Abstract
Background
Regulatory T (Treg) cells are a small subset of CD4+ T cells that regulate immune homeostasis and self-tolerance. Hyperactivated Treg cells can cause autoimmune diseases such as type 1 diabetes. In the context of cancer, these cells interact with diverse immune cells within the tumor microenvironment. Recently, Treg cells have garnered substantial attention as a potential target for immunotherapy; however, previous studies have been limited to specific disease groups. Therefore, this study aims to propose reference intervals for Treg cells in healthy donors.
Methods
Peripheral blood samples were collected from 90 healthy adults at Chungbuk National University Hospital. Eight-color flow cytometry was performed using a DURAClone IM Treg tube kit (Beckman Coulter, USA). The reference intervals for Treg cell detection, using various fluorescent monoclonal antibodies, were established using the Robust statistical method in accordance with the Clinical Laboratory Standards Institute EP28-A3c guidelines.
Results
The reference intervals for Treg cells according to the immunofluorescent antibody combination were determined as follows for each subset of CD3+CD4+ T lymphocytes: CD3+CD4+CD25+ (1.34–11.42%), CD3+CD4+CD39+ (0.82–9.20%), CD3+CD4+FoxP3+ (0.61–8.77%), CD3+CD4+Helios+ (1.10–14.73%), CD3+CD4+CD25+FoxP3+ (0.43–3.80%), and CD3+CD4+FoxP3+Helios+ (0.37–6.29%). No significant differences with respect to sex or age were observed.
초록
배경
조절 T세포(regulatory T cell)는 인간 면역체계에서 억제 기능을 통해 면역 항상성 및 자가관용을 조절하는 CD4+ T세포 중 하나이다. 과도하게 활성화된 조절 T세포의 기능 이상은 1형 당뇨와 같은 자가면역질환을 유발하기도 하고, 종양질환에서는 종양미세환경 속에서 다양한 면역세포들과 상호작용을 하기도 한다. 최근에는 면역치료제의 표적으로서도 관심을 받고 있지만, 이전 연구들은 특정질환에만 한정되어 있었다. 이에 이번 연구에서는 정상 성인군에서의 참고치를 제안하고자 한다.
방법
충북대학교병원에서 90명의 건강인 대조군의 말초혈액 검체를 이용하였다. Duraclone IM Treg tube 키트(Becton Dickinson, USA)를 이용해 8-컬러 유세포검사법을 시행하였으며, 각종 형광단클론항체를 사용하여 CLSI EP28-A3c에 따라 Robust 통계 방법을 이용해 참고치를 설정하였다.
Regulatory T (Treg) cells play an important role in maintaining self-tolerance and homeostasis, which are major immune mechanisms [1]. Treg cells exert an immunosuppressive effect by interacting with effector T cells [2]. Therefore, a deficiency in Treg cells may lead to the development of autoimmune or infiammatory diseases, such as type 1 diabetes, infiammatory bowel disease, and immune dysregulation polyendocrinopathy enteropathy syndrome [3-5]. In the context of cancer, Treg cells are overexpressed as they suppress the tumor’s specific immune response, and a high number of Treg cells is associated with poor prognosis [6].
Treg cells are a subpopulation of CD4+ T cells, accounting for a variable population ranging from 2–4% of CD4+ T cells [7]. The absolute count of Treg cells is notably low. Treg cells comprise both thymic Treg cells originating in the thymus and peripheral Treg cells derived from naïve T cells. During development, the maturation of Treg cells is commonly characterized by expression of forkhead box P3 (FoxP3) and CD25 (interleukin [IL]-2 receptor alpha chain) [5]. Formerly referred to as CD4+CD25+ suppressor T cells, a deficiency in Treg cells has been known to induce autoimmune diseases [5]. FoxP3, discovered in 2000, is a master regulator in Treg cell development, and its expression is significant for identifying the Treg cell lineage [8]. Consequently, these CD25+FoxP3+ immunophenotypic characteristics have been used for the quantitative measurement of Treg cells using fiow cytometry [7, 9].
Treg cells have recently garnered attention for their potential therapeutic applications in immune response regulation as immune checkpoints. They could serve as valuable targets for treating infiammation or autoimmune diseases by inhibiting their activation. Conversely, these cells could be targeted to induce antitumor immune responses through downstream strategies [3]. Nevertheless, some studies have reported the abnormal expression of Treg cells in specific diseases such as type I diabetes mellitus [7]. Additionally, data on the reference intervals required to interpret abnormality are limited. Therefore, this study aims to establish and propose reference intervals for Treg cells in healthy Korean adults according to the Clinical Laboratory Standards Institute (CLSI) EP28-A3c [10].
Ninety healthy adults who visited Chungbuk National University Hospital for routine medical examinations between September 2022 and December 2023 were included in the study. The participants were aged 18–65 years, and blood samples were randomly collected regardless of sex. All enrolled participants had no underlying health conditions that could affect the results of a complete blood count. The complete blood count and differential white blood cell count of all samples were determined using a routine hematology analyzer (DX800, Beckman Coulter, Inc., Fullerton, CA, USA) and were within the reference ranges. This study was approved by the Institutional Review Board of Chungbuk National University Hospital (CBNUH 2022-07-017-003).
Peripheral blood was collected from healthy adults and placed in tubes containing EDTA. Treg cells were measured using a DuraClone IM Treg Tube (Beckman Coulter, Inc., Fullerton, CA, USA) according to the manufacturer’s instructions [11]. Briefiy, 50 μL of peripheral blood was added to Duraclone IM Treg Tube 1 and incubated at room temperature (RT) in the dark for 15 minutes. Subsequently, 3 mL of Dulbecco’s phosphate-buffered saline (DPBS) was added, and the supernatant was aspirated after centrifugation at 500×g for 5 minutes. After vortexing, 5 μL of PerFix-nc Buffer 1 was added and incubated at RT in the dark for 15 minutes. Subsequently, 400 μL of PerFix-nc Buffer 2 was added, and the sample was transferred to Duraclone IM Tube 2, followed by incubation
at RT in the dark for 60 minutes. Upon adding 3 mL of DPBS, the sample was incubated for 5 minutes and centrifuged at 500×g for 5 minutes, and pellet cells were obtained while aspirating the supernatant. Finally, 3 mL of PerFix-nc Buffer 3 was added, followed by centrifugation at 500×g for 5 minutes. The supernatant was then aspirated, and 500 μL of PerFix-nc Buffer 3 was added. The resuspended cells were analyzed using the Navios fiow cytometer (Beckman Coulter, Inc.). Eight-color fiow cytometric immunophenotyping was employed with the following monoclonal antibodies to detect Treg populations: CD45RA (clone 2H4LDH11LDB9)-FITC, CD25 (clone B1.49.9)-PE, CD39 (clone BA54)-PC5.5, CD4 (clone SFCI12T4D11)-PC7, FoxP3 (clone 259D)-A647, CD3 (clone UCHT-1)-AA750, Helios (clone 22F6)-PBE, and CD45 (clone J33)-KrO. Data were analyzed using Kaluza analysis software (Beckman Coulter, Inc.).
A gating strategy was established based on the immunophenotypic characteristics of Treg cells, i.e., CD3+CD4+CD25+FoxP3+ Helios+CD39+ (Fig. 1). First, lymphocytes were gated in the CD45bright/SSClow histogram, and then the CD3+CD4+ population was selected. Subsequently, cells positive for CD25, FoxP3, CD39, and Helios were isolated. Two types of Treg cells were quantified using the following combinations of antibody markers: CD3+CD4+CD25+FoxP3+ and CD3+CD4+FoxP3+Helios+.
Ninety healthy donors, with an average age of 45.8 years, were included in the study. Baseline characteristics of the donors are presented in Table 1. No unusual findings were observed in the complete blood count. The study group included 45 healthy donors of both sexes, with a male-to-female ratio of 1:1. No significant difference was observed in terms of age between the men and women; however, the hemoglobin levels were significantly lower (P=0.000), while the platelet levels were slightly higher (P=0.015) in women. Notably, no significant differences were observed in the white blood cell or lymphocyte counts.
Gaussian distribution was assessed to establish a reference interval. The distribution curves for the results are depicted in Fig. 2. When combining one additional marker (CD25+, CD39+, FoxP3+, or Helios+) with the CD3+CD4+ population, the central 95% interval was calculated using EP Evaluator software program. Given the utilization of 90 samples, a robust methodology was implemented. The transformed parametric method was applied for all markers, except for Helios, which was calculated using the parametric method. The central 95% interval of the distribution curves for the CD3+CD4+CD25+FoxP3+ and CD3+CD4+FoxP3+Helios+ cells was calculated using the transformed parametric method. The confidence interval for all figures was 0.15.
The reference interval for CD3+CD4+ was determined to be 19.62–43.51% of the total lymphocytes. Meanwhile, the reference intervals for Treg cells were determined according to the combinations of four antibodies and the gating strategy (Table 2). Using CD3+CD4+ T cells as the total population, the reference intervals for different subsets were determined as follows: CD3+CD4+CD25+ (1.34–11.42%), CD3+CD4+CD39+ (0.82–9.20%), CD3+CD4+FoxP3+ (0.61–8.77%), CD3+CD4+Helios+ (1.10–14.73%), CD3+CD4+CD25+FoxP3+ (0.43–3.80%), and CD3+CD4+FoxP3+Helios+ (0.37–6.29%).
To examine potential variations based on gender and age, subgroups were formed, and a comparison of values for FoxP3+CD25+ and FoxP3+Helios+ was conducted. No differences were found based on gender (Fig. 3A). In terms of age, comparisons were made in the 10-year interval age groups (20s to 60s): 20s (N=8), 30s (N=17), 40s (N=26), 50s (N=32), and 60s (N=7). A significant difference was found in the CD3+CD4+FoxP3+Helios+ cell populations between the 30s and 60s age groups (P=0.028; Fig. 3B). The average for the 30s age group was 3.16%, while the average for the 60s age group was 1.79%. Although the difference in the mean of these two groups was significant, when two larger groups (age <60 years and age ≥60 years) were compared, no significant difference was noted. In addition, no significant differences were found among other age groups. Hence, the segmented reference values according to age have not been presented as no statistical difference was observed in other generations.
Similar to previous studies, fiow cytometry was used to establish Treg cell reference intervals. Distinguishing various T-cell subtypes requires a methodology with high sensitivity and specificity, and fiow cytometry fulfills this criterion [12]. In contrast to the previously used methodology utilizing a combination of CD3+CD4+CD25+ to identify Treg cells, our study proposed 1.34–11.42% (mean: 5.46%) of the CD3+CD4+ gated T cells as a reference interval. This value was similar to that reported by Schatorjé et al. [13]. They reported a median Treg value of 10% for all age groups (pediatric to adult participants), and this value remained stable regardless of age. However, the CD25+FoxP3+ detection method is considered more sensitive. In our study, the reference interval for CD25+FoxP3+ was 0.43–3.80% (mean, 1.80%) of CD3+CD4+ gated T cells. This value was lower than that observed in a Han Chinese population (2.17–7.94%; mean, 4.80%) [14], but was higher than that observed in an Italian population (0.59–0.79%; mean, 0.7%) [15]. The potential variations associated with the differences in the analysis strategy used, such as antigen combination, race, or study sample size should be considered [16].
The reference range differed depending on the antibody combination employed. This could be due to the differences in the antigen expression levels during Treg cell development [5]. FoxP3 is a unique antigen that continues to be expressed during the development of Treg cells, and high expression indicates activation [17]. Another study on the classification and quantification of native and activated Treg cells based on the level of FoxP3 expression in CD4+CD25high cells reported a mean of Treg cell percentage (Foxp3high) of 1.53% (SD, 0.37), which was similar to the value reported in our study [18]. Since antigen expression exhibits heterogeneity, analytical variations may arise in the results of fiow cytology analysis [19]. Therefore, a gating strategy tailored to each laboratory should be established.
Previously, Helios and CD39 were not widely used as immunophenotypic markers for Treg cells. Helios, an Ikaros family transcription factor, is associated with a native Treg cell phenotype derived from the thymus [3]. Consequently, Helios can also serve as a Treg cell lineage marker, similar to FoxP3. However, due to the heterogeneous expression of all antigens, we presented the reference interval of Helios in conjunction with FoxP3. In our study, the distribution of CD3+CD4+Helios+ was wider compared that of other markers, and the expression of CD3+CD4+FoxP3+Helios+ Treg cells peaked in the 30s and decreased in the 60s. A similar trend was observed in other CD3+CD4+CD25+FoxP3+ T reg cells, but it was not statistically significant. This is likely attributed to a decline in the thymus function with age [20], and further validation according to age is needed using larger sample size. Another marker, CD39 serves as a functional marker presenting a strong suppression status in CD4+CD25highFoxP3+ Treg cells [21]. CD39 functions as an adenosine triphosphate diphosphohydrolase enzyme contributing to adenosine-mediated immune suppression. Its expression increases in conditions such as infiammation, autoimmune disorders, and cancer. Moreover, recent studies have reported that Helios is valuable for enhancing sensitivity in detecting activated Treg cells [22, 23]. Therefore, these two markers appear to be particularly beneficial when classifying activated Treg cells rather than resting Treg cells.
This study has some limitations. First, it did not include the required 120 participants to establish the reference values. Although a robust method was employed to compensate for this limitation, further validation with a larger sample size is necessary. Additionally, the heterogeneous expression patterns of each antigen may have led to ambiguities in classification. Finally, the differences between examiners may have occurred, representing a limitation in fiow cytometry analysis.
In conclusion, we have established reference intervals for Treg cells in peripheral blood using various immunophenotypic markers. Given the potential variability in these values depending on the expression levels of immunophenotypic antigens, caution is recommended when interpreting the results. Nevertheless, as CD3+CD4+FoxP3+CD25+ makers are consistently expressed regardless of the Treg cell developmental process, these markers should be utilized as reference intervals for Treg cells. Furthermore, since this study employed multicolor fiow cytometry to analyze multiple antigens simultaneously, the findings are expected to apply to various clinical conditions such as primary immunodeficiencies, autoimmune diseases, and cancers.
Acknowledgments
This work was supported by a research grant from the Korean Society of Diagnostic Immunology in 2022.
REFERENCES
1. Sakaguchi S. 2004; Naturally arising CD4+ regulatory t cells for immunologic self-tolerance and negative control of immune responses. Annu Rev Immunol. 22:531–62. DOI: 10.1146/annurev.immunol.21.120601.141122. PMID: 15032588.
2. Liston A, Gray DH. 2014; Homeostatic control of regulatory T cell diversity. Nat Rev Immunol. 14:154–65. DOI: 10.1038/nri3605. PMID: 24481337.
3. Sakaguchi S, Mikami N, Wing JB, Tanaka A, Ichiyama K, Ohkura N. 2020; Regulatory T cells and human disease. Annu Rev Immunol. 38:541–66. DOI: 10.1146/annurev-immunol-042718-041717. PMID: 32017635.
4. Petzold C, Riewaldt J, Watts D, Sparwasser T, Schallenberg S, Kretschmer K. 2013; Foxp3+ regulatory T cells in mouse models of type 1 diabetes. J Diabetes Res. 2013:940710. DOI: 10.1155/2013/940710. PMID: 23691523. PMCID: PMC3647588. PMID: 89598a8b4dc84f05a6d34d32ff40b6f4.
5. Deng B, Zhang W, Zhu Y, Li Y, Li D, Li B. 2022; FOXP3+ regulatory T cells and age-related diseases. FEBS J. 289:319–35. DOI: 10.1111/febs.15743. PMID: 33529458.
6. Tanaka A, Sakaguchi S. 2017; Regulatory T cells in cancer immunotherapy. Cell Res. 27:109–18. DOI: 10.1038/cr.2016.151. PMID: 27995907. PMCID: PMC5223231.
7. Grant J, Bourcier K, Wallace S, Pan D, Conway A, Seyfert-Margolis V, et al. 2009; Validated protocol for FoxP3 reveals increased expression in type 1 diabetes patients. Cytometry B Clin Cytom. 76:69–78. DOI: 10.1002/cyto.b.20446. PMID: 18690669.
8. Scheinecker C, Göschl L, Bonelli M. 2020; Treg cells in health and autoimmune diseases: new insights from single cell analysis. J Autoimmun. 110:102376. DOI: 10.1016/j.jaut.2019.102376. PMID: 31862128.
9. Pitoiset F, Barbié M, Monneret G, Braudeau C, Pochard P, Pellegrin I, et al. 2018; A standardized flow cytometry procedure for the monitoring of regulatory T cells in clinical trials. Cytometry B Clin Cytom. 94:777–82. DOI: 10.1002/cyto.b.21622. PMID: 29316248.
10. Clinical, Laboratory Standards Institute. 2010. Defining, establishing, and verifying reference intervals in the clinical laboratory; Approved Guideline -Third Edition. Clinical and Laboratory Standards Institute;Wayne, PA: CLSI document EP28-A3c.
11. Kronenberg K, Riquelme P, Hutchinson JA. Standard protocols for immune profiling of peripheral blood leucocyte subsets by flow cytometry using DuraClone IM reagents. https://protocolexchange.researchsquare.com/article/pex-757/v1. Updated on Mar 2021. DOI: 10.21203/rs.3.pex-757/v1.
12. Salzer U, Sack U, Fuchs I. 2019; Flow cytometry in the diagnosis and follow up of human primary immunodeficiencies. EJIFCC. 30:407–22. PMID: 31814814. PMCID: PMC6893889.
13. Schatorjé EJ, Gemen EF, Driessen GJ, Leuvenink J, van Hout RW, de Vries E. 2012; Paediatric reference values for the peripheral T cell compartment. Scand J Immunol. 75:436–44. DOI: 10.1111/j.1365-3083.2012.02671.x. PMID: 22420532.
14. Niu HQ, Zhao XC, Li W, Xie JF, Liu XQ, Luo J, et al. 2020; Characteristics and reference ranges of CD4+ T cell subpopulations among healthy adult Han Chinese in Shanxi Province, North China. BMC Immunol. 21:44. DOI: 10.1186/s12865-020-00374-9. PMID: 32746780. PMCID: PMC7397677. PMID: 25eaf19dcc2b4eaf93fc93cd1f576acd.
15. Sorrenti V, Marenda B, Fortinguerra S, Cecchetto C, Quartesan R, Zorzi G, et al. 2016; Reference values for a panel of cytokinergic and regulatory lymphocyte subpopulations. Immune Netw. 16:344–57. DOI: 10.4110/in.2016.16.6.344. PMID: 28035210. PMCID: PMC5195844.
16. Chng WJ, Tan GB, Kuperan P. 2004; Establishment of adult peripheral blood lymphocyte subset reference range for an Asian population by single-platform flow cytometry: influence of age, sex, and race and comparison with other published studies. Clin Diagn Lab Immunol. 11:168–73. DOI: 10.1128/CDLI.11.1.168-173.2004. PMID: 14715565. PMCID: PMC321350.
17. Castillo-Aleman YM, Bencomo-Hernandez AA, Ventura-Carmenate Y. 2022; CD4+Foxp3+Helios+ regulatory T cells: role in immunostasis and clinical applications. J Cell Mol Immunol. 1:5–7. DOI: 10.46439/immunol.1.002. PMID: 36624866. PMCID: PMC9825804.
18. Petsiou A, Paschou SA, Vartholomatos G, Chatzigianni K, Kolaitis N, Giotaki E, et al. 2020; A modified flow cytometry method for objective estimation of human CD4+ regulatory T cells (CD4+ Tregs) in peripheral blood, via CD4/CD25/CD45RO/FoxP3 labeling. Cytometry B Clin Cytom. 98:259–69. DOI: 10.1002/cyto.b.21841. PMID: 31571372.
19. Wing JB, Tanaka A, Sakaguchi S. 2019; Human FOXP3+ regulatory T cell heterogeneity and function in autoimmunity and cancer. Immunity. 50:302–16. DOI: 10.1016/j.immuni.2019.01.020. PMID: 30784578.
20. Palmer DB. 2013; The effect of age on thymic function. Front Immunol. 4:316. DOI: 10.3389/fimmu.2013.00316. PMID: 24109481. PMCID: PMC3791471. PMID: 0637faf2771c40cdb35839a37967814f.
21. Mandapathil M, Lang S, Gorelik E, Whiteside TL. 2009; Isolation of functional human regulatory T cells (Treg) from the peripheral blood based on the CD39 expression. J Immunol Methods. 346:55–63. DOI: 10.1016/j.jim.2009.05.004. PMID: 19450601. PMCID: PMC2703678.
22. Elkord E, Abd Al Samid M, Chaudhary B. 2015; Helios, and not FoxP3, is the marker of activated Tregs expressing GARP/LAP. Oncotarget. 6:20026–36. DOI: 10.18632/oncotarget.4771. PMID: 26343373. PMCID: PMC4652984.
23. Himmel ME, MacDonald KG, Garcia RV, Steiner TS, Levings MK. 2013; Helios+ and Helios− cells coexist within the natural FOXP3+ T regulatory cell subset in humans. J Immunol. 190:2001–8. DOI: 10.4049/jimmunol.1201379. PMID: 23359504.
Fig. 1
Gating strategy for regulatory T cells (Tregs). (A) Lymphocytes are gated on the CD45brightSSClow histogram, and the CD3+CD4+ population is defined. (B) Additional markers, CD25+, CD39+, FoxP3+, and Helios+, are used to detect the Treg cell subpopulation. (C) Combinations of two antibodies are used for detecting Tregs.

Fig. 2
Distribution curves from flow cytometric immunophenotyping with various monoclonal antibodies. (A) CD3+CD4+CD25+, (B) CD3+CD4+FoxP3+, (C) CD3+CD4+CD39+, (D) CD3+CD4+Helios+, (E) CD3+CD4+CD25+FoxP3+, and (F) CD3+CD4+FoxP3+Helios+ cells. These results indicate the percentage of CD3+CD4+ T cells for the given variable.
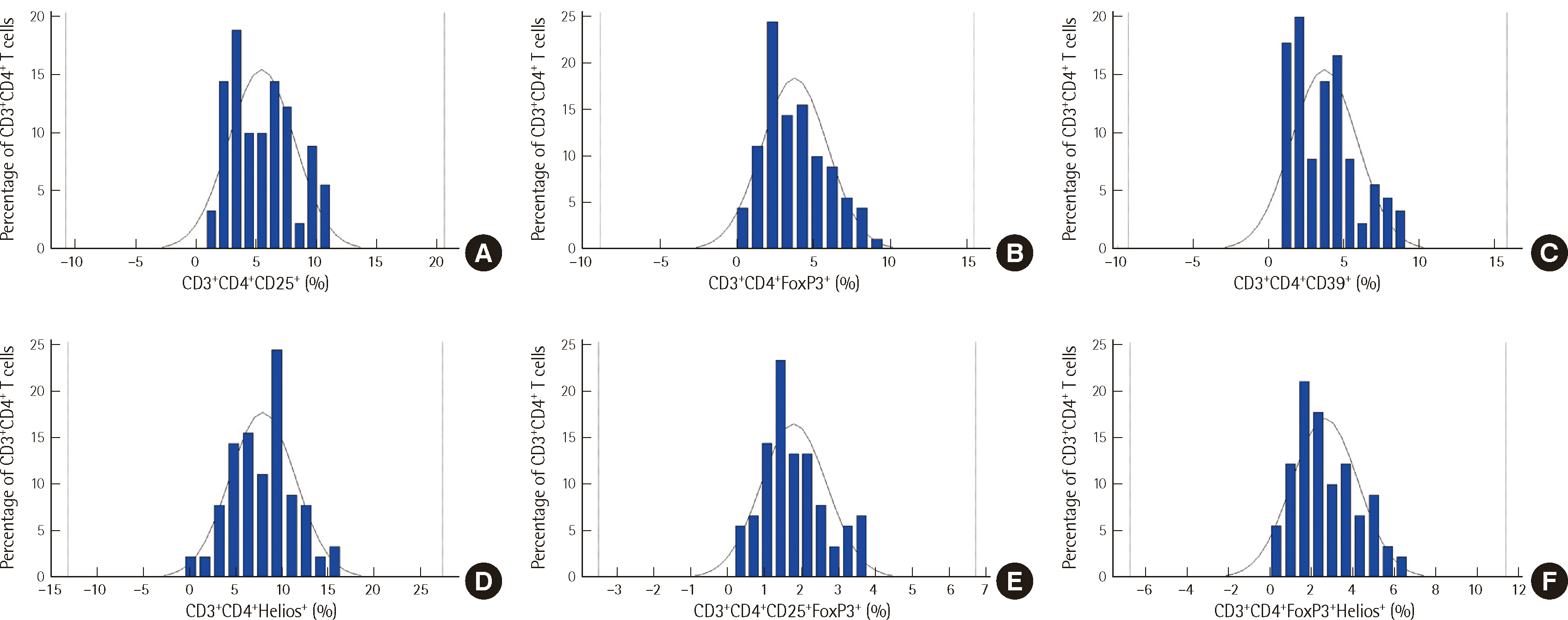
Fig. 3
Assessment of the proportion of CD3+CD4+CD25+FoxP3+/CD3+CD4+FoxP3+Helois+ Treg cells based on gender and age. (A) Comparison of Treg cell subgroups based on gender. No significant differences have been observed between the male and female groups. (B) Comparison of Treg cell subgroups based on age. No significant differences were observed in the CD4+CD25+FoxP3+ subpopulation on the basis of age (the 20s, 30s, 40s, 50s, and 60s). However, significant differences were observed in the CD3+CD4+FoxP3+Helios+ subpopulation between the 30s and 60s subgroups (P=0.028). The interquartile range is presented as the median, and the thick marks indicate the minimum and maximum values.
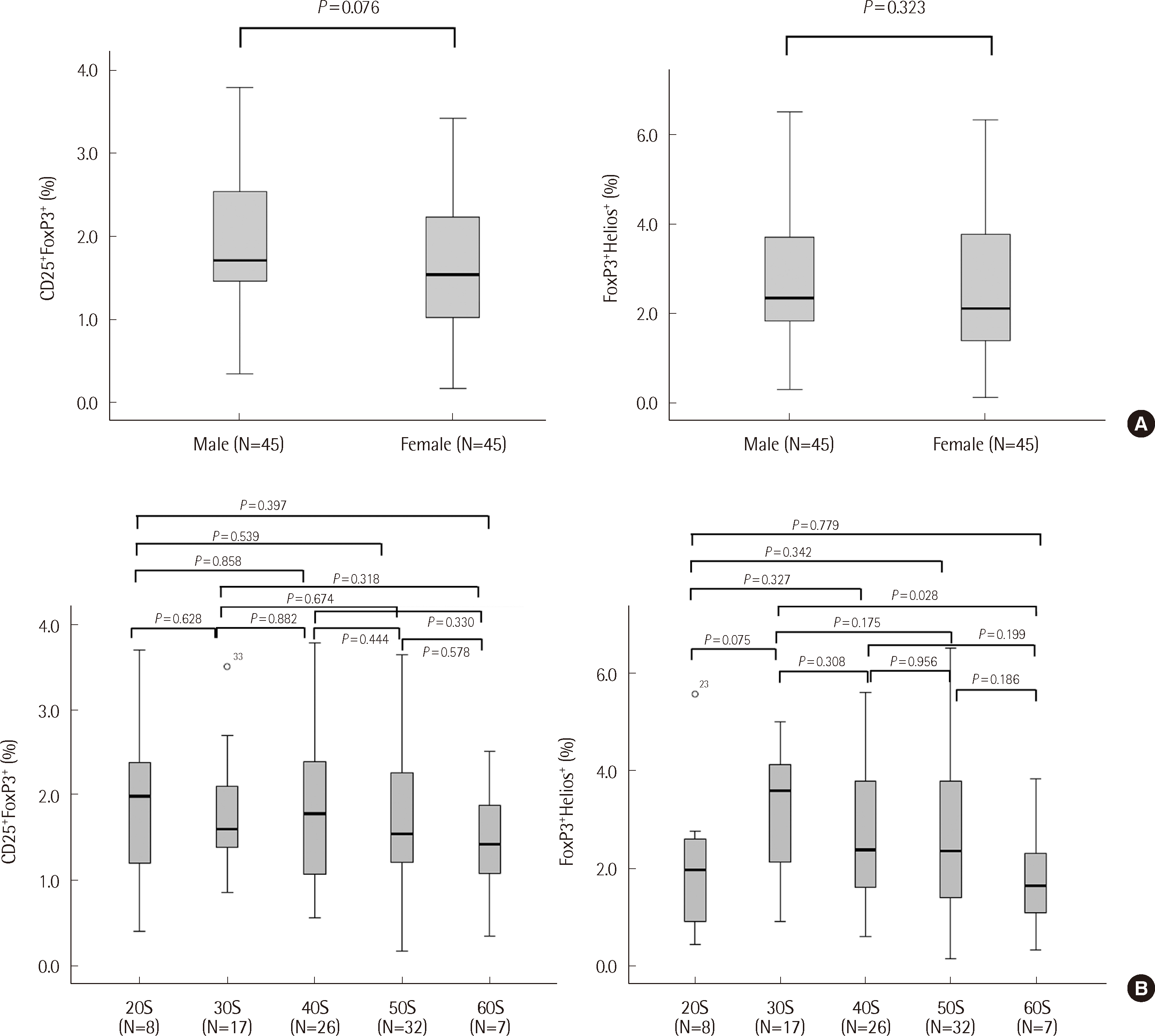
Table 1
Baseline characteristics of 90 healthy donors
Table 2
Proposed reference range of peripheral blood regulatory T-cell population in 90 healthy donors
| Variable* | Mean (%) | SD | Range (%) | Method† | Lower limit (%) | Upper limit (%) | Confidence ratio | ||
|---|---|---|---|---|---|---|---|---|---|
| Value | 90% CI | Value | 90% CI | ||||||
| CD3+CD4+CD25+ | 5.46 | 2.61 | 0.75–11.17 | TP | 1.34 | 0.97–1.75 | 11.42 | 10.31–12.59 | 0.15 |
| CD3+CD4+CD39+ | 3.72 | 2.11 | 0.86–8.89 | TP | 0.82 | 0.63–1.04 | 9.20 | 7.99–10.56 | 0.18 |
| CD3+CD4+FoxP3+ | 3.81 | 2.08 | 0.16–9.29 | TP | 0.61 | 0.38–0.89 | 8.77 | 7.81–9.77 | 0.15 |
| CD3+CD4+Helios+ | 7.92 | 3.48 | 0.79–16.03 | P | 1.10 | 0.07–2.14 | 14.73 | 13.70–15.77 | 0.15 |
| CD3+CD4+CD25+FoxP3+ | 1.80 | 0.86 | 0.17–3.79 | TP | 0.43 | 0.31–0.56 | 3.80 | 3.43–4.19 | 0.15 |
| CD3+CD4+FoxP3+Helios+ | 2.67 | 1.52 | 0.14–6.51 | TP | 0.37 | 0.22–0.57 | 6.29 | 5.59–7.03 | 0.15 |




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download