Abstract
Background
To address the public’s fear of coronavirus disease 2019 (COVID-19), understanding the clinical features of the disease is essential. However, research on the clinical features of COVID-19, including illness duration and post-acute COVID-19, in Korean pediatric patients has been limited. Therefore, this study investigated the clinical features of COVID-19 based on the medical records of pediatric patients with a history of COVID-19 who visited a single center.
Methods
In total, 311 patients were included in this study. The presence and duration of 19 symptoms were examined. Additionally, clinical features were investigated by dividing the patients into different age ranges. Patients aged 6 and above were further categorized according to the presence of asthma, while adolescent patients were divided into vaccinated and unvaccinated groups.
Results
Fever and cough were the most common symptoms. The mean illness duration was 2–4 days. Only 3.5% of the patients were asymptomatic. Post-acute COVID-19 was observed in 13.2% of the patients. The incidence of most symptoms tended to increase with age. Post-acute COVID-19 was observed more frequently in patients with asthma than in those without asthma. Vaccinated patients experienced less fever, vomiting, and fatigue than unvaccinated patients.
Coronavirus disease 2019 (COVID-19) is an infectious disease caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). The World Health Organization declared COVID-19 pandemic on March 11, 2020 [1]. In Korea, the number of daily confirmed cases reached a maximum of 621,035 in mid-March 2022 and gradually decreased afterwards; however, it saw a resurgence beginning in the summer of 2023, coinciding with the relaxation of the mask mandate. According to the Korea Disease Control and Prevention Agency (KDCA), as of August 31, 2023, the cumulative number of confirmed cases stood at 34,571,873. Among them, 3,270,242 involved children aged 0–9 years, and 4,246,913 involved children aged 10–19 years, accounting for 19% of the total cumulative cases [2].
Pediatric patients with COVID-19 experience a milder course of the disease than that in adults [3,4]. However, anxiety surrounding COVID-19 has become widespread due to disruptions in daily routines. A study on anxiety among Korean adolescents during the COVID-19 pandemic found that the fear of infection was one of the factors related to anxiety [5]. One of the contributing factors to this fear of COVID-19 is ignorance about the disease. To overcome this fear, the public and physicians must possess sufficient knowledge about COVID-19 [6].
Hence, this study aimed to provide a trigger point for research on the general course of COVID-19 in children and adolescents in Korea by investigating the clinical features of patients with a history of COVID-19 who visited a single healthcare center.
Ethical statements: This study was approved by the Institutional Review Board (IRB) of the Daegu Catholic University Medical Center (IRB No: CR-22-135). The requirement for patient consent was waived because this study was a retrospective chart review study in which there are minimal risks to subjects.
Among patients who visited the outpatient clinic of the Department of Pediatrics at Daegu Catholic University Medical Center from March 1 to May 31, 2022, we screened 317 patients under the age of 20 who reported having previously been diagnosed with COVID-19 using SARS-CoV-2 rapid antigen kit for professional use or polymerase chain reaction. We excluded four patients within 7 days of the COVID-19 diagnosis because the follow-up period was too short and could provide inaccurate information about the presence or duration of symptoms. Two patients suspected of having other superimposed infections within 1 week before and after the COVID-19 diagnosis were also excluded. Finally, 311 medical records were analyzed retrospectively. The following demographic data and clinical symptoms were obtained from the electronic medical records: sex, age, history of vaccination against COVID-19, fever, cough, runny or stuffy nose, dyspnea, chest discomfort, chest pain, myalgia, headache, dizziness, sore eyes, sore throat, nausea, vomiting, abdominal pain, diarrhea, loss of smell, loss of taste, fatigue, COVID-19-associated hospitalization, and underlying diseases. Loss of smell and taste included both partial and complete loss. Clinical symptoms that rely on verbal expression capability were evaluated for individuals aged 3 years and older. Underlying diseases were evaluated based on medical records; however, only patients who reported being diagnosed with asthma and whose bronchial hyperresponsiveness was confirmed through a methacholine provocation test were classified into the asthma group. Bronchial hyperresponsiveness was defined as a provocative concentration of methacholine causing a 20% fall in forced expiratory volume in 1 second (PC20) ≤16 mg/mL [7]. Patients with no history of asthma in their medical records were classified into the non-asthma group. Post-acute COVID-19 was defined as the persistence of one or more clinical symptoms for over 4 weeks [8].
Patients were divided into infant and toddler (aged 0–2), preschooler (aged 3–5), middle childhood (aged 6–11), and adolescent (aged 12 years and older) groups to compare the symptoms of COVID-19 by age. In addition, children aged 6 years and older were divided into an asthma group and a non-asthma group, while adolescents were divided into a vaccinated group that had completed two primary doses and an unvaccinated group that had not. The clinical features of the two groups were compared.
The chi-square test was used to compare categorical variables. The Fisher exact test was performed if more than 20% of the cells had an expected frequency of less than five. When comparing continuous data from the two groups, the independent samples t-test was performed if the distribution of the variable was normal, and the Mann-Whitney U test was used if the distribution was not normal. The one-way analysis of variance was performed to compare normally distributed continuous variables between three or more groups, and the Kruskal-Wallis test was used to compare non-normally distributed continuous data. Post-hoc analysis was performed to compare three or more groups. Variables with fewer than 20 available data points in any group were not analyzed to achieve alpha levels of 0.05 and 80% power when comparing the groups. The Cochran-Armitage trend test was used to analyze trends between age categories.
When comparing the asthma and non-asthma groups, age and sex were adjusted for using 1:1 propensity score matching.
When comparing the vaccinated and unvaccinated groups, propensity score matching could not be performed because the number of unvaccinated participants was smaller than the number of vaccinated participants. Therefore, to adjust for age and sex in the clinical features that showed differences in the univariate analysis, age, sex, and vaccination history were added as covariates, and multivariate regression analysis was performed.
Statistical analyses were performed using IBM SPSS Statistics version 25.0 software (IBM Co.) and R version 4.2.0 software package (R Project for Statistical Computing). A p-value less than 0.05 was considered statistically significant. When post-hoc analysis was performed, the Bonferroni correction was applied to adjust for type I errors. A p-value <0.008 in the comparison of the four groups and a p-value <0.017 in the comparison of the three groups were considered statistically significant.
Table 1 presents the demographic characteristics of the 311 patients included in this study. The median age was 8 years. Considering age categories, those aged 6–11 was the majority (48.9%), followed by those aged 12 and older, those aged 3–5 years, and those aged 0–2 years. One hundred and sixty-seven participants (52.7%) were male. The median duration from COVID-19 diagnosis to the clinic visit was 30 days (interquartile range [IQR], 20–44 days).
According to reports from patients or caregivers, the most common sources of infection were educational institutions or childcare centers (43.4%) and family or relatives (37.3%). Six infections occurred in public places: two in restaurants, three in hospitals, and one in a swimming pool. Concurrent COVID-19 illness in families or cohabitators occurred in 90% of patients.
South Korea has approved the use of SARS-CoV-2 vaccine for children aged 12 years and older since Oct 2021 [9]. Of 73 patients eligible for vaccination, 42 patients (58%) had been vaccinated. Most of those vaccinated patients had completed two primary vaccinations, and three had received booster doses.
The most frequently reported underlying disease was allergic rhinitis (39.2%), followed by precocious puberty (14.8%). All children with immunodeficiency exhibited selective IgG3 deficiency. Genetic comorbidities were Marfan syndrome in one patient, Prader–Willi syndrome in one patient, Wolf–Hirschhorn syndrome in one patient, Lamb–Shaffer syndrome in one patient, and 3p deletion syndrome in one patient. Thirty-eight patients had no underlying disease.
The most common clinical symptom was fever, observed in 263 patients (84.6%), followed by cough (76.2%) and sore throat (56.7%). Rhinorrhea and nasal stuffiness occurred in 44.7% and 46.3% of the patients, respectively. Symptoms lasted for 2–4 days and improved within 7 days. Eleven patients (3.5%) remained asymptomatic. Forty-one patients (13.2%) developed post-acute COVID-19 (Table 2). Hospitalization occurred in four cases, with stays lasting 7 days in two cases, 6 days in one case, and 4 days in one case. Among the 311 patients, 18 were diagnosed with overlapping clinical syndromes. Overlapping syndrome included croup and bronchitis in five patients each, asthma exacerbation in four patients, acute pharyngitis in one patient, and acute gastroenteritis in one patient.
When evaluating patients with post-acute COVID-19, the most prevalent symptom reported was persistent coughing, observed in 26 out of 41 cases (63%). This was followed by fatigue in eight patients (20%), nasal stuffiness in six patients (15%), and headache and rhinorrhea in four patients (10%). Other symptoms included loss of taste, chest discomfort, nausea, sore throat, dyspnea, loss of smell, diarrhea, abdominal pain, dizziness, and myalgia. In addition, one patient reported only a feeling of sputum production lasting more than 4 weeks without any other symptoms (Fig. 1).
The symptoms were compared by dividing the patients into four age groups. Fever was significantly less frequent at 12 years of age and older than at 3–5 years of age (p<0.008) and showed a tendency to decrease as age increased (p=0.013). The incidences of cough, nasal stuffiness, chest discomfort, myalgia, headache, dizziness, sore eyes, sore throat, nausea, loss of smell, and loss of taste increased with age. There was no trend in rhinorrhea according to age category, but the 6- to 11-year-old group showed a significantly lower incidence rate than the other groups (p<0.008). The incidence rates of dyspnea, chest pain, vomiting, abdominal pain, diarrhea, and asymptomatic presentation did not differ between the age categories, and there was no trend. The frequency of post-acute COVID-19 tended to increase with age; however, there was no significant difference between the groups (Fig. 2).
There were no significant differences between the two groups regarding the duration from the diagnosis of COVID-19 to the clinic visit, age at diagnosis, sex, or vaccination history (p=0.128, p=0.698, p=1.000, respectively). Allergic rhinitis and atopic dermatitis were more frequently observed in the asthma group than that in the non-asthma group. When comparing clinical features, no significant differences were observed between the two groups for all symptoms, including cough, rhinorrhea, nasal stuffiness, and dyspnea. However, the post-acute COVID-19 rate was significantly higher in the asthma group (22.6%) than in the non-asthma group (3.8%) (p=0.004) (Table 3).
Among the 12 patients with asthma and post-acute COVID-19, six reported only a cough lasting more than 4 weeks, and two reported rhinorrhea and cough lasting more than 4 weeks. The remaining four patients each reported that cough and fatigue, rhinorrhea and chest discomfort, loss of taste, and the feeling of having sputum persisted for more than 4 weeks.
There was no difference in mean duration from diagnosis of COVID-19 to clinic visit between the vaccinated and unvaccinated groups (p=0.110). The median age of the vaccinated group was 15 years (IQR, 14.0–16.3 years), significantly older than that of the unvaccinated group (13 years old: IQR, 12.0–14.0 years; p=0.001); there was no difference in sex between the groups (Table 4). In the vaccinated group, fever occurred in 24 patients (63.2%) with a peak value of 38.5 °C (IQR, 38.0–39.0 °C), significantly lower than that observed in the 27 unvaccinated patients (87.1%), with a peak value of 39 °C (IQR, 38.1–39.5 °C) (p=0.024, p=0.047, respectively). The frequencies of cough, rhinorrhea, nasal stuffiness, dyspnea, chest discomfort, chest pain, myalgia, headache, dizziness, sore eyes, and sore throat did not differ between the two groups. Among the gastrointestinal symptoms, the frequency of nausea, abdominal pain, and diarrhea did not differ between the two groups; however, the frequency of vomiting differed significantly between the two groups: one patient (2.6%) in the vaccinated group and seven patients (22.6%) in the unvaccinated group (p=0.019). The frequencies of alterations in smell and taste did not differ between the two groups. The frequency of fatigue was significantly lower in the vaccinated group: 15 patients (39.5%) in the vaccinated group and 20 patients (64.5%) in the non-vaccinated group (p=0.038). The incidence rates of asymptomatic and post-acute COVID-19 did not differ between the two groups.
Age and sex adjustments were made to investigate the effects of vaccination on fever, vomiting, and fatigue. In the vaccination group, the independent decrease in the risk of fever was observed regardless of age and sex (odds ratio [OR], 0.25; 95% confidence interval [CI], 0.07–0.88; p=0.030), and the peak value of fever also decreased (β, –0.30; 95% CI, –0.94 to –0.03; p=0.038). The risk of vomiting also decreased independent of age and sex in the vaccination group (OR, 0.09; 95% CI, 0.01–0.80; p=0.031). Fatigue was related to both sex (OR, 3.36; 95% CI, 1.19–9.46; p=0.022) and vaccination status (OR, 0.34; 95% CI, 0.12–0.95; p=0.040); the risk of fatigue was lower in females and in patients in the vaccination group.
In this study, the median illness duration for all symptoms investigated was 2–4 days. Post-acute COVID-19 was observed in 41 patients (13.2%), and the most common symptom was cough. There were no significant differences in the acute symptoms between the asthma and non-asthma groups; however, the incidence of post-acute COVID-19 was significantly higher in the asthma group. Fever, vomiting, and fatigue were less observed in vaccinated group than that in unvaccinated group.
In general, the most frequently observed symptoms in children and adolescents with COVID-19 were fever (20.6%–80.2%) and cough (33.7%–54.2%) [4,10-12]. Although the frequencies were moderately different, fever (84.6%) and cough (76.2%) were the most common in our study. However, a prospective cohort study in the United Kingdom revealed headache and fatigue as the predominant symptoms (62.2% and 55.0%, respectively) [13]. The mean illness duration was 6 days in foreign studies and 4 days in this study, indicating that the symptoms disappeared within 1 week [4,10,13].
The common cold is a viral illness on the upper respiratory tract such as the nose, sinuses, and throat. In general, the severity of symptoms of cold peaks within 2–3 days, and then the symptoms decrease and disappear [14]. Although it was not compared with a control group consisting of patients with other respiratory viral infections during the same period in this study, based on previous research about the common cold, the duration of illness of COVID-19 in children and adolescents shown in our study is similar to that of the common cold.
The proportion of asymptomatic patients varied among the studies. Data from hospitalized Korean children showed that 4.9%–48.2% of patients were asymptomatic. KDCA data from February to April 2020 showed that 38.6% of patients under the age of 20 were asymptomatic. Foreign studies reported that 66.4% of patients in an American study and 13% of patients in one meta-analysis were asymptomatic [4,10-12,15]. In our study, asymptomatic patients were similar to the finding in two of three studies on hospitalized Korean patients but lower than that reported in other studies. The lower number of asymptomatic patients in these three studies might stem from enrolling only patients who visited the hospital. Another Korean study with hospitalized children reported that 48.2% of patients were asymptomatic. Because the study was conducted on patients when all patients were transferred to community treatment centers or hospitals according to the guidelines of the Korean quarantine authorities, the rate may have been higher than other Korean studies with patients who visited hospital. Therefore, the real number of asymptomatic patients is expected to be higher than reported in this study.
There is a lack of consensus on the definition of long-lasting symptoms after acute COVID-19, and the prevalence varies widely [16]. Even when only studies investigating symptoms lasting more than 4 weeks are considered, the results differ between studies. A national cohort study of patients under 18 years of age in Denmark reported that 12%–51% of patients showed post-acute COVID-19 symptoms depending on age [17], and a prospective cohort study of patients aged 5–17 years in the United Kingdom found that 3%–5% experienced post-acute COVID-19 symptoms [13]. In the current study, 13.2% of all patients had post-acute COVID-19 symptoms, and this finding is similar to that of the Danish study. In a meta-analysis of several case-control studies that surveyed persistent symptoms after SARS-CoV-2 infection, symptoms, such as cough, abdominal pain, myalgia, diarrhea, fatigue, and dizziness, did not show statistically significant differences between the patient and control groups. Loss of smell, headache, sore throat, and sore eyes occurred more frequently in the patient group than in the control group. The authors argued that the incidence of persistent symptoms following SARS-CoV-2 infection may be much less than that reported in the literature [18]. The most frequent symptoms of post-acute COVID-19 in our study were cough and fatigue. Some of the cases among the 13.2% of post-acute COVID-19 cases observed in our study may not have been caused by SARS-CoV-2 infection because these variables did not differ between the patient and control groups in a previously mentioned meta-analysis. When analyzing the data of the participants in our study for the significant variables mentioned above, only five patients had post-acute COVID-19, and the frequency decreased to 1.6%. Therefore, the frequency of post-acute COVID-19 cases is expected to significantly reduce in future studies addressing this issue.
The disease course differs significantly between children and adults. It is speculated that this is due to age-related differences in the immune system, the level of expression of the angiotensin-converting enzyme 2 receptor, and the characteristics of the respiratory system [19]. An American study reported results similar to those of our study. Cough, rhinorrhea, dyspnea, myalgia, headache, sore throat, nausea or vomiting, abdominal pain, diarrhea, and loss of smell or taste were more frequently observed in those aged 10 years and older. Only fever was seen more frequently in children younger than 10 years of age [20]. In our study, the frequency of 12 out of 19 symptoms tended to increase with age, and no age-related trend was observed for six symptoms. Only fever tended to decrease with age, as observed in a previous study in the United States. We believe that this phenomenon reflects not only the hypothesis mentioned above but also the fact that symptoms can be expressed better as age increases.
Asthma was initially hypothesized to be a risk factor for severe COVID-19, given the association between asthma exacerbations and common respiratory viral diseases. Fortunately, however, the overall clinical outcomes of COVID-19 patients with asthma are similar to those of patients without asthma [21]. In a case-control study that compared asthma and non-asthma groups in COVID-19 patients aged 6–18 years, there was no statistically significant difference between the two groups in the incidence of cough and dyspnea during the acute period. The incidence of fever, sore throat, chest pain, and gastrointestinal problems were also similar to our results [22]. Unlike the acute phase symptoms, we observed that post-acute COVID-19 occurred more often in the asthma group than in the non-asthma group. One study of 29 children with a mean age of 13 years showed that the prevalence of asthma was higher in COVID-19 patients who complained of respiratory symptoms for more than 6 weeks than in the general population under 18 years old [23]. In a prospective cohort study of approximately 1,000 adults with COVID-19 in the United States, asthma patients had a 1.54 times higher risk of developing post-acute COVID-19 [24]. This suggests that asthma may affect the development of prolonged symptoms after COVID-19. There is another opinion as well. In a retrospective cohort study with adult patients with COVID-19, 75% of those with asthma and 76.8% of those without asthma continue to complain of symptoms even 90 days after infection, and some researchers have asserted that post-acute COVID-19 does not occur more often in patients with asthma in their review article [21,25].
Even before the COVID-19 pandemic, cough, which was the most common symptom of post-acute COVID-19 in this study, was usually referred to as postinfectious cough if it persisted for more than 3 weeks following viral infection [26]. There are no studies comparing persistent symptoms including cough after viral infection in patients with and without typical asthma. However, there have been studies that analyzed patients who coughed over 3 weeks by dividing them into postinfectious cough and cough variant asthma. One study of 195 patients with prolonged cough in Japan reported that 99 patients (50.8%) were diagnosed with postinfectious cough, and 40 patients (20.5%) were diagnosed with cough variant asthma. Chinese research investigating 104 patients with subacute cough following cold reported that 45 patients (43.2%) were diagnosed with postinfectious cough, and 17 patients (16.3%) were diagnosed with cough variant asthma [27,28]. In both studies, the number of patients with cough variant asthma were smaller than that of postinfectious cough. It suggests that SARS-CoV-2 may have different effects in asthma unlike other respiratory viruses. However, since the previous two studies excluded patients already diagnosed with asthma, it is difficult to simply compare their results with that of our study. In the future, additional well-designed studies are needed to determine whether post-acute COVID-19, especially prolonged cough, is more prevalent in asthma patients than other viral infections.
One study suggested that the reason why post-acute COVID-19 is more observed in asthma is related to immunoglobulin. A prospective cohort study in Switzerland reported that asthma was associated with risk of developing post-acute COVID-19 (OR, 9.74; p=0.003), and IgG3 in asthma patients was significantly lower (p=0.038) than patients without asthma [29]. Authors of the study suggested that IgG3 secreted from B cells contributes to the development of post-acute COVID-19. This is also supported by an Italian cohort study that showed differences in epigenetic and transcriptional signatures in B cells between patients who did and did not develop post-acute COVID-19 [30].
Despite reports that the overall effectiveness of SARS-CoV-2 vaccination in children is 90%–100% and that it is more effective and safer in children than in adults [29,30], breakthrough infections may occur. In our study, 42 of the 73 patients eligible for vaccination (58%) were vaccinated, and 41 of them were infected with SARS-CoV-2 despite being fully vaccinated more than twice. One review article reported that breakthrough infections were milder than infections in the unvaccinated group [31]. Likewise, in our study, vaccinated adolescents had lower incidences of fever, vomiting, and fatigue than those of unvaccinated adolescents. Similar results were obtained after adjusting for age and sex, which are consistent with the results of previous studies. According to the UK Health Security Agency, of eight studies with COVID-19 patients, six showed that vaccinated patients less developed post-acute COVID than unvaccinated patients [32]. Our study did not show a significant difference between the two groups, contrary to previous studies. The results in this study cannot confirm the effectiveness of vaccination in adolescents and cannot refute or support earlier studies showing that vaccination may reduce post-acute COVID-19. Because our study included a small number of patients in the vaccinated and unvaccinated groups (38 and 31, respectively). Therefore, future studies should assess the effectiveness of vaccination, targeting only Korean children and adolescents. Such studies will hopefully counter and refute rumors and conspiracy theories spreading among the public.
This study had several limitations. First, the number of cases in this study was less than 1% of the 23,719 daily average number of cases of COVID-19 among Korean patients under the age of 20 reported in the first half of 2022; this sample size is too small to adequately represent Korean pediatric patients. Second, recall bias existed in our study because it was a retrospective study based on reports from medical records. Additionally, our data included missing values, which was an obstacle to the statistical analysis. Finally, selection bias could not be excluded because only patients who visited a single tertiary healthcare center were included; thus, many patients with asymptomatic SARS-CoV-2 infection with no underlying diseases may not have been enrolled in this study. However, severely ill patients may have been underrecruited because our hospital was not COVID-19-dedicated.
Nevertheless, our report has the advantage of examining the duration of each symptom and frequency of post-acute COVID-19 in children and adolescents who visited a single center regardless of hospitalization. Additionally, we showed that the rate of post-acute COVID-19 was significantly higher in the asthma group than in the non-asthma group. In the case of vaccination, even if breakthrough infection occurred, they had milder symptoms compared to those who were not vaccinated. Our findings provide information on the clinical manifestations of COVID-19 in Korean children for patients and their caregivers. Our results also highlight the need to observe patients with asthma for more than 4 weeks. In the future, large-scale, long-term studies are required to more accurately identify the clinical features and prognosis of COVID-19 in Korean pediatric patients to reduce public anxiety and unnecessary socioeconomic costs.
Notes
Author contributions
Conceptualization: JEJ, HLC, YYJ. Data curation: JEJ, YHK, NL, YK. Formal analysis: YYJ. Investigation: JEJ, YYJ. Methodology: Project administration; Resources; Software; Supervision: YYJ. Validation; Visualization: JEJ, YYJ. Writing - original draft: JEJ, YYJ. Writing - review & editing: YYJ. Approval of final manuscript: all authors.
References
1. World Health Organization (WHO). General speeches [Internet]. WHO; c2023 [cited 2023 Jun 4]. Available from: https://www.who.int/director-general/speeches/.
2. Korea Disease Control and Prevention Agency (KDCA). Cumulative confirmed cases of COVID-19 [Internet]. KDCA; c2023 [cited 2023 Jun 13]. Available from: https://ncov.kdca.go.kr/.
3. Liguoro I, Pilotto C, Bonanni M, Ferrari ME, Pusiol A, Nocerino A, et al. SARS-COV-2 infection in children and newborns: a systematic review. Eur J Pediatr. 2020; 179:1029–46.
4. Irfan O, Muttalib F, Tang K, Jiang L, Lassi ZS, Bhutta Z. Clinical characteristics, treatment and outcomes of paediatric COVID-19: a systematic review and meta-analysis. Arch Dis Child. 2021; 106:440–8.
5. Lee KS, Sung HK, Lee SH, Hyun J, Kim H, Lee JS, et al. Factors related to anxiety and depression among adolescents during COVID-19: a web-based cross-sectional survey. J Korean Med Sci. 2022; 37:e199.
6. Heiat M, Heiat F, Halaji M, Ranjbar R, Tavangar Marvasti Z, Yaali-Jahromi E, et al. Phobia and fear of COVID-19: origins, complications and management, a narrative review. Ann Ig. 2021; 33:360–70.
7. Crapo RO, Casaburi R, Coates AL, Enright PL, Hankinson JL, Irvin CG, et al. Guidelines for methacholine and exercise challenge testing-1999. This official statement of the American Thoracic Society was adopted by the ATS Board of Directors, July 1999. Am J Respir Crit Care Med. 2000; 161:309–29.
8. Nalbandian A, Sehgal K, Gupta A, Madhavan MV, McGroder C, Stevens JS, et al. Post-acute COVID-19 syndrome. Nat Med. 2021; 27:601–15.
9. Korea Disease Control and Prevention Agency (KDCA). Revision of COVID-19 vaccination implementation standards and related FAQ information (as of March 14, 2022) [Internet]. KDCA; c2024 [cited 2024 Apr 15]. https://www.kdca.go.kr/.
10. Choi YY, Choi SH, Choi JH, Kim DH, Lee JK, Eun BW, et al. SARS-CoV-2-naive Korean children and adolescents hospitalized with COVID-19 in 2021. J Korean Med Sci. 2022; 37:e303.
11. Sol IS, Lee E, Yang HJ, Lee YJ, Yum HY, Lee MH, et al. Clinical characteristics of pediatric patients infected with SARS-CoV-2 versus common human coronaviruses: a national multicenter study. Clin Exp Pediatr. 2023; 66:134–41.
12. Moon G, Shin D, Choi SH. Clinical characteristics of pediatric patients with the coronavirus disease 2019 during the third and fourth waves of the epidemic in Korea: a single center retrospective study. Pediatr Infect Vaccine. 2022; 29:131–40.
13. Molteni E, Sudre CH, Canas LS, Bhopal SS, Hughes RC, Antonelli M, et al. Illness duration and symptom profile in symptomatic UK school-aged children tested for SARS-CoV-2. Lancet Child Adolesc Health. 2021; 5:708–18.
14. Heikkinen T, Jarvinen A. The common cold. Lancet. 2003; 361:51–9.
15. Forrest CB, Burrows EK, Mejias A, Razzaghi H, Christakis D, Jhaveri R, et al. Severity of acute COVID-19 in children <18 years old March 2020 to December 2021. Pediatrics. 2022; 149:e2021055765.
16. Ha EK, Kim JH, Han MY. Long COVID in children and adolescents: prevalence, clinical manifestations, and management strategies. Clin Exp Pediatr. 2023; 66:465–74.
17. Borch L, Holm M, Knudsen M, Ellermann-Eriksen S, Hagstroem S. Long COVID symptoms and duration in SARS-CoV-2 positive children: a nationwide cohort study. Eur J Pediatr. 2022; 181:1597–607.
18. Behnood SA, Shafran R, Bennett SD, Zhang AX, O’Mahoney LL, Stephenson TJ, et al. Persistent symptoms following SARS-CoV-2 infection amongst children and young people: a meta-analysis of controlled and uncontrolled studies. J Infect. 2022; 84:158–70.
19. Kwak BO, Kim DH. Coronavirus disease 2019: reasons for better clinical course for children compared to adults. Pediatr Infect Vaccine. 2021; 28:1–6.
20. Stokes EK, Zambrano LD, Anderson KN, Marder EP, Raz KM, El Burai Felix S, et al. Coronavirus disease 2019 case surveillance: United States, January 22-May 30, 2020. MMWR Morb Mortal Wkly Rep. 2020; 69:759–65.
21. Palmon PA, Jackson DJ, Denlinger LC. COVID-19 infections and asthma. J Allergy Clin Immunol Pract. 2022; 10:658–63.
22. El-Sayed ZA, El-Owaidy RH, Harb WN, Shousha GA. COVID-19 in a group of children with asthma: presentation, severity, and outcome. Am J Clin Exp Immunol. 2022; 11:92–102.
23. Leftin Dobkin SC, Collaco JM, McGrath-Morrow SA. Protracted respiratory findings in children post-SARS-CoV-2 infection. Pediatr Pulmonol. 2021; 56:3682–7.
24. Jacobs ET, Catalfamo CJ, Colombo PM, Khan SM, Austhof E, Cordova-Marks F, et al. Pre-existing conditions associated with post-acute sequelae of COVID-19. J Autoimmun. 2023; 135:102991.
25. Eggert LE, He Z, Collins W, Lee AS, Dhondalay G, Jiang SY, et al. Asthma phenotypes, associated comorbidities, and long-term symptoms in COVID-19. Allergy. 2022; 77:173–85.
26. Braman SS. Postinfectious cough: ACCP evidence-based clinical practice guidelines. Chest. 2006; 129(1 Suppl):138S–146S.
27. Mikami M, Tomita K, Yamasaki A. A history of recurrent episodes of prolonged cough as a predictive value for determining cough variant asthma in a primary care setting. Yonago Acta Med. 2021; 64:353–9.
28. Lai K, Lin L, Liu B, Chen R, Tang Y, Luo W, et al. Eosinophilic airway inflammation is common in subacute cough following acute upper respiratory tract infection. Respirology. 2016; 21:683–8.
29. Tian F, Yang R, Chen Z. Safety and efficacy of COVID-19 vaccines in children and adolescents: a systematic review of randomized controlled trials. J Med Virol. 2022; 94:4644–53.
30. Xu W, Tang J, Chen C, Wang C, Wen W, Cheng Y, et al. Safety and efficacy of the COVID-19 vaccine in children and/or adolescents: a meta-analysis. J Infect. 2022; 84:722–46.
31. Amanatidou E, Gkiouliava A, Pella E, Serafidi M, Tsilingiris D, Vallianou NG, et al. Breakthrough infections after COVID-19 vaccination: insights, perspectives and challenges. Metabol Open. 2022; 14:100180.
32. Mahase E. Covid-19: vaccinated people are less likely to get long COVID, review finds. BMJ. 2022; 376:o407.
Fig. 1.
Proportion of patients with post-acute coronavirus disease 2019 symptoms. If a patient had multiple symptoms, multiple variables corresponding to the symptoms were selected.
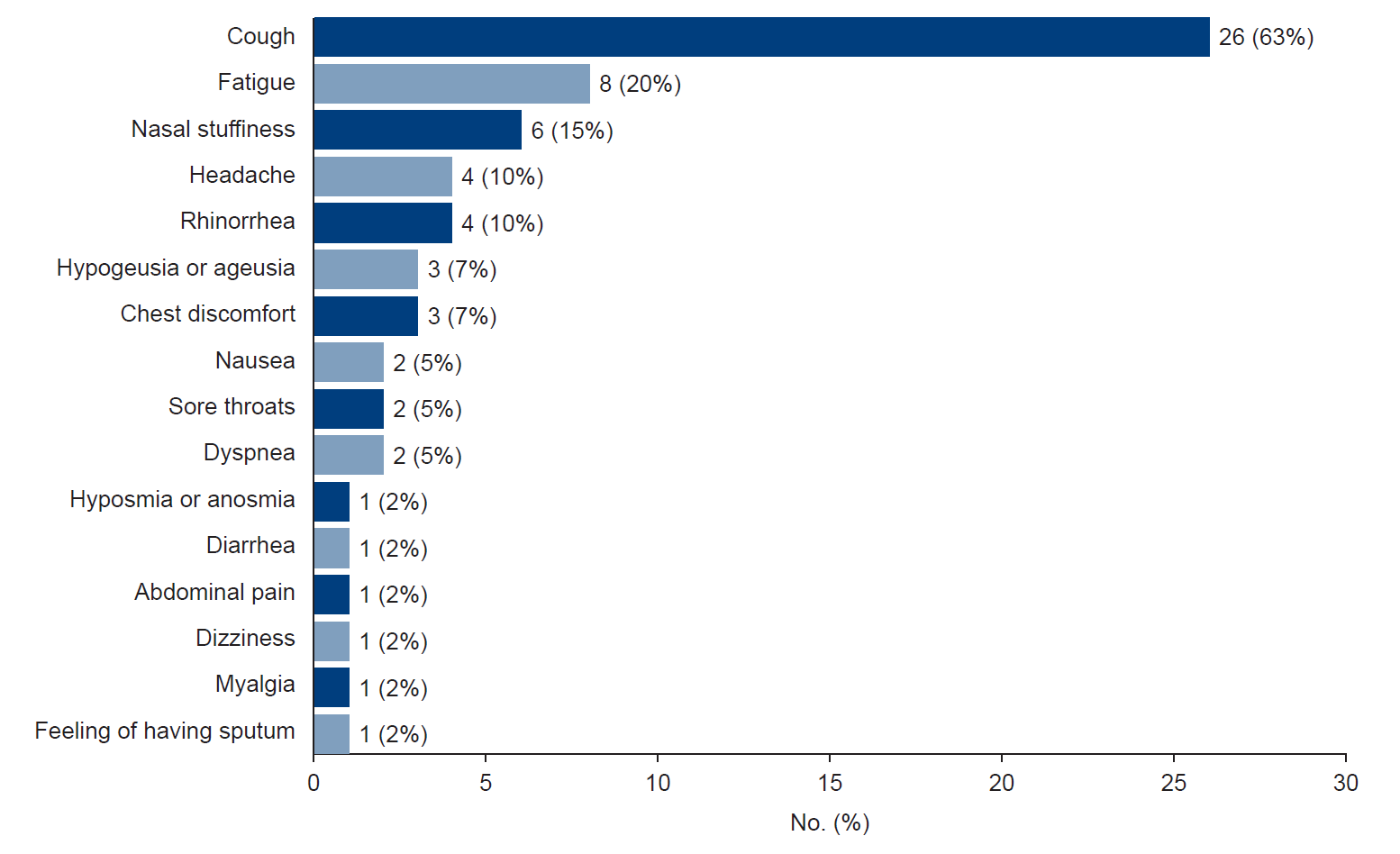
Fig. 2.
Clinical features by age category: (A) fever, (B) cough, (C) rhinorrhea, (D) nasal stuffiness, (E) dyspnea, (F) chest discomfort, (G) chest pain, (H) myalgia, (I) headache, (J) dizziness, (K) sore eyes, (L) sore throat, (M) nausea, (N) vomiting, (O) abdominal pain, (P) diarrhea, (Q) loss of smell, (R) loss of taste, (S) fatigue, (T) asymptomatic, and (U) post-acute coronavirus disease 2019 (COVID-19). *p<0.008; **p<0.017.
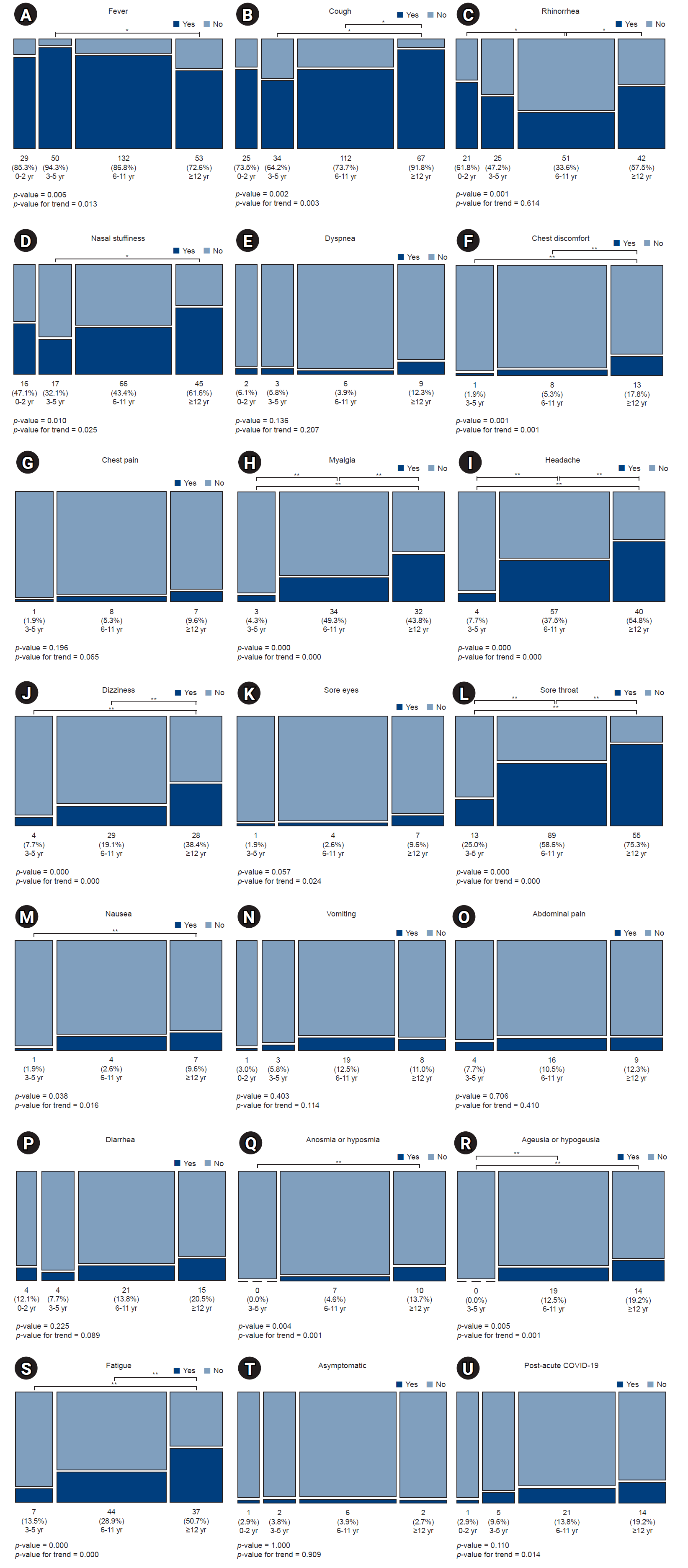
Table 1.
Demographic characteristics
Values are presented as median (interquartile range) or number (%).
COVID-19, coronavirus disease 2019.
a) Considering South Korea’s vaccination approval requirements, only those aged 12 or older were analyzed.
Table 2.
Clinical manifestations of the analyzed patients
Table 3.
Clinical features according to the presence of asthma
Table 4.
Clinical features according to vaccination status




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download