and higher sensitivity to the hormone. These mice had a lower risk of obesity and glucose intolerance than WT mice [16]. Furthermore, Esp-/Esp- mutants lacking a single Osc allele lost their characteristic phenotype.
In a study by Ferron et al. [17], GluOC was administered to WT mice that were exposed to a high-fat diet. In another group of mice, the centre responsible for appetite control had been damaged, which resulted in unrestrained hunger and excessive food intake, leading to obesity. In both cases, mice treated with injectable GluOC therapy had lower fat mass, higher insulin sensitivity, and lower serum triglyceride levels than those fed the same way but without GluOC therapy [17].
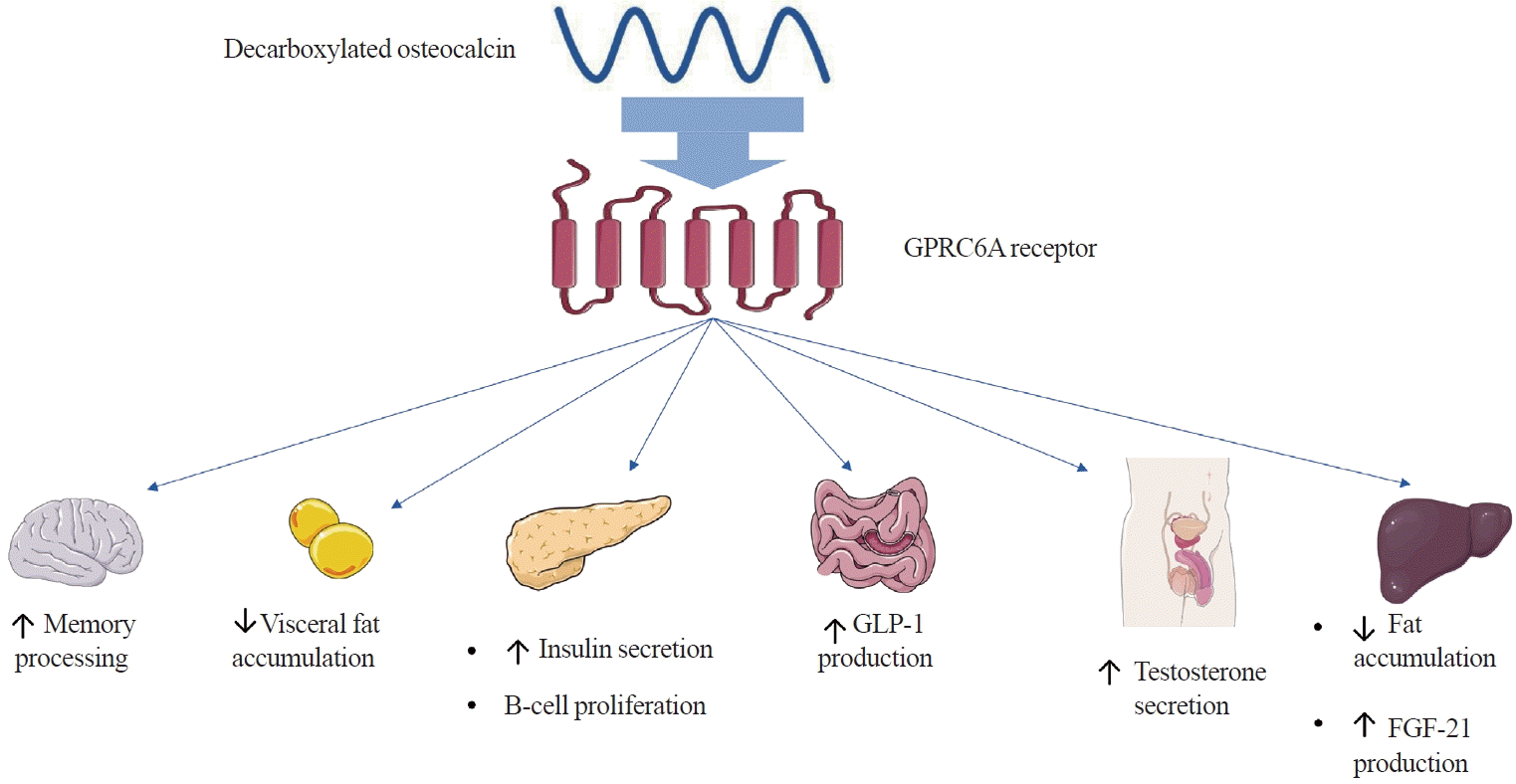
Studies using isolated pancreatic islet cells and primary adipocytes derived from WT mice have established the biochemical mechanism of action of GluOC and its effects on energy metabolism. GluOC has been shown to increase insulin secretion by promoting an increase in insulin 1 (Ins1) and Ins2 gene expression and an increase in intracellular Ca2+ ion concentrations by binding to the G protein-coupled receptor family C group 6 member A (GPRC6A) receptor in pancreatic islets. Furthermore, genetically modified mice lacking the Gprc6a gene developed metabolic syndrome, similarly to Osc-/Osc- mice, and had reduced testosterone levels, suggesting that the GPRC6A receptor may be a major mediator of GluOC regulatory functions (Fig. 3) [18].
Apart from pancreatic islets, the GPRC6A receptor in mice is also found in cells present in other organs, including the liver, small intestine, skeletal muscles, bones, the brain, and testis, which indicates that GluOC may affect the function of these organs [19]. Mice lacking GPRC6A expression in hepatocytes presented excessive hepatic fat accumulation and glycogen depletion, impaired glucose tolerance with normal insulin sensitivity, and reduced serum FGF21 levels. These findings imply that GluOC regulates hepatic metabolism and FGF21 release (Fig. 3) [20]. In the small intestine, GluOC stimulates enteroendocrine L cells to produce glucagon-like peptide-1, an incretin hormone that increases blood insulin concentrations and promotes pancreatic islet β-cell proliferation (Fig. 3) [21-23]. GluOC, via GPRC6A present on Leydig cells, has also been shown to promote testosterone synthesis in mice, thereby affecting male fertility [24].
GluOC promotes the expression of adiponectin genes in white adipocytes and peroxisome proliferator-activated receptor gamma coactivator 1-alpha (Pgc1α) and uncoupling protein 1 (Ucp1) in brown adipocytes, which is consistent with the observed lower accumulation of visceral adipose tissue in mice with higher blood GluOC concentrations (Fig. 3) [16,17,25]. When low doses (<10 ng/mL) of GluOC were administered to mice, increased adiponectin expression was also observed, as well as adipose tissue triglyceride lipase and peroxisome proliferator-activated receptor γ. In contrast, high doses (>20 ng/mL) induced programmed adipocyte necrosis. These experimental results suggest that GluOC may have applications in the treatment of obesity and metabolic syndrome [26-28].
An important aspect of the study of the endocrine functions of GluOC is the identification of its role in the muscle-bone feedback loop (muscle-bone crosstalk) [29]. A study by Mera et al. [30] showed that serum GluOC concentrations in mice increased after exercise; however, this effect was decreasingly evident as individuals matured. It was also observed that injecting GluOC immediately before exercise increased the exercise capacity of young mice, while in older mice, it restored it to levels comparable to those found in young adults. Additionally, long-term GluOC injection promoted an increase in muscle mass in mice. At the metabolic level, GluOC, mediated by GPRC6A, has been shown to promote the uptake and catabolism of glucose and fatty acids in myofibres, which is essential for muscle adaptation to exercise. Additionally, GluOC increases the release of interleukin-6 (IL-6) by myofibres. Meanwhile, IL-6, a cytokine produced by muscle fibres during exercise, affects bone metabolism by promoting bone resorption, thereby increasing serum GluOC concentrations. This effect occurs through upregulation of receptor activator of nuclear factors κB ligand (RANKL) expression and downregulation of osteoprotegerin (OPG) expression in murine osteoblasts, thus disrupting the OPG/RANKL concentration ratio. This creates a feedback loop involving bone and muscle metabolic products as part of a broad network of interactions [30-33].
Previous research into the biological functions of GluOC has also raised the important issue of its influence on central nervous system function [34]. Oury et al. [35] demonstrated that GluOC penetrates the blood-brain barrier and directly regulates the expression of the tryptophan hydroxylase-2 (Tph2), tyrosine hydroxylase (Th), glutamate decarboxylase-1 (Gad1), and Gad2 genes, thereby affecting neurotransmitter concentrations. OC-deficient mice had higher concentrations of γ-aminobutyric acid in all brain structures and lower concentrations of dopamine and serotonin in the brainstem compared to WT mice. Impaired neurotransmitter synthesis may have been responsible for the observed behavioural impairment of Osc-/Osc- mice—namely, a greater tendency towards anxiety and depressive behaviour. They also exhibited poorer memory and slower learning. Furthermore, intracerebroventricular administration of GluOC to Osc-/Osc- mice resulted in altered expression of the Tph2, Th, Gad1, and Gad2 genes and normalised behaviour. Changes in behaviour and neurotransmitter concentrations were not observed in Gprc6a-/Gprc6a- mice with normal GluOC levels [35]. Moreover, research by Khrimian et al. [36] suggested that OC plays a key role in early brain development. It is also essential for the formation and maturation of neuronal circuits.
Following Lee et al. [16], other researchers have also created OC-deficient mouse models. However, apart from changes in bone structure (particularly in hydroxyapatite crystals), they found no significant phenotypic changes suggesting that OC is a hormonally active substance. The phenotypic variations between the mouse models may be due to differences in the genetic engineering methods used to create them [37,38].
The concentration of GluOC, just like that of any other hormone, must be tightly regulated by reciprocal feedback. Experiments have shown that the main regulators of GluOC in the body are two hormones that also affect energy metabolism—specifically, insulin and leptin [39].
The results of a series of experiments by Ferron et al. showed that mouse osteoblasts have receptors for insulin (InsR) on their surface. Their metabolic activity depends on OST-PTP, as mentioned earlier. Insulin, by binding to InsR on osteoblasts, inhibits the activity of forkhead box O 1 (FoxO1), a nuclear transcription factor, which then leads to a decrease in OPG expression and the OPG/RANKL concentration ratio, thereby promoting bone resorption. Another observation was increased expression of the T cell immune regulator 1, ATPase H+ transporting V0 subunit a3 (Tcirg1) gene, which encodes a subunit of the vacuolar proton pump found on osteoclasts and required for acidification of the bone ECM. As mentioned earlier, lowering the pH of the ECM is necessary for the process of OC decarboxylation, thereby causing an increase in GluOC concentration. This creates a feedback loop in mice, where insulin via InsR in osteoblasts influences GluOC production, while GluOc, in turn, promotes insulin production and beta-cell proliferation in the pancreas [40].
In humans, however, the Esp gene is a pseudogene and it does not encode a functional protein [41]. Therefore, a search began to identify an enzyme, present in human osteoblasts, that could be equivalent to mouse OST-PTP. This protein turned out to be protein-tyrosine phosphatase 1B (PTP1B), an enzyme capable of dephosphorylating INSR, a specific InsR present in human osteoblasts. It has been shown that, as in mice, deactivation of INSR results in decreased phosphorylation of FOXO1 and increased expression of OPG. Thus, insulin signalling regulated by tyrosine phosphatase occurs in human osteoblasts, as it does in mice. Additionally, it has been observed that in individuals affected by osteopetrosis, a disease that limits bone resorption and results in decreased ability of osteoclasts to acidify the ECM, serum GluOC and insulin concentrations after meals were also reduced, corresponding to the results of experiments in a mouse model [40].
Leptin, known as the “satiety hormone,” is mainly secreted by adipose tissue. It crosses the blood-brain barrier by binding to a specific receptor (leptin receptor long isoform [LepRb]) in the hypothalamus, among others, thereby regulating food intake and energy expenditure [42]. Acting through activation of the sympathetic nervous system and the β2-adrenergic receptor (Adrβ2) located in osteoblasts, leptin has been shown to have a direct effect on bone metabolism [43]. Genetically modified mice lacking the leptin gene and the receptor for leptin had high bone mass associated with an impaired balance of bone formation and osteogenesis, while mice overexpressing the leptin gene had low bone mass. A similar relationship was observed in humans with an inactivating mutation in the leptin gene [44,45]. Additionally, leptin, via Adrβ2, was found to increase Esp expression in a mouse model, leading to reduced serum GluOC levels. Genetically modified mice lacking Adrβ2 had increased GluOC concentrations [46].
Due to the promising results in mouse models, observational analyses have been carried out on the human population to understand the extracellular functions of OC.
Iki et al. [47] showed statistically significant negative correlations between serum GluOC levels and fasting plasma glucose (FPG), glycated hemoglobin (HbA1c), and homeostatic model assessment-insulin resistance (HOMA-IR) in more than 1,500 Japanese men over 65 years of age with type 2 diabetes mellitus (T2DM). Concordant results were also obtained in another study that, apart from men, also included postmenopausal women with T2DM. Total OC concentration (tOC) was shown to be an independent predictor for HOMA-IR, HbA1c (negative relationship) and HOMA for beta-cell function (HOMA-β) (positive relationship) [48,49].
A study by Zheng et al. [50], conducted on 225 children of both sexes with congenital osteoporosis, demonstrated similar findings regarding the correlations of tOC and GluOC with FPG and HOMA-IR as those found in adults with T2DM. Furthermore, GluOC and tOC exhibited statistically significant negative correlations with body mass index (BMI). This study also analysed children’s grip strength, which was significantly positively correlated with the GluOC/tOC ratio, similarly to the percentage body fat-free mass [50].
In a group of 79 obese children aged 7 to 12 years analysed by Wang et al. [51], negative correlations were found between tOC concentration and percentage body fat and visceral fat area, and a positive correlation was observed between tOC and lean body mass. Furthermore, children with severe and moderate obesity had statistically significantly lower tOC concentrations than those with mild obesity and overweight [51]. Moreover, among the population of children with type 1 diabetes mellitus, lower GluOC, and tOC concentrations were identified as independent factors associated with increased body fat [52].
Lower tOC concentrations have been identified as an independent risk factor for the development of metabolic syndrome [53-55]. Additionally, low tOC concentrations were found to be an independent risk factor for hypertriglyceridemia, hyperglycemia, and reduced high-density lipoprotein cholesterol concentrations [55].
In an analysis of the relationship between muscle strength and the GluOC/tOC ratio in women over 70 years of age, the ratio was found to be positively associated with muscle strength of the hip flexors, hip extensors, and quadriceps of the thigh, as well as measures of executive functioning and global cognition [56,57].
The effects of OC, the most common non-collagenous protein in bone tissue, have been shown to extend far beyond the skeletal system. A mouse model lacking OC has revealed that this protein can have a significant clinical impact, particularly on energy metabolism, which has been confirmed in observational studies in the human population. GluOC levels have been found to exhibit negative correlations with FPG and HOMA-IR, and tOC has shown negative correlations with percentage body fat and visceral fat area. Positive correlations were observed between tOC and lean body mass, and between GluOC and HOMA-β. A negative correlation between tOC and BMI was confirmed in a meta-analysis including the results of 28 studies [58]. A meta-analysis based on the results of 24 studies showed that serum tOC levels are significantly reduced in patients with T2DM and negatively correlated with the development of T2DM [59]. Furthermore, observations on the association of GluOC/tOC with muscle strength in both children with congenital osteoporosis and women over 70 years of age are consistent with those made in mouse models.
To conclude, research on OC has confirmed that it is actively involved in the regulation of energy metabolism and may have an impact on muscle tissue development. However, further research on GluOC is required to more precisely identify its role in the pathophysiology of diseases such as obesity, T2DM, metabolic syndrome, and sarcopenia, and to discover its potential use in the treatment of these conditions in the future.
REFERENCES
1. Bilezikian JP, Martin TJ, Clemens TL, Rosen CJ. Principles of bone biology. 4th ed. London: Academic Press;2020. Chapter 20, Phosphorus homeostasis and related disorders. p. 469–507.
3. Shimada T, Kakitani M, Yamazaki Y, Hasegawa H, Takeuchi Y, Fujita T, et al. Targeted ablation of Fgf23 demonstrates an essential physiological role of FGF23 in phosphate and vitamin D metabolism. J Clin Invest. 2004; 113:561–8.

4. Fukumoto S. FGF23-related hypophosphatemic rickets/osteomalacia: diagnosis and new treatment. J Mol Endocrinol. 2021; 66:R57–65.

5. D’Onofrio L, Maddaloni E, Buzzetti R. Osteocalcin and sclerostin: background characters or main actors in cardiovascular disease? Diabetes Metab Res Rev. 2020; 36:e3217.

6. Wang JS, Mazur CM, Wein MN. Sclerostin and osteocalcin: candidate bone-produced hormones. Front Endocrinol (Lausanne). 2021; 12:584147.

7. Dirckx N, Moorer MC, Clemens TL, Riddle RC. The role of osteoblasts in energy homeostasis. Nat Rev Endocrinol. 2019; 15:651–65.

8. Mosialou I, Shikhel S, Liu JM, Maurizi A, Luo N, He Z, et al. MC4R-dependent suppression of appetite by bone-derived lipocalin 2. Nature. 2017; 543:385–90.

9. Manolagas SC. Osteocalcin promotes bone mineralization but is not a hormone. PLoS Genet. 2020; 16:e1008714.

10. Nakashima K, de Crombrugghe B. Transcriptional mechanisms in osteoblast differentiation and bone formation. Trends Genet. 2003; 19:458–66.

11. Atalay S, Elci A, Kayadibi H, Onder CB, Aka N. Diagnostic utility of osteocalcin, undercarboxylated osteocalcin, and alkaline phosphatase for osteoporosis in premenopausal and postmenopausal women. Ann Lab Med. 2012; 32:23–30.

12. Hauschka PV, Lian JB, Cole DE, Gundberg CM. Osteocalcin and matrix Gla protein: vitamin K-dependent proteins in bone. Physiol Rev. 1989; 69:990–1047.

13. Hoang QQ, Sicheri F, Howard AJ, Yang DS. Bone recognition mechanism of porcine osteocalcin from crystal structure. Nature. 2003; 425:977–80.

14. Desbois C, Hogue DA, Karsenty G. The mouse osteocalcin gene cluster contains three genes with two separate spatial and temporal patterns of expression. J Biol Chem. 1994; 269:1183–90.

15. Ducy P, Desbois C, Boyce B, Pinero G, Story B, Dunstan C, et al. Increased bone formation in osteocalcin-deficient mice. Nature. 1996; 382:448–52.

16. Lee NK, Sowa H, Hinoi E, Ferron M, Ahn JD, Confavreux C, et al. Endocrine regulation of energy metabolism by the skeleton. Cell. 2007; 130:456–69.

17. Ferron M, Hinoi E, Karsenty G, Ducy P. Osteocalcin differentially regulates beta cell and adipocyte gene expression and affects the development of metabolic diseases in wildtype mice. Proc Natl Acad Sci U S A. 2008; 105:5266–70.
18. Pi M, Chen L, Huang MZ, Zhu W, Ringhofer B, Luo J, et al. GPRC6A null mice exhibit osteopenia, feminization and metabolic syndrome. PLoS One. 2008; 3:e3858.

19. Pi M, Nishimoto SK, Darryl Quarles L. Explaining divergent observations regarding osteocalcin/GPRC6A endocrine signaling. Endocrinology. 2021; 162:bqab011.

20. Pi M, Xu F, Ye R, Nishimoto SK, Williams RW, Lu L, et al. Role of GPRC6A in regulating hepatic energy metabolism in mice. Sci Rep. 2020; 10:7216.

21. Mizokami A, Yasutake Y, Gao J, Matsuda M, Takahashi I, Takeuchi H, et al. Osteocalcin induces release of glucagon-like peptide-1 and thereby stimulates insulin secretion in mice. PLoS One. 2013; 8:e57375.

22. Kim W, Egan JM. The role of incretins in glucose homeostasis and diabetes treatment. Pharmacol Rev. 2008; 60:470–512.

24. Oury F, Sumara G, Sumara O, Ferron M, Chang H, Smith CE, et al. Endocrine regulation of male fertility by the skeleton. Cell. 2011; 144:796–809.

25. Ferron M, Lacombe J. Regulation of energy metabolism by the skeleton: osteocalcin and beyond. Arch Biochem Biophys. 2014; 561:137–46.

26. Otani T, Mizokami A, Kawakubo-Yasukochi T, Takeuchi H, Inai T, Hirata M. The roles of osteocalcin in lipid metabolism in adipose tissue and liver. Adv Biol Regul. 2020; 78:100752.

27. Otani T, Mizokami A, Hayashi Y, Gao J, Mori Y, Nakamura S, et al. Signaling pathway for adiponectin expression in adipocytes by osteocalcin. Cell Signal. 2015; 27:532–44.

28. Otani T, Matsuda M, Mizokami A, Kitagawa N, Takeuchi H, Jimi E, et al. Osteocalcin triggers Fas/FasL-mediated necroptosis in adipocytes via activation of p300. Cell Death Dis. 2018; 9:1194.

29. Novotny SA, Warren GL, Hamrick MW. Aging and the muscle-bone relationship. Physiology (Bethesda). 2015; 30:8–16.

30. Mera P, Laue K, Ferron M, Confavreux C, Wei J, Galan-Diez M, et al. Osteocalcin signaling in myofibers is necessary and sufficient for optimum adaptation to exercise. Cell Metab. 2016; 23:1078–92.

31. Karsenty G, Mera P. Molecular bases of the crosstalk between bone and muscle. Bone. 2018; 115:43–9.

32. Mera P, Laue K, Wei J, Berger JM, Karsenty G. Osteocalcin is necessary and sufficient to maintain muscle mass in older mice. Mol Metab. 2016; 5:1042–7.

33. Teitelbaum SL, Ross FP. Genetic regulation of osteoclast development and function. Nat Rev Genet. 2003; 4:638–49.

34. Nakamura M, Imaoka M, Takeda M. Interaction of bone and brain: osteocalcin and cognition. Int J Neurosci. 2021; 131:1115–23.

35. Oury F, Khrimian L, Denny CA, Gardin A, Chamouni A, Goeden N, et al. Maternal and offspring pools of osteocalcin influence brain development and functions. Cell. 2013; 155:228–41.

36. Khrimian L, Obri A, Ramos-Brossier M, Rousseaud A, Moriceau S, Nicot AS, et al. Gpr158 mediates osteocalcin’s regulation of cognition. J Exp Med. 2017; 214:2859–73.

37. Moriishi T, Ozasa R, Ishimoto T, Nakano T, Hasegawa T, Miyazaki T, et al. Osteocalcin is necessary for the alignment of apatite crystallites, but not glucose metabolism, testosterone synthesis, or muscle mass. PLoS Genet. 2020; 16:e1008586.

38. Diegel CR, Hann S, Ayturk UM, Hu JC, Lim KE, Droscha CJ, et al. An osteocalcin-deficient mouse strain without endocrine abnormalities. PLoS Genet. 2020; 16:e1008361.

39. Ducy P. The role of osteocalcin in the endocrine cross-talk between bone remodelling and energy metabolism. Diabetologia. 2011; 54:1291–7.

40. Ferron M, Wei J, Yoshizawa T, Del Fattore A, DePinho RA, Teti A, et al. Insulin signaling in osteoblasts integrates bone remodeling and energy metabolism. Cell. 2010; 142:296–308.

41. Cousin W, Courseaux A, Ladoux A, Dani C, Peraldi P. Cloning of hOST-PTP: the only example of a protein-tyrosinephosphatase the function of which has been lost between rodent and human. Biochem Biophys Res Commun. 2004; 321:259–65.

43. Sharan K, Yadav VK. Hypothalamic control of bone metabolism. Best Pract Res Clin Endocrinol Metab. 2014; 28:713–23.

44. Elefteriou F, Takeda S, Ebihara K, Magre J, Patano N, Kim CA, et al. Serum leptin level is a regulator of bone mass. Proc Natl Acad Sci U S A. 2004; 101:3258–63.

45. Ducy P, Amling M, Takeda S, Priemel M, Schilling AF, Beil FT, et al. Leptin inhibits bone formation through a hypothalamic relay: a central control of bone mass. Cell. 2000; 100:197–207.

46. Hinoi E, Gao N, Jung DY, Yadav V, Yoshizawa T, Myers MG Jr, et al. The sympathetic tone mediates leptin’s inhibition of insulin secretion by modulating osteocalcin bioactivity. J Cell Biol. 2008; 183:1235–42.

47. Iki M, Tamaki J, Fujita Y, Kouda K, Yura A, Kadowaki E, et al. Serum undercarboxylated osteocalcin levels are inversely associated with glycemic status and insulin resistance in an elderly Japanese male population: Fujiwara-kyo Osteoporosis Risk in Men (FORMEN) Study. Osteoporos Int. 2012; 23:761–70.

48. Ma XY, Chen FQ, Hong H, Lv XJ, Dong M, Wang QY. The relationship between serum osteocalcin concentration and glucose and lipid metabolism in patients with type 2 diabetes mellitus: the role of osteocalcin in energy metabolism. Ann Nutr Metab. 2015; 66:110–6.

49. Kanazawa I, Yamaguchi T, Tada Y, Yamauchi M, Yano S, Sugimoto T. Serum osteocalcin level is positively associated with insulin sensitivity and secretion in patients with type 2 diabetes. Bone. 2011; 48:720–5.

50. Zheng WB, Hu J, Zhao DC, Zhou BN, Wang O, Jiang Y, et al. The role of osteocalcin in regulation of glycolipid metabolism and muscle function in children with osteogenesis imperfecta. Front Endocrinol (Lausanne). 2022; 13:898645.

51. Wang JW, Tang QY, Ruan HJ, Cai W. Relation between serum osteocalcin levels and body composition in obese children. J Pediatr Gastroenterol Nutr. 2014; 58:729–32.

52. Takashi Y, Ishizu M, Mori H, Miyashita K, Sakamoto F, Katakami N, et al. Circulating osteocalcin as a bone-derived hormone is inversely correlated with body fat in patients with type 1 diabetes. PLoS One. 2019; 14:e0216416.

53. Alfadda AA, Masood A, Shaik SA, Dekhil H, Goran M. Association between osteocalcin, metabolic syndrome, and cardiovascular risk factors: role of total and undercarboxylated osteocalcin in patients with type 2 diabetes. Int J Endocrinol. 2013; 2013:197519.

54. Liu X, Yeap BB, Brock KE, Levinger I, Golledge J, Flicker L, et al. Associations of osteocalcin forms with metabolic syndrome and its individual components in older men: the health in men study. J Clin Endocrinol Metab. 2021; 106:e3506–18.

55. Tan A, Gao Y, Yang X, Zhang H, Qin X, Mo L, et al. Low serum osteocalcin level is a potential marker for metabolic syndrome: results from a Chinese male population survey. Metabolism. 2011; 60:1186–92.

56. Levinger I, Scott D, Nicholson GC, Stuart AL, Duque G, McCorquodale T, et al. Undercarboxylated osteocalcin, muscle strength and indices of bone health in older women. Bone. 2014; 64:8–12.

57. Bradburn S, McPhee JS, Bagley L, Sipila S, Stenroth L, Narici MV, et al. Association between osteocalcin and cognitive performance in healthy older adults. Age Ageing. 2016; 45:844–9.





 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download