Abstract
The accurate detection of genetic mutations is very important in acute leukemia. Occasionally, tumor fraction may not be high at diagnosis, thus leading to false negative genetic test results. We present a case of acute lymphoblastic leukemia with relapse where the percentage of blasts in bone marrow aspirate was only 7.1% at the initial diagnosis. Karyotyping and next-generation sequencing results appeared negative at diagnosis; however, a retrospective review of the initial tests revealed that some of the mutations detected later at relapse were present even at the initial diagnosis but at very low fractions. Given the recently revised World Health Organization classification (fifth edition) and International Consensus Classification, the probability of encountering acute leukemia with a low blast percentage in bone marrow is expected to increase. This case indicates that efforts should be made to reduce false negative assessments for low tumor fraction leukemia to obtain a more accurate diagnosis and improve treatment.
초록
급성 백혈병에서 유전 변이를 정확하게 검출하는 것은 매우 중요하다. 간혹 종양 분율이 진단 시 높지 않은 경우, 유전 검사에서 위음성이 초래될 수 있다. 본 증례는 B림프모구백혈병에서 초진 시 골수 흡인 검체에 모세포가 7.1%로 낮았던 예이다. 초진 시의 핵형분석 및 차세대염기서열분석 결과는 음성으로 분석되었었다. 그러나 후향적으로 검토할 때, 재발 시에 검출된 변이 중 일부가 초진 시에도 매우 낮은 분율로 존재했던 것을 확인하였다. 최근 개정된 급성 백혈병에 대한 WHO 및 International Consensus Classification의 진단 기준을 고려할 때, 초진 시 골수 내 모세포의 분율이 낮은 경우들은 더 많아질 것으로 예상된다. 본 증례는 이러한 종양 분율이 낮은 급성 백혈병에 대해, 위음성을 줄임으로써 환자의 정확한 진단과 치료에 기여하기 위한 노력들이 필요함을 시사한다.
The accurate detection of genetic mutations is becoming increasingly important in diagnosing and managing acute myeloid leukemia (AML) and acute lymphoblastic leukemia (ALL). Many mutations, such as breakpoint cluster region (BCR)::Abelson murine leukemia viral oncogene homolog 1 (ABL1) rearrangement, have been shown to represent distinct entities with different characteristics and prognoses. Consequently, various genetic assays from conventional karyotyping and reverse-transcriptase polymerase chain reaction (RT-PCR) to comprehensive next-generation sequencing (NGS) have been adopted in the field of hemato-oncology.
The genetic analysis of acute leukemia may yield false negative results because of various reasons. One thing to consider is that the tumor fraction in the sample affects the proportion of DNA containing somatic mutations. If the blast percentage is low in the bone marrow sample, DNA from cancer cells will be diluted by DNA from normal cells, thus increasing the probability of false negative results.
In this study, we present a case of B-cell ALL (B-ALL) where genetic mutations were not captured at initial diagnosis but were identified at relapse. The retrospective review revealed that some of the mutations were present even at the initial diagnosis but were hidden because of low tumor fraction.
A 12-year-old female presented with fever and bone pain in May 2019. Whole body magnetic resonance imaging (MRI) and positron emission tomography/computed tomography showed multifocal bone marrow edema with fiuorodeoxyglucose uptake but without any definite mass lesion. Complete blood cell count revealed the following: white blood cells, 3.87×109/L; hemoglobin, 119 g/L; and platelets, 307×109/L. Blasts were not observed from peripheral blood smear; however, in bone marrow aspirate, blasts were 7.1% of total nucleated cells (Fig. 1A, Table 1). Although this was not a high proportion, the diagnosis of B-ALL was made because the fiow cytometric immunophenotyping of the blasts yielded positive results for immature B-cell markers (Table 2). The G-banding karyotyping and NGS targeting of 497 genes related to hematologic malignancy were performed using bone marrow aspirate, but no significant mutations were detected. She achieved complete remission in June 2019 and underwent consolidation chemotherapy until December 2021. However, immunoglobulin heavy chain (IGH)/kappa light chain (IGK) gene clonality assay still indicated measurable residual disease (MRD) (Fig. 2). IGH clone was negative near the last chemotherapy and increased to 0.07% in July 2022. IGK clone was positive for one clone, 0.068% near the last chemotherapy, and positive for all previously detected clones, with the largest being 0.24% in July 2022.
The patient revisited our hospital with chest wall pain in October 2022. Whole body MRI showed diffusely increased T2 signal intensity and increased enhancement in almost the whole axial skeleton. Complete blood cell count revealed the following: white blood cells, 8.82×109/L; hemoglobin, 132 g/L; and platelets, 159×109/L. Blasts comprised 9% of white blood cells in peripheral blood smear. Bone marrow study confirmed relapse with blasts occupying 84.6% of total nucleated cells and with an immunophenotype similar to that from the initial diagnosis (Fig. 1B, Tables 1 and 2). Genetic analysis with bone marrow aspirate at relapse showed some relevant mutations. G-banding karyotyping revealed 47,XX,+del(1)(p13),t(9;22)(q34;q11.2)[17]/46,XX[8]. RT-PCR and targeted RNA fusion panel confirmed the presence of e1a2-type BCR::ABL1 rearrangement. NGS panel targeting 531 genes related to hematologic malignancy revealed multiple exon deletions in Ikaros family zinc finger 1 (IKZF1; exons 4–7) and retinoblastoma 1 (RB1; exons 18–27). NGS also showed the duplication of chromosome 1q, which is consistent with the karyotyping results (Fig. 3). IGH/IGK gene clonality assay showed clones with the same gene combinations as those at diagnosis (Fig. 2).
None of the genetic alterations at relapse had been captured at diagnosis. The test data at diagnosis were retrospectively reviewed. Karyotyping could not be further verified because the remnant data were only for the initially reviewed 20 cells. However, NGS data showed that some of the mutations at relapse were present even at the initial diagnosis. The deletion of IKZF1 and the duplication of chromosome 1q were too subtle to be reliably captured with routine NGS pipelines but could be observed by the manual inspection of normalized depth plots (Fig. 3). The subtleness could be explained by the low proportion of blasts at the diagnosis.
After reporting the mutations, including BCR::ABL1 rearrangement, imatinib was added to the patient’s chemotherapy regimen. The patient achieved morphologic remission in December 2022. Quantitative polymerase chain reaction for BCR::ABL1 rearrangement converted to negative in February 2023, and the patient received allogeneic hematopoietic stem cell transplantation in May 2023. Although some MRD is suspected from IGH/IGK gene clonality assay, with one IGK clone being 0.003% at the latest assessment, the patient has not relapsed as of the latest follow-up in August 24, 2023 (Fig. 2).
The reliable detection of genetic aberrations even in cases of low tumor fraction is an important issue in acute leukemia. Although a diagnosis of ALL is generally not recommended if the bone marrow blast percentage is less than 20%, there is no definite lower limit [1]. A diagnosis of ALL is occasionally given even under a low blast percentage if clinical and laboratory evidence indicates the disease. For AML, there were already some mutations, such as runt-related transcription factor 1 (RUNX1)–RUNX1 partner transcriptional co-repressor 1 (RUNX1T1) rearrangement, that justified the diagnosis of AML even with a blast percentage of less than 20% [2]. The recently revised diagnostic criteria went even further. The fifth edition of the World Health Organization classification removed the 20% blast cutoff for AML with any defining genetic abnormalities, except AML with BCR::ABL1 rearrangement and AML with CCAAT enhancer binding protein alpha (CEBPA) mutation [3]. The International Consensus Classification also lowered the blast percentage cutoff to 10% for many AML-defining genetic aberrations [4]. Under these changes, there will be more cases of acute leukemia with a low fraction of blasts in the bone marrow. These cases will be more prone to false negative results, and the omission of defining genetic aberrations may even lead to diagnoses other than acute leukemia. Therefore, genetic analyses should be more sensitive to catch relevant mutations even in cases of low tumor fraction.
In our case, the proportion of blasts in the bone marrow at initial diagnosis was below 10%. A retrospective review revealed a previously hidden deletion of IKZF1 and a duplication of chromosome 1q, which are consistent with those detected at relapse. RB1 deletion was not observed from the manual inspection of normalized depth plots (Fig. 3). It could have been hidden in even more minor subclones at diagnosis or acquired at relapse; this situation is very rare but has been previously reported [5]. There was no definite evidence of BCR::ABL1 rearrangement at diagnosis. However, from karyotyping at relapse, the Philadelphia chromosome was present in all cells with a partial duplication of chromosome 1, thus lowering the possibility of the late acquisition of the rearrangement. If the rearrangement had been present from diagnosis, a more sensitive genetic analysis might have led to the earlier detection of the rearrangement, thus facilitating administration of a more suitable treatment and the prevention of relapse. This indicates that for acute leukemia with low tumor fraction, efforts are needed to prevent false negative assessments and to ensure that genetic mutations present in tiny proportions are sensitively captured.
Karyotyping is a conventional method for detecting chromosomal duplications, deletions, and rearrangements, which constitute a critical part of relevant mutations in acute leukemia. However, as shown in this case, its sensitivity is low, particularly for low tumor fraction samples. To prevent tumor cells from being overlooked because of the presence of normal cells, reviewing more than 20 cells may be considered. Furthermore, if at least one cell with abnormality is observed, a further confirmation of the mutation will be appropriate rather than simply dismissing it as a false finding.
Other than karyotyping, assays with higher sensitivity need to be more actively applied. Fluorescent in situ hybridization can have a limit of detection of 5% or lower depending on the specific technique, and RT-PCR can detect one aberrant cell among 100,000 normal cells [6]. Concerning NGS technology, more progress is needed to reliably detect low-fraction copy number variants and differentiate them from false positives. However, for single-nucleotide variants and short indels, efforts have been made to lower the limit of detection to less than 1% [7, 8]. NGS can also be applied to RNA-based fusion gene detection with a maximum sensitivity of 1 per 10,000 cells [9]. Even if some genetic tests are inevitably prone to false negative results, the active application of these sensitive assays can lower the combined false negative rate.
The IGH/IGK gene clonality assay is a genetic test that does not target diagnosis-defining mutations but aids in MRD follow-up. An uncommon finding in this case was that even when IGH clonality was converted to negative, IGK clonality still indicated MRD. In a previous study, cases where IGH clonality decreased beyond 10-6 with remaining IGK or immunoglobulin lambda locus (IGL) clonality at the end of consolidation comprised 10.6% of patients with pediatric B-ALL [10]. IGK or IGL MRD without IGH MRD was not correlated with three-year event-free survival in the study. However, the patient in the current case relapsed approximately 3.5 years after initial diagnosis, thus possibly indicating the need for follow-up of not only IGH but also IGK/IGL clonality for long-term prognostic predictions.
This study reported a case of acute leukemia where low tumor fraction at diagnosis led to false negative genetic assessments. More sensitive assays would have prevented false negative results and may have led to more adequate treatment and better outcomes. This case indicates that efforts for higher sensitivity need to be made, particularly for acute leukemia with low tumor fraction.
Acknowledgments
This work was supported by a grant from the National Research Foundation of Korea (NRF-2021R1I1A1A01045980).
REFERENCES
1. Borowitz MJ, Chan JKC, Downing JR, Le Beau MM, Arber DA, Harris NL, et al. Swerdlow SH, Campo E, editors. 2017. Precursor lymphoid neoplasms. WHO classification of tumours of haematopoietic and lymphoid tissues. Revised 4th ed. IARC;Lyon: p. 200. DOI: 10.53347/rid-9250.
2. Arber DA, Brunning RD, Le Beau MM, Falini B, Vardiman JW, Porwit A, et al. Swerdlow SH, Campo E, editors. 2017. Acute myeloid leukaemia with recurrent genetic abnormalities. WHO classification of tumours of haematopoietic and lymphoid tissues. Revised 4th ed. IARC;Lyon: p. 130. DOI: 10.53347/rid-9250.
3. Khoury JD, Solary E, Abla O, Akkari Y, Alaggio R, Apperley JF, et al. 2022; The 5th edition of the World Health Organization classification of haematolymphoid tumours: myeloid and histiocytic/dendritic neoplasms. Leukemia. 36:1703–19. DOI: 10.1038/s41375-022-01613-1. PMID: 35732831. PMCID: PMC9252913.
4. Arber DA, Orazi A, Hasserjian RP, Borowitz MJ, Calvo KR, Kvasnicka HM, et al. 2022; International consensus classification of myeloid neoplasms and acute leukemias: integrating morphologic, clinical, and genomic data. Blood. 140:1200–28. DOI: 10.1182/blood.2022015850. PMID: 35767897. PMCID: PMC9479031.
5. Forero-Castro M, Montaño A, Robledo C, García de Coca A, Fuster JL, de Las Heras N, et al. 2020; Integrated genomic analysis of chromosomal alterations and mutations in B-cell acute lymphoblastic leukemia reveals distinct genetic profiles at relapse. Diagnostics (Basel). 10:455. DOI: 10.3390/diagnostics10070455. PMID: 32635531. PMCID: PMC7400270. PMID: 8c7bfe55c6f543d1a24c92c9571527e1.
6. Nashed AL, Rao KW, Gulley ML. 2003; Clinical applications of BCR-ABL molecular testing in acute leukemia. J Mol Diagn. 5:63–72. DOI: 10.1016/S1525-1578(10)60454-0. PMID: 12707370. PMCID: PMC1907317.
7. Kim J, Kim D, Lim JS, Maeng JH, Son H, Kang HC, et al. 2019; The use of technical replication for detection of low-level somatic mutations in next-generation sequencing. Nat Commun. 10:1047. DOI: 10.1038/s41467-019-09026-y. PMID: 30837471. PMCID: PMC6400950. PMID: 0578a86addbe436fb5132e2cadf2e2e8.
8. Lee KS, Seo J, Lee CK, Shin S, Choi Z, Min S, et al. 2022; Analytical and clinical validation of cell-free circulating tumor DNA assay for the estimation of tumor mutational burden. Clin Chem. 68:1519–28. DOI: 10.1093/clinchem/hvac146. PMID: 36306340.
9. de Boer EN, Johansson LF, de Lange K, Bosga-Brouwer AG, van den Berg E, Sikkema-Raddatz B, et al. 2020; Detection of fusion genes to determine minimal residual disease in leukemia using next-generation sequencing. Clin Chem. 66:1084–92. DOI: 10.1093/clinchem/hvaa119. PMID: 32613252.
10. Chen H, Gu M, Liang J, Song H, Zhang J, Xu W, et al. 2023; Minimal residual disease detection by next-generation sequencing of different immunoglobulin gene rearrangements in pediatric B-ALL. Nat Commun. 14:7468. DOI: 10.1038/s41467-023-43171-9. PMID: 37978187. PMCID: PMC10656538. PMID: e360ad2e17b0408d94050a198b8b92a5.
Fig. 1
Bone marrow aspiration (Wright–Giemsa stain, ×400) at initial diagnosis (A) and upon relapse (B) with a representative blast magnified in the upper right corner (×2,000).
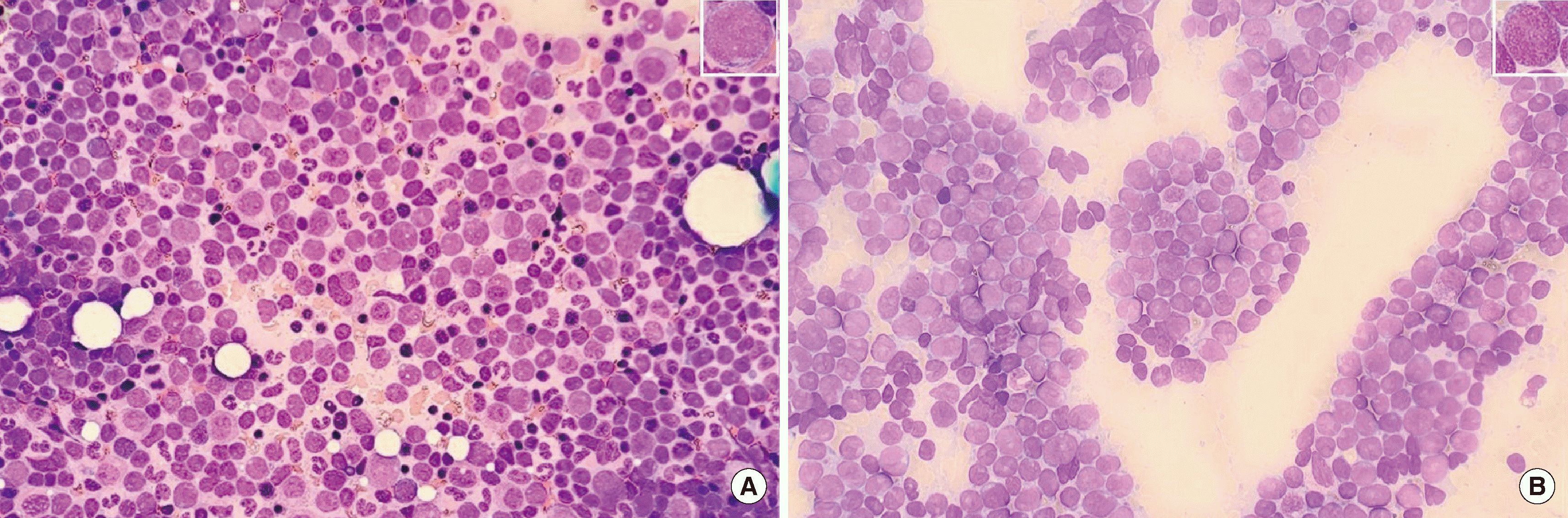
Fig. 2
The patient’s immunoglobulin heavy chain/kappa light chain (IGH/IGK) gene clonality assay results along with the clinical course.
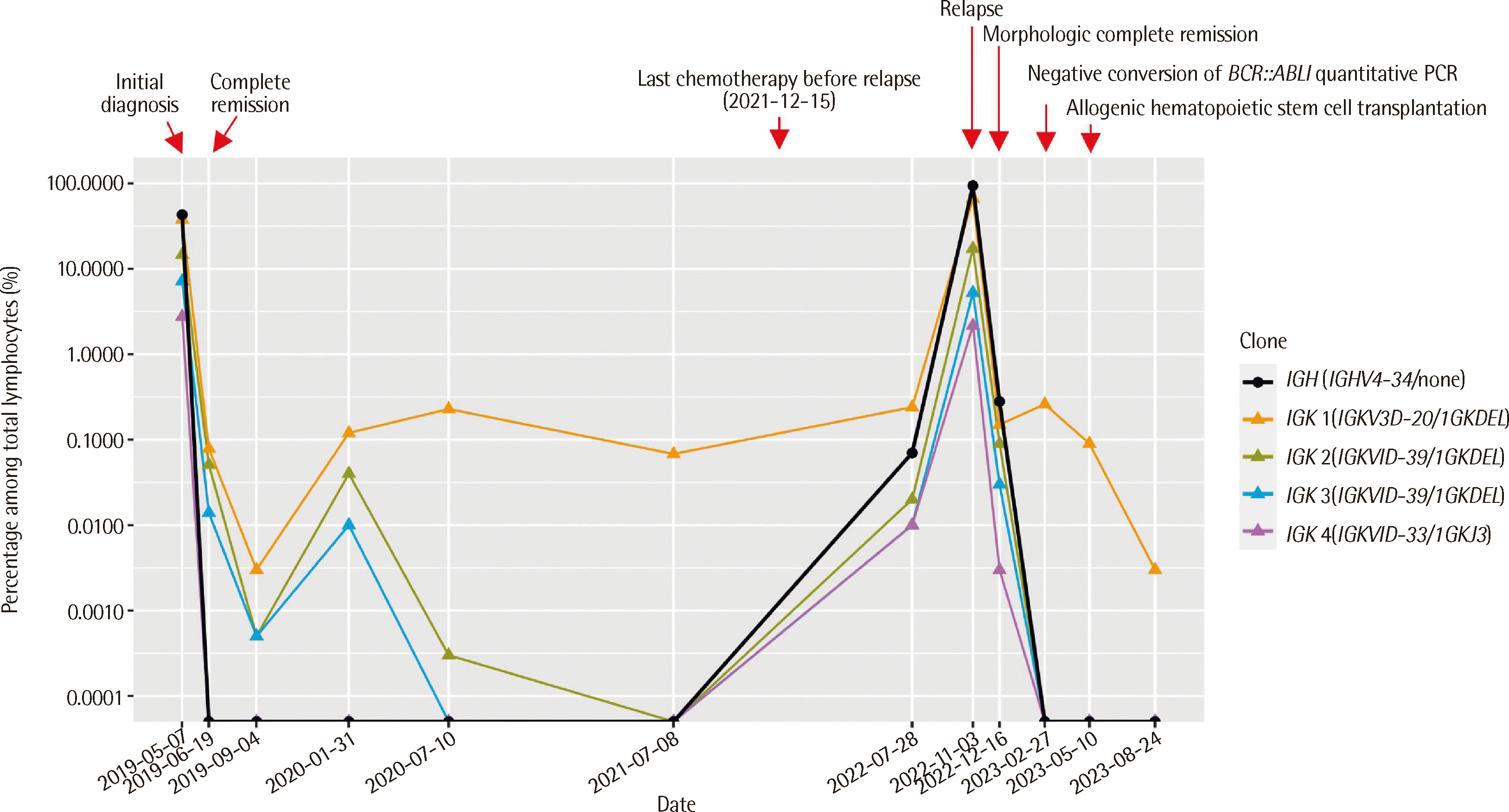
Fig. 3
The patient’s genetic tests reviewed. Karyotyping at diagnosis (A) and relapse (B). Normalized depth plots of IKZF1 at diagnosis (C) and relapse (D). Normalized depth plots of RB1 at diagnosis (E) and relapse (F). Whole chromosome normalized depth plots at diagnosis (G) and relapse (H). The normalized depth plot of chromosome 1 at diagnosis (I).
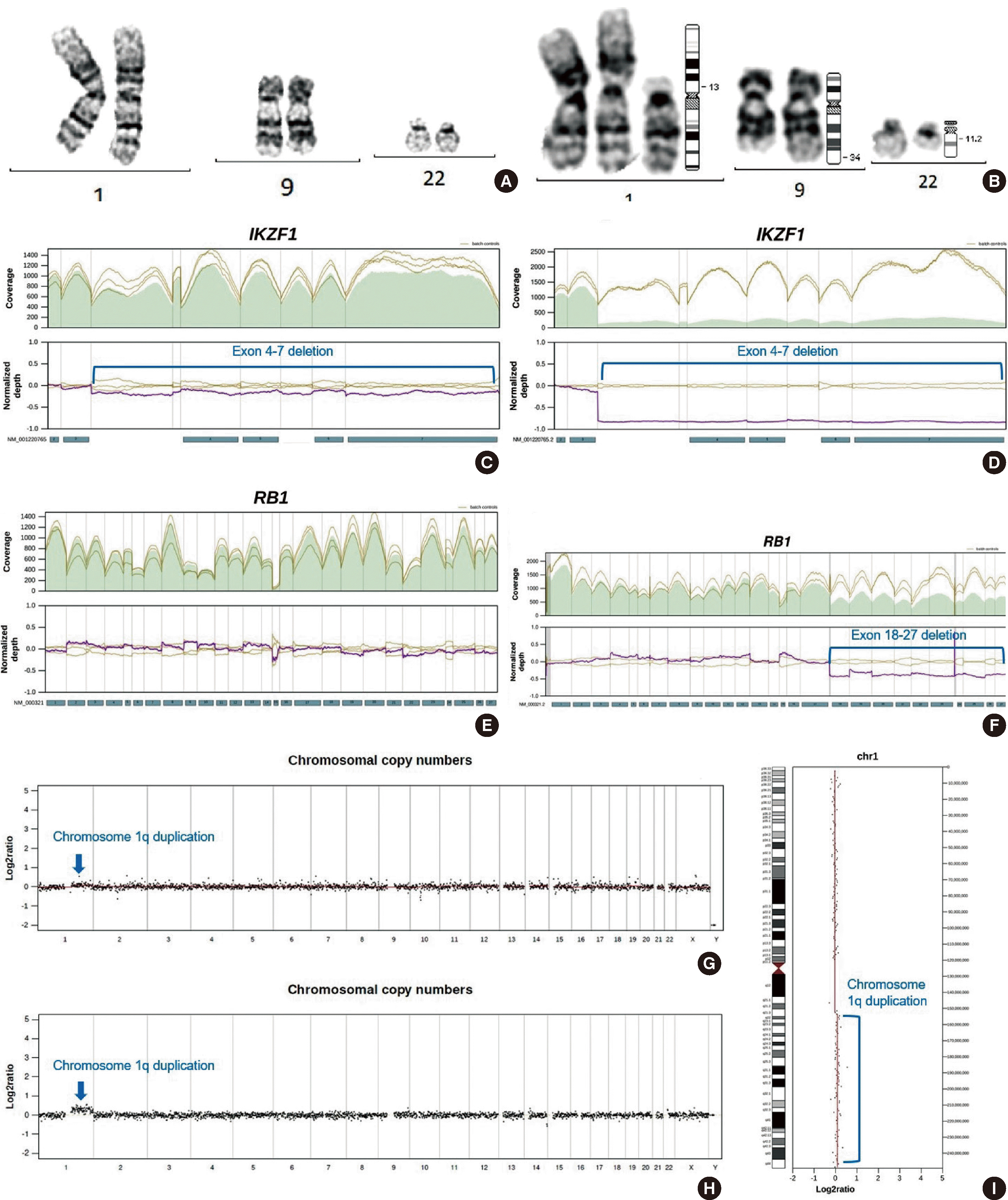
Table 1
Comparison of test results at initial diagnosis and relapse
Table 2
Flow cytometric immunophenotyping of leukemic blasts at diagnosis and relapse
| CD45 | Side scatter | CD34 | TdT | CD10 | CD19 | cCD79a | cCD22 | CD20 | CD38 | |
|---|---|---|---|---|---|---|---|---|---|---|
| Diagnosis | Neg to T* | Low | Pos | Neg | Pos | Pos | Pos (D* to T*) | Pos (D* to T*) | Neg | Pos |
| Relapse | Neg to D* | Low to T* | Pos | Pos | Pos | Pos | Pos | Pos (D* to T*) | Neg | Pos (D*) |
| CD3 | CD5 | CD7 | HLA-DR | MPO | CD13 | CD33 | CD117 | CD14 | CD11c | |
|---|---|---|---|---|---|---|---|---|---|---|
| Diagnosis | Neg | Neg | Neg | Pos | Neg | Pos | Pos (D*) | Pos(D* to T*) | Neg | NA† |
| Relapse | Neg | NA† | Neg | Pos | Neg | Pos (T*) | Pos (partial) | Neg | Neg | Neg |




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download