Abstract
Walking can have a positive impact on cognitive function in adolescents. This study aimed to compare the effects of walking with sneakers and barefoot on cognitive ability in adolescents. Fifty-nine adolescent male students were included in the study and assigned to the control (n = 20), sneaker (n = 19), and barefoot (n = 20) groups. The barefoot and sneakers group performed a 40-min walking exercise four times a week for 12 weeks during the morning physical activity time, while the control group performed self-study. Electroencephalogram (EEG) and brain activity variables were measured before and after the exercise program. The results showed that after 12 weeks, the barefoot group had a significant decrease in Gamma and H-beta waves and a significant increase in sensorimotor rhythm (SMR) and Alpha waves. Conversely, the control group showed a significant decrease in SMR waves and increase in Theta waves. The sneaker group showed a significant decrease in SMR waves alone. In an eyes-open resting state, the barefoot group showed a significant increase in H-beta, M-beta, SMR, and Alpha waves. The barefoot group also had a significant increase in cognitive speed and concentration and a significant decrease in brain stress. Taken together, barefoot walking can effectively enhance cognitive ability in adolescents, as demonstrated by the significant variation in EEG activity. This research highlights the potential benefits of barefoot walking as a simple and effective form of exercise for enhancing cognitive function in adolescents.
Adolescence is a stage of neurodevelopmental period characterized by pronounced neuroplasticity and significant structural and functional changes in the brain, particularly in regions associated with advanced cognitive function [1,2]. While physical activity is essential for healthy development [3] during adolescence, the practice of walking has become less common in modern societies [4]. This is concerning as research has demonstrated that regular physical activity, including walking, can have significant physiological and psychological benefits for adolescents [5,6]. Therefore, it is crucial to understand the impact of walking on the developing adolescent brain and its potential role in promoting healthy brain development.
Recently, there has been growing interest in the potential benefits of barefoot walking for physical and cognitive health [7,8]. Barefoot walking has been widely recommended for teenagers and can effectively prevent disease and improve health by stimulating the feet without the side effects of shoes [9,10], and can be performed at all ages without complicated rules or high physical fitness; therefore, the possibility of discontinuing this activity is significantly lower than that in other forms of exercise and can be performed repeatedly over long periods [11]. Several studies have investigated the effects of barefoot walking on various aspects of health, including balance control, gait efficiency, and foot and ankle function [12,13]. For example, a study by Petersen et al. [14] found that barefoot walking can improve balance and postural control in healthy young adults. Another study by McNab et al. [15] showed that barefoot walking can increase gait efficiency and reduce the risk of falls in older adults. However, to the best of our knowledge, no previous studies have investigated the effects of barefoot walking on electroencephalogram (EEG) activity in adolescents. EEG is a noninvasive technique that can provide insights into the neural mechanisms underlying cognitive function, and has been used in previous studies to investigate the effects of various interventions on brain activity in both adults and children [16-20].
Therefore, the present study aims to investigate the potential effects of barefoot walking on EEG activity in adolescents. Our objective is to test the differences in EEG activity during walking with and without shoes, with a hypothesis that barefoot walking would potentially enhance cognitive function in adolescents.
The study included 62 volunteers who had no difficulty in performing physical activity but did not participate in regular exercise. During the study, participants who discontinued owing to personal circumstances were excluded, and the measurement results were analyzed for a total of 59 participants, 20 in the control, 19 in the sneaker, and 20 in the barefoot groups. Each participant provided written assent, and a parent or guardian provided written informed consent.
The research plan was approved by the Keimyung University Bioethics Committee (KMU IRB/40525-201909-HR-055-02), and the physical characteristics of the participants, who were aged in 13-years-old, are shown in Table 1.
During morning physical activity, participants engaged in a 40-min exercise session, comprising 5 min of warm-up exercises, followed by 30 min of continuous walking, and concluded with 5 min of cool-down exercises for 12 weeks at a frequency of four times per week. Meanwhile, the control group performed self-study for the same amount of time. EEG were measured before and after the 12 weeks for 40 min, the same duration as the morning exercise. The EEG measurements were employed to assess changes in brainwave patterns and understand their association with cognitive function. To minimize the influence of environmental factors during the experimental period, barefoot activities, and intentional physical activities other than the set exercise, were not performed.
Before entering this study, the participants were fully educated for one week on walking barefoot and in sneakers, and preliminary adaptation exercises in the playground, such as walking, jumping, and running lightly with bare feet, were conducted to remove any physical and psychological anxiety factors [21]. The playground area, totaling 200 square meters with a 60-meter straight path and a 40-meter curved path, was intentionally chosen to meet our exercise requirements without inducing undue stress on the participants. After completing the preliminary adaptation period, the 12-week exercise program was conducted at a frequency of four times a week from 8:10 am to 8:50 am for a total of 40 min. Exercise intensity was gradually increased in accordance with the principle of training; however, it was set slightly lower than the standard suggested by the American College of Sports Medicine in consideration of foot impact in the barefoot group participants and potential injury [9]. The change in heart rate was measured using a wireless heart rate monitor (RS800CX; Polar System) to maintain the appropriate walking exercise intensity. Exercise intensity was determined using Karvonen's formula. The individual target heart rate was set at 50% of the heart rate reserve for weeks 1–4, 50%–60% for weeks 5–8, and 60%–70% for weeks 9–12, and the rating of perceived exertion was maintained in the range of 10–14, which mean participants were felt it from light to hard.
EEG activity was recorded to examine the changes in the left and right hemispheres in the resting state (eyes open and closed) and during spatial perception and memory tasks. Alpha, Beta, Theta, and Gamma waves were measured. We used the EEG system QEEG-8 (LXE3208; LAXTHA Inc.) with eight electrode areas (Fp1, Fp2, F3, F4, T3, T4, O1, O2), one reference electrode (A2), and a ground electrode placed at the back of the neck. The sampling frequency was set at 256 Hz. The method used for the analysis of the recorded brain waves was based on the International Brain Education Association Brain Test, and signals were processed with the commercial software Complexity Ver. 2 (LAXTHA Inc.). The quantitative analysis indicators of the EEG were obtained by changing the t-score for statistical standardization. In addition, to examine spatial perception and memory during the presentation of eight questions and 24 items, the change in brain activity was analyzed using a brain test (LAXTHA Inc.) based on brain wave variation. As for the assessed variables, changes in cognitive intensity, cognitive speed, concentration, and brain stress were analyzed. During EEG recording of brain activity, body movement and noise generation can affect measurements; therefore, these factors were limited as much as possible while recording. In addition, drinking, visual stimuli, auditory stimuli, excited psychological states, and exercise affecting the heart rate can affect the recorded values; therefore, the mental and physical stability of the participant were maintained before recording to ensure accurate measurements [22]. The pre-exercise EEG was conducted at 8:10 AM on the day preceding the exercise treatment, while the post-exercise EEG took place at the same time, 8:10 AM, one day after the completion of the 12-week exercise treatment [23,24].
Statistical analysis was performed using the Prism 9 (v. 9.4.0) software for Mac. A three-way repeated measure analysis of variance (ANOVA) was conducted to determine the effects of sneaker and barefoot exercise training on EEG brain activity compared to the control. For simple two-way interactions and main effects, a Bonferroni adjustment was applied and statistical significance set at p < 0.05. After testing the homogeneity of the measurements (p > 0.05), all data were presented as the mean ± standard deviation and/or standard error. All data were normally distributed (p > 0.05) as assessed using Shapiro–Wilk’s normality test. Additionally, Levene’s test for equality of variances was used to assess variance homogeneity. Statistical significance was set at p < 0.05.
There was a statistically significant interaction between time and EEG scores in a stable with eyes-closed in the three groups (F(25, 672) = 6.63, p < 0.001). However, the effect of the 12-week program was significantly different between control (F(5, 228) = 32.66, p < 0.001), sneaker (F(5, 216) = 14.00, p < 0.001), and barefoot groups (F(5, 228) = 53.08, p < 0.001). In the control group, the sensorimotor rhythm (SMR) wave score was decreased after the 12-week period (mean ± standard deviation [Md] = 10.95, 95% confidence intervals [CI] diff from 2.71 to 19.19, p = 0.003), and the Theta score increased (Md = −9.15, 95% CI diff from −17.39 to 0.91, p = 0.02). The Gamma (Md = −13.75, 95% CI diff from −19.98 to −7.52, p < 0.001) and H-beta (Md = −9.70, 95% CI diff from −15.93 to −3.47, p < 0.001) scores were significantly decreased in the barefoot group, whereas the Alpha score was increased (Md = 13.65, 95% CI diff from 7.42 to 19.88, p < 0.001). Finally, the SMR score was significantly increased in both the barefoot (Md = 9.90, 95% CI diff from 3.67 to 16.13, p < 0.001) and sneakers group (Md = −13.05, 95% CI diff from −24.42 to −1.69, p = 0.02) (Fig. 1).
There was a significant interaction between time and EEG scores in the eyes-open resting state in the three groups (F(25, 672) = 1.88, p < 0.01). However, the effect of the 12-week program was significantly different in the barefoot (F(5, 228) = 5.84, p < 0.001) and sneakers group (F(5, 216) = 3.21, p = 0.01), but not in the control (F(5, 228) = 1.72, p = 0.13). In the barefoot group, H-beta (Md = 9.20, 95% CI diff from 2.17 to 16.23, p = 0.004), M-beta (Md = 13.00, 95% CI diff from 5.97 to 20.03, p < 0.001), SMR (Md = 13.05, 95% CI diff from 6.02 to 20.08, p < 0.001), and Alpha (Md = 11.95, 95% CI diff from 4.92 to 18.98, p < 0.001) scores were significantly increased. In the sneaker group, however, there were no significant differences in the EEG scores (Fig. 2).
There was a significant interaction between brain activity and time in the three groups (F(25, 672) = 6.85, p < 0.001). The effect the 12-week program was significantly different between the control (F(5, 228) = 11.48, p < 0.001), sneaker (F(5, 216) = 13.29, p < 0.001), and barefoot groups (F(5, 228) = 10.36, p < 0.001). In the control group, the brain stress score (Md = 8.30, 95% CI diff from 2.96 to 13.64, p < 0.001) was increased and the concentration score (Md = −7.55, 95% CI diff from −12.89 to −2.21, p < 0.01) decreased. In the barefoot group, cognitive speed (Md = 17.10, 95% CI diff from 12.03 to 22.17, p < 0.001) and concentration (Md = 8.80, 95% CI diff from 3.73 to 13.87, p < 0.001) scores were increased, whereas the brain stress score was decreased (Md = −11.60, 95% CI diff from −16.67 to −6.53, p < 0.001). However, mental performance in the sneaker group did not vary significantly (Fig. 5).
In this study, we aimed to investigate the effects of barefoot walking on cognitive ability in adolescents. Our findings showed that barefoot walking led to significant changes in EEG scores, including a decrease in Gamma and H-beta waves and an increase in SMR and Alpha waves, during both eyes-closed and eyes-open resting states. Additionally, the barefoot group showed significant improvements in cognitive concentration and speed and a decrease in brain stress after the 12-week exercise period. These results suggest that barefoot walking can effectively enhance cognitive ability in adolescents.
The EEG scores of the barefoot group during the eyes-closed resting state showed a significant decrease in Gamma and H-beta waves and a significant increase in SMR and Alpha waves after the 12-week training period. This observation aligns with the intriguing link between serotonin, which is a pivotal neurotransmitter, and brainwave frequencies such as gamma and H-beta waves, an area of significant interest in neuroscience. The presence of gamma waves in EEG signals has been widely associated with higher-order cognitive functions, such as memory encoding, attentional control, and perceptual processing [25-28]. The observed increase in gamma wave activity following barefoot walking suggests a potential enhancement in cognitive performance and information processing. These findings support the notion that engaging in barefoot walking could have cognitive benefits beyond mere locomotion. Additionally, the detection of H-beta waves in EEG recordings is indicative of attentional processes and sensory integration [29]. The observed changes in H-beta wave activity following barefoot walking suggest an augmentation in attentional focus and sensory processing abilities. This may be attributed to the multisensory stimulation experienced during barefoot walking, which engages various sensory receptors in the feet and promotes heightened sensory awareness. Furthermore, the stimulation of the toes during barefoot walking [30] can dilate brain capillaries and increase brain blood flow, thus improving oxygen supply to the brain and promoting brain activation [31,32]. These mechanisms may explain the decrease in Gamma and H-beta waves and increase in SMR and Alpha waves observed in our study.
In the eyes-open resting state, the barefoot group showed significant increases in H-beta, M-beta, SMR, and Alpha waves after the 12-week training period. These results indicate that barefoot walking can effectively activate cognitive ability, especially in regions associated with information processing and complex reasoning. The stimulation of nerve cells in the sensory cortex, thalamus, and cerebellum through the activation of the toes during barefoot walking may play a key role in this process [33]. Additionally, the increase in cognitive concentration and speed and decrease in brain stress observed in the barefoot group after the 12-week training period suggest that barefoot walking can improve cognitive function, even under conditions that require brain utilization.
The balanced activity of the left and right hemispheres of the brain is important for overall cognitive function and brain health [31]. Our results showed no significant differences in EEG scores between the left and right hemispheres of the brain during spatial perception and memory tests, indicating a balance in brain activity. The increase in H-beta, SMR, and Alpha waves observed in both hemispheres of the barefoot group is particularly noteworthy, as this pattern is associated with increased attention, sensory sensitivity, and cognitive ability [34]. Exercise-induced SMR activation promotes motor learning by reinforcing neural pathways involved in movement control [35]. These results stimulate brain regions responsible for cognitive function, including attention, memory, and executive function. Regular exercise has been linked to improved cognitive performance and may help reduce the risk of cognitive decline and neurodegenerative diseases. Furthermore, these findings suggest that barefoot walking can effectively activate the whole brain, leading to improvements in various cognitive functions.
Aerobic exercise has been shown to improve cognitive function and brain health in numerous studies [36-38]. Our findings suggest that barefoot walking can be a particularly effective form of aerobic exercise for improving cognitive function and brain health in adolescents. Barefoot walking can activate the enteric nervous system, increase brain blood flow, and stimulate the toes [32] to activate nerve cells in the sensory cortex, thalamus, and cerebellum, leading to improved overall cognitive ability [39]. Furthermore, barefoot walking stimulates the toes more than walking in sneakers, thereby enhancing lower skeletal muscle strength [32,40]. These mechanisms can facilitate increased blood flow to the brain [41]. Furthermore, the reduction in mental fatigue and brain stress observed in the barefoot group suggests that this form of exercise may be particularly useful for individuals who experience high levels of stress or mental fatigue.
There are limitations to note with this study. Firstly, the study was conducted on a relatively small sample size. Secondly, the study was conducted on a specific age group, and it is unclear whether the results can be extended to other age groups. Third, this study did not measure serotonin levels. Finally, the study was conducted over a short period, and therefore, the long-term effects of the intervention could not be assessed. Further research with larger sample sizes and a longer intervention period is necessary to confirm and extend our findings.
Our study suggests that barefoot walking can effectively enhance cognitive ability in adolescents. These findings may be attributed to the activation of the enteric nervous system, increased brain blood flow, and stimulation of nerve cells in the sensory cortex, thalamus, and cerebellum during barefoot walking. Nevertheless, further research is necessary to validate these results and to explore the potential underlying mechanisms responsible for the improvement of cognitive ability in adolescents during barefoot walking (Fig. 6).
Notes
FUNDING
This work was supported by the Ministry of Education of the Republic of Korea and the National Research Foundation of Korea (NRF-2021R1I1A1A01047419, RS-2023-00221673) and by Korean Basic Science Institute (National research Facilities and Equipment Center) grant funded by the Ministry of Education (2023RIA6C1018008).
REFERENCES
1. Black M, Adjei NK. 2022; Longitudinal analysis of cognitive development across childhood and adolescence: evidence from the UK Millennium Cohort Study. Lancet. 400 Suppl 1:S23. DOI: 10.1016/S0140-6736(22)02233-4.
2. Sijtsma H, Lee NC, van Kesteren MTR, Braams BR, van Atteveldt NM, Krabbendam L, van Buuren M. 2023; The effect of incorrect prior information on trust behavior in adolescents. Neuropsychologia. 179:108423. DOI: 10.1016/j.neuropsychologia.2022.108423. PMID: 36574534.
3. Lee SJ, Kim TW, Park TH, Lee IH, Jang EC, Kwon SC, Lee HJ, Choi JH, Lee JB. 2022; Thermotherapy as an alternative to exercise for metabolic health in obese postmenopausal women: focus on circulating irisin level. Korean J Physiol Pharmacol. 26:501–509. DOI: 10.4196/kjpp.2022.26.6.501. PMID: 36302624. PMCID: PMC9614401.
4. Smallcombe JW, Biddle GJH, Slater T, Thackray AE, Dunstan DW, Barrett LA, Tolfrey K. 2022; Breaking sitting time with physical activity increases energy expenditure but does not alter postprandial metabolism in girls. Med Sci Sports Exerc. 54:1850–1860. DOI: 10.1249/MSS.0000000000002979. PMID: 35714076.
5. Hawkins JL, Williams GN, Milner CE. 2023; Changes in walking biomechanics after a 30-min exercise bout in sedentary compared with active young women. Med Sci Sports Exerc. 55:722–726. DOI: 10.1249/MSS.0000000000003083. PMID: 36374524.
6. Bennett HJ, Ringleb SI, Bobzien J, Haegele JA. 2022; Are gait biomechanics related to physical activity engagement? An examination of adolescents with autism spectrum disorder. Med Sci Sports Exerc. 54:447–455. DOI: 10.1249/MSS.0000000000002810. PMID: 34628448.
7. Hollander K, Heidt C, VAN DER Zwaard BC, Braumann KM, Zech A. 2017; Long-term effects of habitual barefoot running and walking: a systematic review. Med Sci Sports Exerc. 49:752–762. DOI: 10.1249/MSS.0000000000001141. PMID: 27801744.
8. Ren X, Kebbach M, Bruhn S, Yang Q, Lin H, Bader R, Tischer T, Lutter C. 2022; Barefoot walking is more stable in the gait of balance recovery in older adults. BMC Geriatr. 22:904. DOI: 10.1186/s12877-022-03628-w. PMID: 36434546. PMCID: PMC9700923.
9. Robbins SE, Hanna AM. 1987; Running-related injury prevention through barefoot adaptations. Med Sci Sports Exerc. 19:148–156. DOI: 10.1249/00005768-198704000-00014.
10. Wearing SC, Hooper SL, Dubois P, Smeathers JE, Dietze A. 2014; Force-deformation properties of the human heel pad during barefoot walking. Med Sci Sports Exerc. 46:1588–1594. DOI: 10.1249/MSS.0000000000000281. PMID: 24504425.
11. Hollander K, Petersen E, Zech A, Hamacher D. 2022; Effects of barefoot vs. shod walking during indoor and outdoor conditions in younger and older adults. Gait Posture. 95:284–291. DOI: 10.1016/j.gaitpost.2021.04.024. PMID: 34020852.
12. Skaaret I, Steen H, Niratisairak S, Swanson D, Holm I. 2021; Postoperative changes in vertical ground reaction forces, walking barefoot and with ankle-foot orthoses in children with Cerebral Palsy. Clin Biomech (Bristol, Avon). 84:105336. DOI: 10.1016/j.clinbiomech.2021.105336. PMID: 33848706.
13. Abdelraouf OR, Abdel-Aziem AA. 2021; Ankle and foot mechanics in individuals with chronic ankle instability during shod walking and barefoot walking: a cross-sectional study. Chin J Traumatol. 24:174–179. DOI: 10.1016/j.cjtee.2021.02.010. PMID: 33757697. PMCID: PMC8173573.
14. Petersen E, Zech A, Hamacher D. 2020; Walking barefoot vs. with minimalist footwear - influence on gait in younger and older adults. BMC Geriatr. 20:88. DOI: 10.1186/s12877-020-1486-3. PMID: 32131748. PMCID: PMC7057536.
15. McNab B, Sadler S, Lanting S, Chuter V. 2022; The relationship between foot and ankle joint flexibility measures and barefoot plantar pressures in healthy older adults: a cross-sectional study. BMC Musculoskelet Disord. 23:729. DOI: 10.1186/s12891-022-05618-w. PMID: 35906599. PMCID: PMC9338503.
16. Liebherr M, Corcoran AW, Alday PM, Coussens S, Bellan V, Howlett CA, Immink MA, Kohler M, Schlesewsky M, Bornkessel-Schlesewsky I. 2021; EEG and behavioral correlates of attentional processing while walking and navigating naturalistic environments. Sci Rep. 11:22325. DOI: 10.1038/s41598-021-01772-8. PMID: 34785702. PMCID: PMC8595363.
17. Ogrim G, Kropotov J, Hestad K. 2012; The quantitative EEG theta/beta ratio in attention deficit/hyperactivity disorder and normal controls: sensitivity, specificity, and behavioral correlates. Psychiatry Res. 198:482–488. DOI: 10.1016/j.psychres.2011.12.041. PMID: 22425468.
18. Villwock A, Bottari D, Röder B. 2022; Event-related potential correlates of visuo-tactile motion processing in congenitally deaf humans. Neuropsychologia. 170:108209. DOI: 10.1016/j.neuropsychologia.2022.108209. PMID: 35283159.
19. Heim S, Keil A, Choudhury N, Thomas Friedman J, Benasich AA. 2013; Early gamma oscillations during rapid auditory processing in children with a language-learning impairment: changes in neural mass activity after training. Neuropsychologia. 51:990–1001. DOI: 10.1016/j.neuropsychologia.2013.01.011. PMID: 23352997. PMCID: PMC3633611.
20. Jang HJ, Cho KO. 2019; Dual deep neural network-based classifiers to detect experimental seizures. Korean J Physiol Pharmacol. 23:131–139. DOI: 10.4196/kjpp.2019.23.2.131. PMID: 30820157. PMCID: PMC6384195.
21. Kim TH, Kim KJ. 2020; Kinetic effects analysis on barefoot jumping and regular jumping rope of elementary school students. J Korean Soc Study Phys Edu. 24:205–217. DOI: 10.15831/JKSSPE.2020.24.4.205.
22. Kim SH, Han DH, Lee YS, Kim BN, Cheong JH, Han SH. 2014; Baduk (the Game of Go) improved cognitive function and brain activity in children with attention deficit hyperactivity disorder. Psychiatry Investig. 11:143–151. DOI: 10.4306/pi.2014.11.2.143. PMID: 24843369. PMCID: PMC4023088.
23. Cusi K, Maezono K, Osman A, Pendergrass M, Patti ME, Pratipanawatr T, DeFronzo RA, Kahn CR, Mandarino LJ. 2000; Insulin resistance differentially affects the PI 3-kinase- and MAP kinase-mediated signaling in human muscle. J Clin Invest. 105:311–320. DOI: 10.1172/JCI7535. PMID: 10675357. PMCID: PMC377440.
24. Rosenfeldt AB, Koop MM, Fernandez HH, Alberts JL. 2021; High intensity aerobic exercise improves information processing and motor performance in individuals with Parkinson's disease. Exp Brain Res. 239:777–786. DOI: 10.1007/s00221-020-06009-0. PMID: 33394100.
25. Siddiqui A, Agrawal S, Sekhri N. 2023; Fentanyl-induced serotonin syndrome 5 days after cessation of serotonergic agents: a case report. A A Pract. 17:e01720. DOI: 10.1213/XAA.0000000000001720. PMID: 37934660.
26. Schwartzmann B, Dhami P, Uher R, Lam RW, Frey BN, Milev R, Müller DJ, Blier P, Soares CN, Parikh SV, Turecki G, Foster JA, Rotzinger S, Kennedy SH, Farzan F. 2023; Developing an electroencephalography-based model for predicting response to antidepressant medication. JAMA Netw Open. 6:e2336094. DOI: 10.1001/jamanetworkopen.2023.36094. PMID: 37768659. PMCID: PMC10539986.
27. Reese M, Christensen S, Anolick H, Roberts KC, Wong MK, Wright MC, Acker L, Browndyke JN, Woldorff MG, Berger M. MADCO-PC and INTUIT Investigators. 2023; EEG pre-burst suppression: characterization and inverse association with preoperative cognitive function in older adults. Front Aging Neurosci. 15:1229081. DOI: 10.3389/fnagi.2023.1229081. PMID: 37711992. PMCID: PMC10499509.
28. Li G, Lu C, Li S, Kang L, Li Q, Bai M, Xiong P. 2023; Correlation study of brain-derived neurotrophic factor, EEG γ activity and cognitive function in first-episode schizophrenia. Brain Res. 1820:148561. DOI: 10.1016/j.brainres.2023.148561. PMID: 37657750.
29. Liu X, Sun L, Zhang D, Wang S, Hu S, Fang B, Yan G, Sui G, Huang Q, Wang S. 2022; Phase-amplitude coupling brain networks in children with attention-deficit/hyperactivity disorder. Clin EEG Neurosci. 53:399–405. DOI: 10.1177/15500594221086195. PMID: 35257602.
30. Lieberman DE. 2012; What we can learn about running from barefoot running: an evolutionary medical perspective. Exerc Sport Sci Rev. 40:63–72. Erratum. DOI: 10.1097/JES.0b013e31824ab210. PMID: 22257937.
31. Lee OK, Kim EJ. 2009; The effect of foot reflex-massage on EEG variation & blood velocity. Korean J Aesthet Cosmetol. 7:129–142.
32. Beyaert C, Pierret J, Vasa R, Paysant J, Caudron S. 2020; Toe walking in children with cerebral palsy: a possible functional role for the plantar flexors. J Neurophysiol. 124:1257–1269. DOI: 10.1152/jn.00717.2019. PMID: 32877265.
33. Goswami U. 2004; Neuroscience and education. Br J Educ Psychol. 74:1–14. DOI: 10.1348/000709904322848798. PMID: 15096296.
34. Xu T, Wang J, Zhang G, Zhang L, Zhou Y. 2023; Confused or not: decoding brain activity and recognizing confusion in reasoning learning using EEG. J Neural Eng. 20:026018. DOI: 10.1088/1741-2552/acbfe0. PMID: 36854180.
35. Zhang X, Jing F, Liu Y, Tang J, Hua X, Zhu J, Tuo H, Lin Q, Gao P, Liu W. 2023; Effects of non-invasive brain stimulation on walking and balance ability in Parkinson's patients: a systematic review and meta-analysis. Front Aging Neurosci. 14:1065126. DOI: 10.3389/fnagi.2022.1065126. PMID: 36704502. PMCID: PMC9871558.
36. VAN Riper SM, Tempest GD, Piccirilli A, Ma Q, Reiss AL. 2023; Aerobic exercise, cognitive performance, and brain activity in adolescents with attention-deficit/hyperactivity disorder. Med Sci Sports Exerc. 55:1445–1455. DOI: 10.1249/MSS.0000000000003159. PMID: 36897828.
37. Zhao N, Yao X, Wang Y, Chen X, Wang Z. 2023; Aerobic exercise combined with memory strategy training improve the cognitive function. Brain Behav. 13:e3234. DOI: 10.1002/brb3.3234. PMID: 37612254. PMCID: PMC10636400.
38. Barzegari A, Amouzad Mahdirejei H, Hanani M, Esmaeili MH, Salari AA. 2023; Adolescent swimming exercise following maternal valproic acid treatment improves cognition and reduces stress-related symptoms in offspring mice: role of sex and brain cytokines. Physiol Behav. 269:114264. DOI: 10.1016/j.physbeh.2023.114264. PMID: 37295664.
39. Hamacher D, Hamacher D, Müller R, Schega L, Zech A. 2019; The effect of a cognitive dual task on the control of minimum toe clearance while walking. Motor Control. 23:344–353. DOI: 10.1123/mc.2018-0006. PMID: 30599803.
40. de Oliveira VGC, Arrebola LS, de Oliveira PR, de Sá CDS, Yi LC. 2019; Effect of plantar flexor muscle strengthening on the gait of children with idiopathic toe walking: a study protocol. Pediatr Phys Ther. 31:373–378. DOI: 10.1097/PEP.0000000000000650. PMID: 31568387.
41. Schlough K, Andre K, Owen M, Adelstein L, Hartford MC, Javier B, Kern R. 2020; Differentiating between idiopathic toe walking and cerebral palsy: a systematic review. Pediatr Phys Ther. 32:2–10. DOI: 10.1097/PEP.0000000000000659. PMID: 31842091.
Fig. 1
EEG scores in the eyes-closed resting state.
The three-way repeated measure ANOVA revealed a statistically significant interaction between time and the EEG score in the eyes-closed resting state in the control (n = 20), barefoot (n = 20), and sneakers group (n = 19) (F(25, 672) = 6.63, p < 0.001). The control group presents significantly decreased SMR and increased Theta scores after the 12 weeks of self-study. Post-training, the barefoot group shows significantly decreased Gamma and H-beta scores while those of SMR and Alpha are increased. Conversely, the sneaker group presents a significant decrease in the SMR score alone. EEG, electroencephalogram; SMR, sensorimotor rhythm. *p < 0.05, **p < 0.01, ***p < 0.001 vs. before the program.
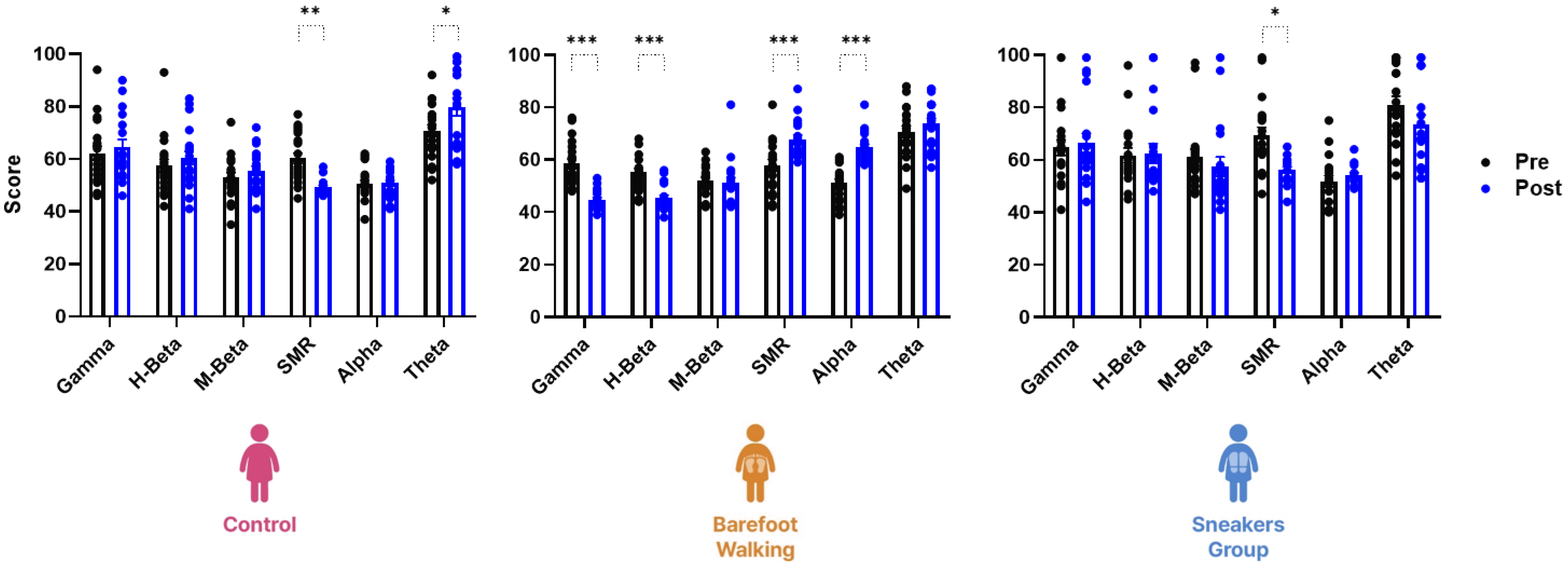
Fig. 2
EEG scores in the eyes-open resting state.
The three-way repeated measure ANOVA revealed a significantly different interaction between EEG score and time for control, barefoot group, and sneakers group (F(25, 672) = 1.88, p < 0.01). After the 12-week program, only the barefoot group presents a significant increase in H-beta (Md = 9.20, 95% CI diff 2.17–16.23, p = 0.004), M-beta (Md = 13.00, 95% CI diff 5.97–20.03, p < 0.001), SMR (Md = 13.05, 95% CI diff 6.02–20.08, p < 0.001), and Alpha (Md = 11.95, 95% CI diff 4.92–18.98, p < 0.001) scores. EEG, electroencephalogram; SMR, sensorimotor rhythm; Md, mean ± standard deviation; CI, confidence intervals. **p < 0.01, ***p < 0.001 vs. before the program.
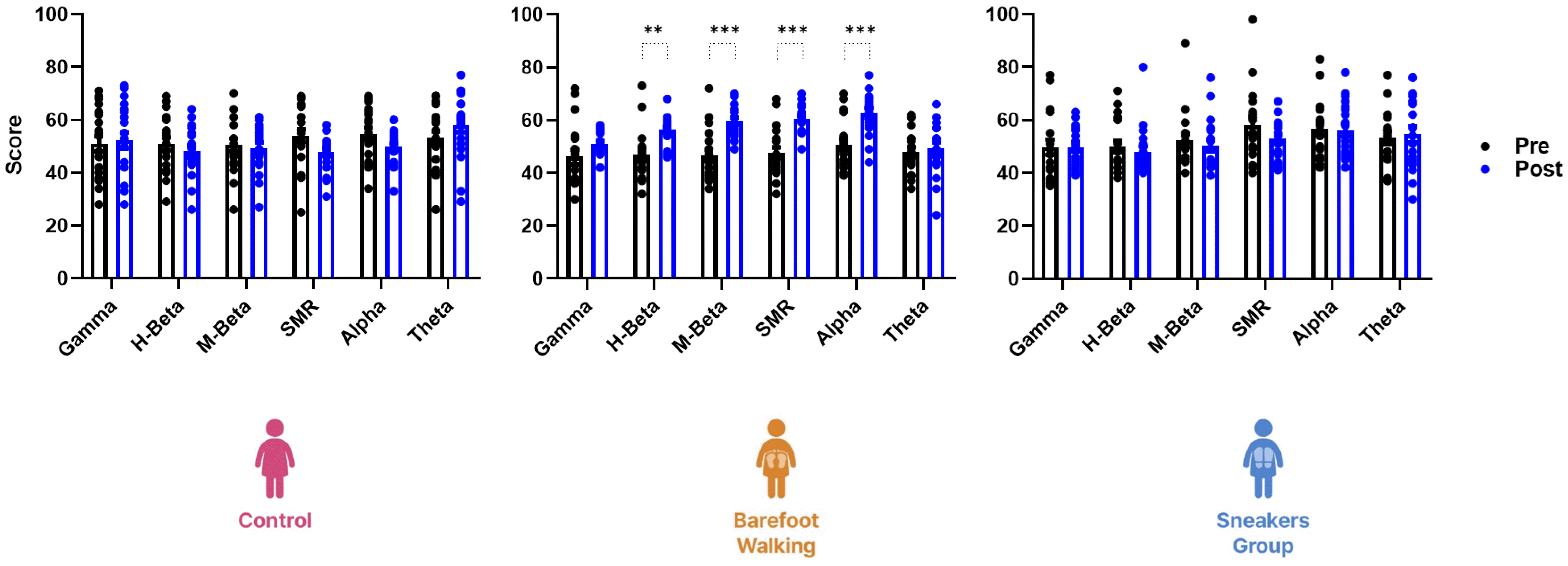
Fig. 3
Left hemisphere EEG scores during spatial perception and memory tests.
The three-way repeated measure ANOVA revealed no significant difference in the EEG scores of the left hemisphere during spatial perception and memory tests before and after the program (F(25, 672) = 1.41, p = 0.09). EEG, electroencephalogram; SMR, sensorimotor rhythm.
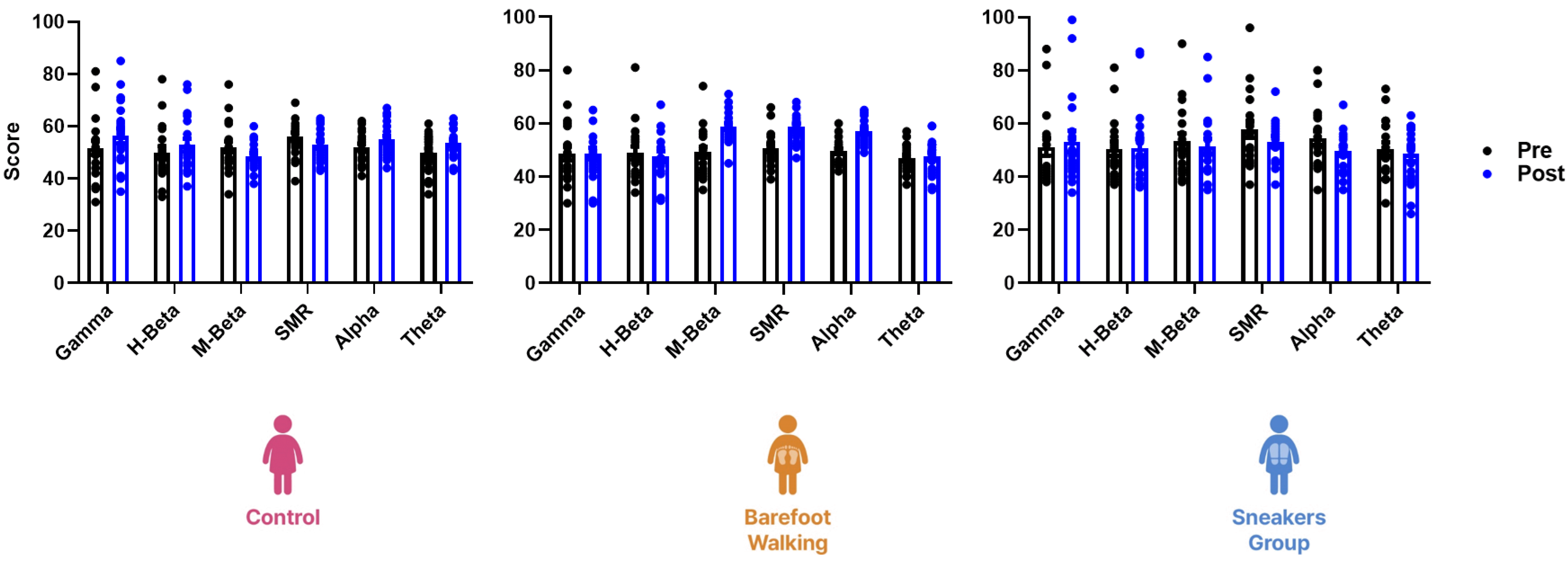
Fig. 4
Right hemisphere EEG scores during spatial perception and memory tests.
The three-way repeated measure ANOVA revealed no significant difference in the EEG scores of the right hemisphere during spatial perception and memory tests before and after the program (F(25, 672) = 0.66, p = 0.90). EEG, electroencephalogram; SMR, sensorimotor rhythm.
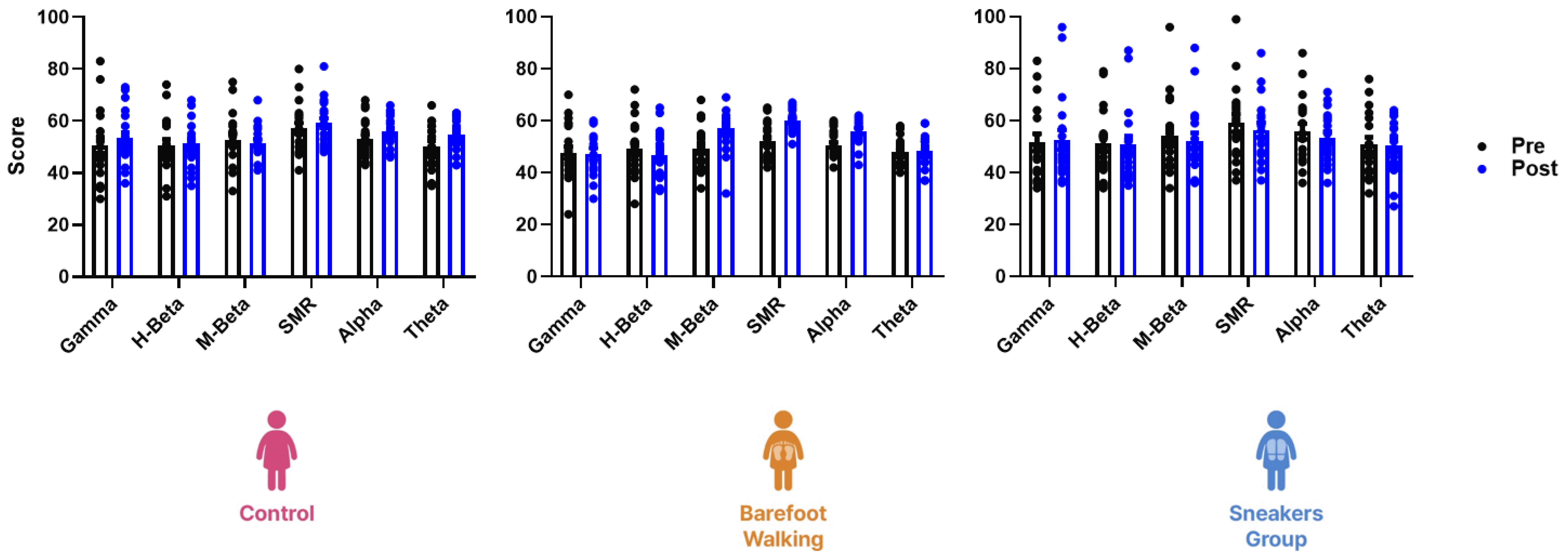
Fig. 5
Brain activity during spatial perception and memory tests.
The three-way repeated measure ANOVA revealed a significant interaction between mental performance and time in the three groups (F(25, 672) = 6.85, p < 0.001). In the control group, the brain stress score is increased, and the concentration score decreased. In the barefoot group, the cognitive speed and concentration scores are increased, and the brain stress score decreased. However, brain engagement in the sneaker group does not vary significantly. **p < 0.01, ***p < 0.001 vs. before the program.
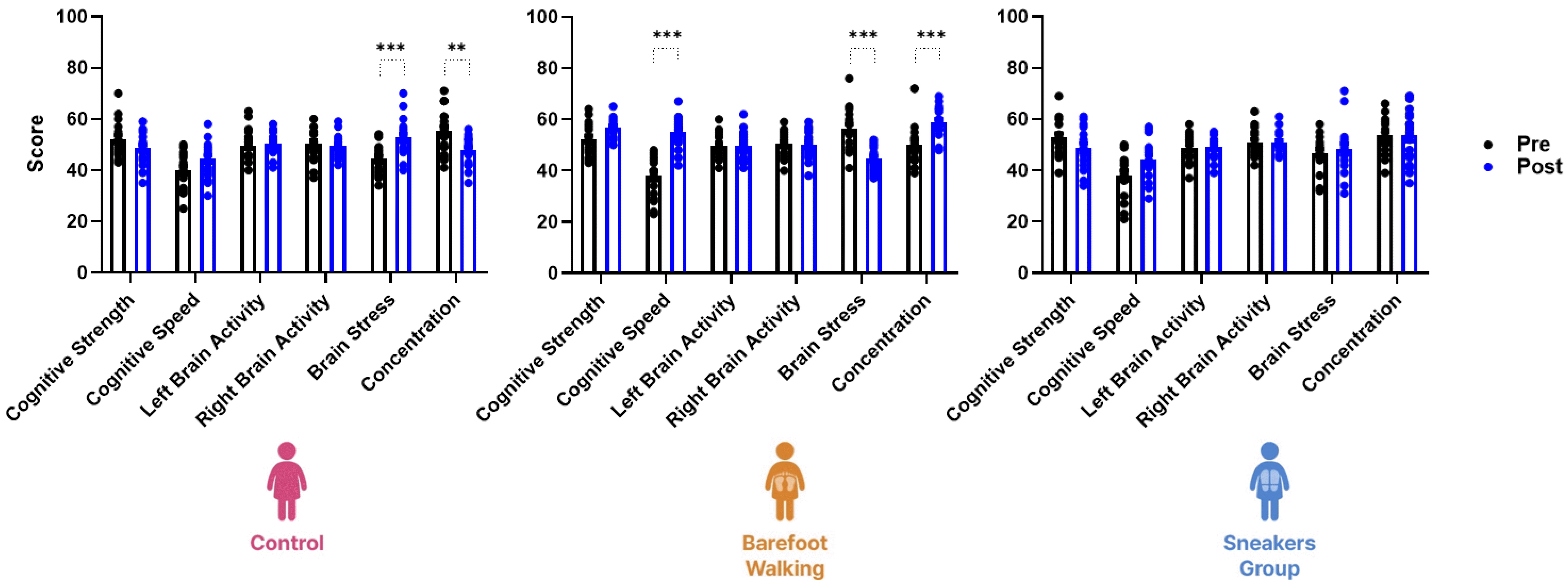
Fig. 6
The study involved 59 adolescent male students who were divided into control (n = 20), sneaker (n = 19), and barefoot (n = 20) groups.
The barefoot and sneaker groups performed a 40-min walking exercise four times a week for 12 weeks during morning physical activity time, while the control group performed self-study. Electroencephalogram (EEG) and brain activity variables were measured before and after the exercise program. After 12 weeks, the barefoot group showed a significant decrease in gamma and H-beta waves and a significant increase in sensorimotor rhythm (SMR) and alpha waves. The control group showed a significant decrease in SMR waves and an increase in theta waves. The sneaker group showed a significant decrease in only SMR waves. In the resting state with eyes open, the barefoot group showed a significant increase in H-beta, M-beta, SMR, and alpha waves. In addition, the barefoot group showed a significant increase in cognitive speed and concentration, and a significant decrease in brain stress. Taken together, barefoot walking can effectively improve cognitive performance in adolescents, as evidenced by significant changes in EEG activity.
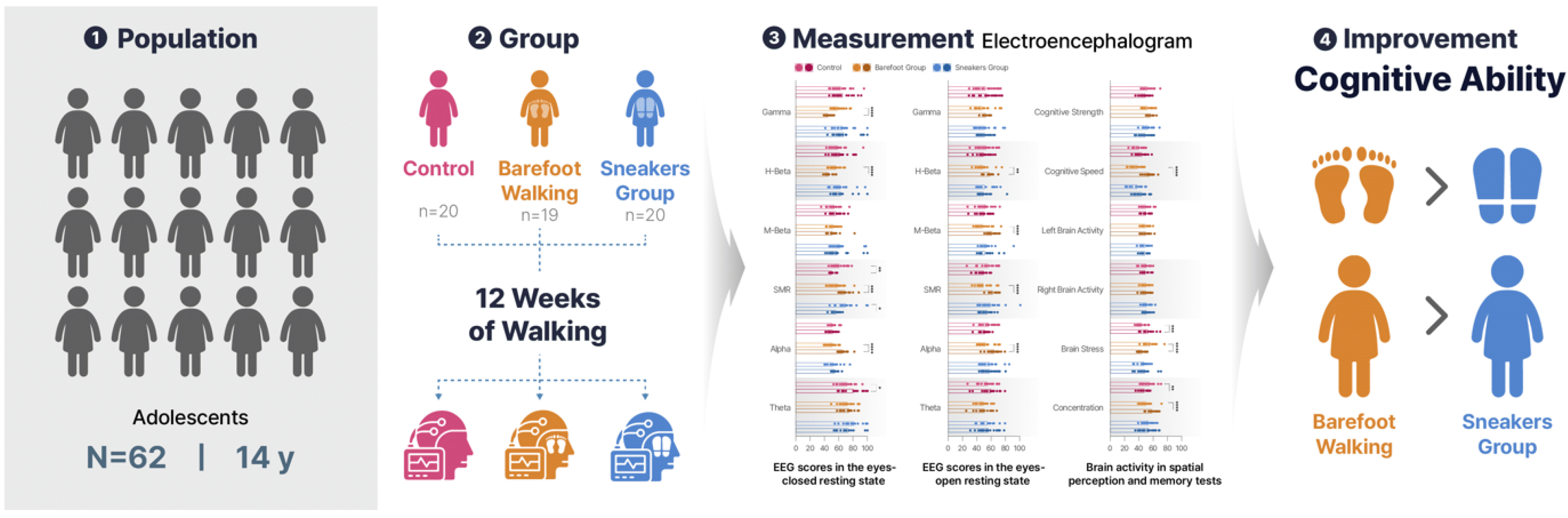
Table 1
Physical characteristics of the subjects




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download