INTRODUCTION
Struma ovarii, a type of ovarian teratoma, is an ovarian tumor with thyroid tissue comprising over 50% of the mass. It is rare, comprising less than 1% of all ovarian tumors and approximately 2% to 3% of all ovarian teratomas [
1-
4]. Struma ovarii is usually not diagnosed before surgery but instead based on postoperative pathological findings after ovarian resection. Most struma ovarii cases are benign, with malignant struma ovarii (MSO) accounting for around 5% to 10% of all cases [
2,
3,
5-
8].
MSO commonly is diagnosed by tumor histology or behavior [
1]. It is not difficult to diagnose ‘histological’ MSO, but immunohistochemical staining must be performed for markers like thyroglobulin (Tg) to confirm its thyroid origin. ‘Biologically,’ MSO is defined by extra-ovarian extension or recurrence or metastasis [
9,
10].
The primary treatment for MSO is surgery; however, there is no established standard post-surgical treatment for MSO. Because MSO is such an extremely rare malignant tumor, it has only been reported sporadically in the form of case reports or series.
Treatment recommendations are often based on such case reports or series. In some cases, total thyroidectomy and radioactive iodine (RAI) may be recommended, emphasizing the need for involvement of an endocrinologist in the treatment of MSO [
1,
11].
However, endocrinologists typically only encounter MSO patients referred by gynecologists after surgery. The number of such cases is very low, so some endocrinologists never encountering a case throughout their career. Therefore, without awareness of MSO, it may be misclassified as a condition solely within the field of gynecology with some thyroid involvement, leading to the inability to provide appropriate treatment.
Here, we investigated 170 patients with struma ovarii who underwent surgery from 1994 to 2023 at the Department of Obstetrics and Gynecology, Samsung Medical Center, and identified 15 cases of MSO among them. We analyzed the incidence, clinical characteristics, treatment, and prognosis of these 15 MSO patients.
Go to :

METHODS
The research was conducted at Samsung Medical Center, Seoul, Korea, and included patients who were diagnosed with struma ovarii based on pathology reports of ovarian specimens from November 1994 to May 2023. The clinical data of patients were extracted from electronic medical records. The age and tumor location of patients with benign struma ovarii and MSO were collected. Subsequently, the size of MSO, treatment modalities, and survival outcomes were analyzed for MSO patients until September 2023.
To compare the laterality of benign struma ovarii and MSO patients, a chi-square test was used; to compare the age at diagnosis, a Mann-Whitney U test was performed. P values less than 0.05 were considered statistically significant. Statistical analysis was conducted using R version 4.3.0 (R Foundation for Statistical Computing, Vienna, Austria).
This study was conducted in accordance with the 1964 Declaration of Helsinki. This study was approved by the Institutional Review Board of Samsung Medical Center (SMC-IRB 2023-07-186), and written informed consent was waived due to the retrospective nature of this study.
Go to :

RESULTS
Clinical characteristics of 170 patients with struma ovarii
Among a total of 170 patients diagnosed with struma ovarii, 155 (91.2%) had benign struma ovarii and 15 (8.8%) had MSO (
Fig. 1). The median age of all patients with struma ovarii was 44 years (range, 12 to 81). Among them, the median age of patients with benign struma ovarii was 44 years (range, 12 to 81), and the median age of patients with MSO was 48 years (range, 30 to 74). However, the difference in median age between benign struma ovarii and MSO was not statistically significant (
P=0.126). Among the patients with struma ovarii, 90 (52.9%) had a tumor in the right ovary and 79 (46.5%) had a tumor in the left ovary; one (0.6%) patient had bilateral struma ovarii. Benign struma ovarii occurred more frequently in the right ovary (54.8%), whereas MSO occurred more frequently in the left ovary (66.7%). There was no statistically significant difference between the two groups (
P=0.255). More than 50% of both benign struma ovarii and MSO patients were asymptomatic (57.9% vs. 64.3%), with abdominal or pelvic pain being the most common symptom (22.2% vs. 21.4%). The median number of children was 1 (range, 0 to 6) of benign struma ovarii and 2 (range, 0 to 4) of MSO. There were no significant differences in clinical manifestations between the two groups of benign struma ovarii and MSO (initial symptom
P=0.559; number of children
P=0.147) (
Table 1).
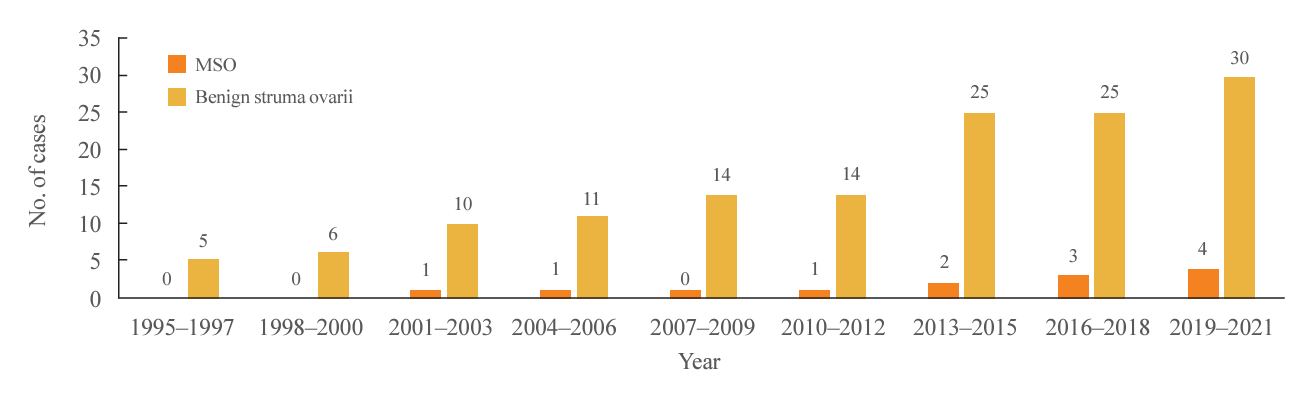 | Fig. 1.Numbers of cases of malignant struma ovarii (MSO) and benign struma ovarii managed by Samsung Medical Center by 3-year periods from 1995 to 2021. 
|
Table 1.
Clinical Manifestation of 170 Patients with Struma Ovarii
|
Variable |
Benign (n=155) |
Malignant (n=15) |
P value |
|
Age at diagnosis, yr |
44 (12–81) |
48 (30–74) |
0.126 |
|
Location |
|
|
0.255 |
|
Left |
69 (44.5) |
10 (66.7) |
|
|
Right |
85 (54.8) |
5 (33.3) |
|
|
Bilateral |
1 (0.6) |
0 |
|
|
Presenting symptom |
|
|
0.559 |
|
No symptom or incidental finding |
73 (57.9) |
9 (64.3) |
|
|
Abdominal/pelvic pain |
28 (22.2) |
3 (21.4) |
|
|
Abnormal vaginal bleeding |
14 (11.1) |
0 |
|
|
Others |
11 (8.7) |
2 (14.3) |
|
|
Number of children |
1 (0–6) |
2 (0–4) |
0.147 |

Clinical characteristics of 15 patients with MSO
The median size of MSO was 3.3 cm (range, 0.5 to 11.0). Among the 15 patients with MSO, papillary thyroid carcinoma (PTC) was found in seven (46.7%). The next common type was follicular thyroid carcinoma (FTC), found in four patients (26.7%), followed by poorly differentiated thyroid carcinoma in one patient and differentiated high-grade thyroid carcinoma in one patient. Additionally, there were two patients with uncertain malignant potential: one with a well-differentiated thyroid tumor of uncertain malignant potential and the other with a struma ovarii of uncertain malignant potential. In both cases, peritoneal involvement was confirmed, leading to the biological diagnosis of MSO in both cases.
Among the 15 patients with MSO, excluding one with a previous surgical history of thyroid cancer at another hospital, ten of the 14 patients underwent thyroid ultrasonography at our hospital. Thyroid tumors were found in five of 10 patients: one was diagnosed with a benign tumor, one with atypia of undetermined significance/follicular lesion of undetermined significance, and three with thyroid cancer.
Among the four patients who did not undergo thyroid ultrasonography at our hospital, two had PTC, and two had FTC. None of these four patients were referred for consultation to the endocrinologists at our institution. Among the 15 patients with MSO, only eight (53.3%) were consulted by an endocrinologist.
There were two patients with previous thyroid dysfunction: one with Graves’ disease and one with hypothyroidism. Neither patient was taking any thyroid medication at the time of MSO diagnosis. All 15 patients were diagnosed with MSO after ovarian resection, and there was no patient in which MSO was suspected by preoperative computed tomography or magnetic resonance imaging.
Treatment modalities for MSO and oncological outcomes in 15 patients with MSO
The extent of gynecological surgery was varied for the 15 patients with MSO. Nine patients underwent total hysterectomy (TH) with bilateral salpingo-oophorectomy (BSO), and seven of them also underwent omentectomy. One patient underwent BSO, two underwent only unilateral salpingo-oophorectomy (USO), and two underwent USO with contralateral ovarian cystectomy. One patient presented to our institution following ovarian cystectomy at another hospital and planned for further treatment but was subsequently lost to follow-up.
Four patients (26.7%) underwent thyroidectomy for thyroid cancer; three underwent total thyroidectomy and one underwent hemithyroidectomy. One patient with peritoneal metastasis and ascites underwent total thyroidectomy and RAI therapy for MSO. No patients received chemotherapy or radiation therapy.
Excluding two patients, one who was lost to follow-up after ovarian cystectomy at another hospital and one who had follow-up loss without postoperative imaging, the median follow-up period was 33 months (range, 4 to 156) in 13 patients. Eleven patients remain disease-free, one is currently undergoing treatment, one has experienced progression with peritoneal seeding, and the remaining two patients were lost to follow-up. There have been no recurrences or deaths due to MSO (
Table 2).
Table 2.
Clinical Manifestations of 15 Patients with MSO
|
No. |
Surgery date |
Age, yr |
Pathologic diagnosis |
Size, cm |
Laterality |
Distant metastasis |
Gynecological surgery |
Thyroid surgery |
RAI |
Pre-Op CA-125 |
Last CA-125a
|
Disease state |
F/U period, mo |
F/U |
|
1 |
2002 |
67 |
FTC |
2.5 |
Right |
M0 |
TH BSO |
ND |
ND |
20.3 |
2.6 |
Remission |
156 |
Transfer |
|
2 |
2004 |
36 |
PTC-FV |
6.0 |
Left |
M0 |
TH USO OMT (previous USO for contralateral ovary tumor in 1999) |
Previous OP for thyroid cancer in 2003 |
NA |
19.5 |
2.3 |
NA |
3 |
NA |
|
3 |
2010 |
56 |
FTC |
5.5 |
Left |
M0 |
TH BSO OMT |
ND |
ND |
3,148.2 |
13.3 |
Remission |
12 |
Transfer |
|
4 |
2014 |
33 |
PTC |
NA |
Left |
M0 |
OC |
ND |
ND |
NA |
NA |
NA |
1 |
F/U loss |
|
5 |
2014 |
34 |
FTC |
3.5 |
Left |
M0 |
TH BSO OMT |
ND |
ND |
NA |
5.6 |
Remission |
81 |
F/U loss |
|
6 |
2015 |
48 |
PTC |
1.5 |
Left |
M0 |
TH BSO |
OP for thyroid cancer (PTMC) in 2021 |
ND |
NA |
NA |
Remission |
88 |
F/U ongoing |
|
7 |
2017 |
47 |
PDTC |
5.0 |
Right |
M0 |
USO OC OMT |
ND |
ND |
15.9 |
3.1 |
Remission |
72 |
F/U ongoing |
|
8 |
2017 |
54 |
PTC |
1.1 |
Left |
M0 |
BSO |
ND |
ND |
NA |
10.9 |
Remission |
59 |
F/U completed |
|
9 |
2019 |
32 |
Struma ovarii with UMP |
4.2 |
Left |
Peritoneal metastasis |
USO OC |
Previous OP for thyroid cancer (PTC) in 2018 |
RAI for thyroid cancer |
24.6 |
7.0 |
Progression |
55 |
F/U ongoing |
|
10 |
2020 |
30 |
PTC |
1.1 |
Left |
M0 |
USO |
ND |
ND |
NA |
NA |
Remission |
33 |
F/U ongoing |
|
11 |
2020 |
73 |
FTC |
2.0 |
Right |
Omental metastasis |
BSO TH OMT |
OP for MSO in 2020 |
RAI for MSO |
NA |
43.4 |
Remission |
12 |
Transfer |
|
12 |
2021 |
74 |
PTC |
0.5 |
Left |
M0 |
TH BSO OMT |
ND |
ND |
29.9 |
NA |
Remission |
13 |
F/U loss |
|
13 |
2022 |
32 |
PTC |
3.2 |
Left |
M0 |
USO |
ND |
ND |
NA |
17.8 |
Remission |
7 |
F/U loss |
|
14 |
2023 |
61 |
DHGTC |
3.4 |
Right |
M0 |
TH BSO OMT |
OP for thyroid cancer (FV-PTC) in 2023 |
RAI for thyroid cancer |
157.0 |
NA |
In treatment |
4 |
In treatment |
|
15 |
2023 |
66 |
WDTT with UMP |
11.0 |
Right |
Peritoneal extension |
TH BSO OMT |
ND |
ND |
179.7 |
9.0 |
Remission |
4 |
F/U ongoing |

Go to :

DISCUSSION
Struma ovarii is a rare type of ovarian germ cell tumor, with MSO being even extremely rare. Most struma ovarii are benign, with MSO representing approximately 5% to 10% of cases [
3,
4,
8]. In the present study, MSO accounted for 15 (8.8%) of the total 170 cases of struma ovarii. Struma ovarii is most commonly diagnosed in patients between the ages of 40 and 60 years. In the present study, the median age of patients with MSO tended to be older than that of patients with benign struma ovarii (48 years vs. 44 years), but this difference was not significant. In our report, we observed an increasing trend in the number of struma ovarii patients at 3-year intervals (
Fig. 1), which is thought to be primarily influenced by the rising number of patients undergoing ovarian surgery at our institution over time.
As reported in other studies, all MSO patients were diagnosed after resection of the ovary, and no patient in the present study in which MSO was suspected by preoperative imaging. According to various reports, some cases of struma ovarii manifest with abdominal/pelvic masses or pain, while others are discovered solely through increased serum cancer antigen 125 (CA-125) concentration, without any symptoms [
4,
12]. Occasionally, MSO is accompanied by irregular menstruation or ascites. In some cases, hyperthyroidism may also be present [
2-
5]. CA-125 is considered more useful as a marker of ovarian germ cell tumors than as a specific tumor marker for MSO [
13]. In this study, among the eight patients with preoperative CA-125 level measurements, CA-125 was elevated in only three (37.5%). Regardless of the preoperative CA-125 concentration, its level decreased in all patients after surgery. Nevertheless, the diagnosis of MSO is determined only through microscopic examination of resected tissue after surgery.
Some studies have reported that struma ovarii is more common in the left ovary [
2,
3]. The subtype of MSO is reported to have PTC as the most prevalent, followed by FTC [
12], and this has been consistently reproduced in our report. The incidence of MSO increases with the size of the tumor, and the size of MSO is predominantly 5 cm or larger [
3,
5,
6]. In the present study, the median tumor size for MSO was 3.3 cm, with a range from 0.5 to 11.0 cm.
The treatment for benign struma ovarii is surgical resection of the ovarian tumor. Salpingectomy, removal of the fallopian tube, may or may not be performed during the procedure. The primary treatment for MSO is surgery, with the first choice being TH with BSO. However, in clinical practice, the surgical approach for MSO appears to vary, ranging from USO to TH with BSO and omentectomy [
12]. In fertile women who desire pregnancy, conservative surgery to preserve the contralateral ovary is necessary. However, in premenopausal women with progressed disease or postmenopausal women with high risk of recurrence, TH with BSO is performed as part of a complete resection [
6,
13].
There is no established standard post-surgical treatment for MSO. Some analyses have found that MSO demonstrates excellent disease-specific survival despite the use of non-standardized management approaches [
8,
9,
14]. However, as around 5% of MSO cases present with distant metastasis, it can have fatal consequences [
3-
5]. Additionally, according to Marti et al. [
14] in 2012, MSO showed a recurrence rate of 7.5% over 25 years in 50 cases of localized malignant ovarian tumors treated with oophorectomy alone. To reduce local and remote recurrence, total thyroidectomy and RAI may be considered as postoperative treatments in gynecologic surgery. After thyroid surgery and RAI, thyroid stimulating hormone (TSH) suppression therapy is administered based on the extent of thyroid cancer progression [
6]. In cases with significant progression, TSH level is suppressed below 0.10 mU/L, while it is maintained between 0.10 and 0.50 mU/L in others [
15]. Tg is used as a tumor marker to monitor the recurrence of MSO after total thyroidectomy and RAI treatment. Serum Tg level should be measured periodically, and in the presence of biochemical or structural persistent disease or metastatic conditions, periodic imaging tests including pelvic involvement should be conducted [
6]. In our study, only one case had preoperative measurement of Tg. This patient was already undergoing Tg monitoring due to PTC in thyroid gland. Following gynecological surgery, this patient experienced a decrease in Tg, which then increased again as peritoneal seeding progressed. Excluding the aforementioned patient, five patients with MSO were followed up for Tg levels after gynecological surgery. Among them, three patients did not undergo thyroid surgery, one patient had surgery for a MSO without abnormalities within thyroid, and one patient underwent hemithyroidectomy for papillary thyroid microcarcinoma of thyroid. Although Tg levels were not measured before gynecological surgery in these patients, all showed a trend of decreasing Tg levels post-surgery.
Recent studies have tried to establish risk stratification for MSO and have proposed treatment options [
1,
13]. In this study, four patients underwent thyroidectomy for thyroid cancer. One patient with peritoneal metastasis and ascites underwent total thyroidectomy and RAI therapy for MSO. Except for one patient who underwent hemithyroidectomy for thyroid cancer, TSH suppression therapy was performed in four patients in this study.
The prognosis of MSO is generally favorable; however, recurrence occurs in 7.5% to 35% and metastasis in 5% to 27% of patients [
6,
14,
16]. According to the report by Jean et al. [
16], FTC has a higher incidence of recurrence and metastasis compared with PTC (recurrence 39% vs. 8%; metastasis 39% vs. 15%). Conversely, in the report by Cui et al. [
12], there were no cases of FTC among the deceased patients with MSO. About one-third of MSO metastases are detected at the time of diagnosis, while the remaining two-thirds are discovered during follow-up [
16]. In cases where the cancer has extended beyond the ovary, when the tumor size exceeds 4 cm, or when it is accompanied by B-rapidly accelerated fibrosarcoma (
BRAF) mutation, the probability of recurrence is elevated [
17]. Several studies have reported on molecular studies in MSO [
12,
18]. According to the report by Cui et al. [
12], gene mutations in the rat sarcoma (
RAS) or B-Raf,
BRAF, or tyrosine kinase (KIT) proto-oncogene, receptor
KIT genes were detected in 62.5% of MSO patients. Moreover, according to Neyrand et al. [
18], molecular alterations such as
RAS or
BRAF or telomerase reverse transcriptase (
TERT) promoter mutations were observed in 87.5% of biologically MSO, and
RAS or
BRAF were observed in 70% of histologically MSO. This provides evidence that MSO shares molecular mechanisms akin to cancers originating in the thyroid gland. However, due to the limited number of cases analyzed in the studies, the established significance of molecular mutations remains inconclusive. In our study,
BRAF mutation test was conducted in one patient with MSO, and both
BRAF mutation test and
TERT promoter mutations test were performed in another patient with MSO, with all results confirmed as ‘not detected.’ There were no cases where molecular studies were conducted using samples diagnosed with benign struma ovarii. Analyzing the molecular studies in struma ovarii might be beneficial in future research.
Considering that MSO takes an average of 4 to 6 years to recur, a follow-up period longer than 10 years is recommended [
6,
16]. In this study, during the median follow-up period of 33 months (range, 4 to 156), 11 patients remained disease-free, one is currently undergoing treatment, and one has experienced progression with peritoneal seeding. There have been no recurrences or deaths due to MSO.
Only 53% of MSO patients in our study were treated by an endocrinologist. It seems likely that many gynecologists consider MSO as a gynecological disease. However, given the possibilities of recurrence and metastasis mentioned above, as well as postoperative treatment considerations, MSO requires a multidisciplinary approach, including endocrinology. MSO should be considered a thyroid cancer, not an ovarian cancer.
In conclusion, MSO is a rare disease that may be unfamiliar to endocrinologists. It generally has a favorable prognosis; however, in some cases, metastasis and recurrence can occur, and it is recommended to be followed for more than 10 years. There is no established standard treatment after gynecologic surgery, but total thyroidectomy and RAI therapy are recommended in patients with advanced stage or aggressive histology. Endocrinologists specialized in treating thyroid cancer can utilize their expertise to estimate and evaluate the risk stratification of MSO. Additionally, they can offer suggestions for post-surgery treatments, including RAI therapy, if necessary. Therefore, the management of MSO requires the involvement of not only gynecologists, but also endocrinologists.
Go to :





 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download