Abstract
Herpes zoster virus infection is common and results in significant morbidity in patients who have undergone solid organ transplantation. Herpes zoster can involve the cranial nerves, and vagus nerve involvement is an infrequent primary manifestation of herpes zoster. Here, we describe a rare presentation of disseminated herpes zoster infection with vagus nerve involvement in a kidney transplant recipient. A 62-year-old man who had undergone kidney transplantation 3 years prior presented to our clinic with sore throat and hoarseness, followed by multiple vesicular-pustular rashes on the face and trunk. Flexible laryngoscopy revealed left paramedian vocal cord paralysis with multiple ulcerative lesions extending from the left pyriform sinus to the epiglottis. Computed tomography of the neck, abdomen, and chest revealed no significant abnormalities that could have caused vocal cord paralysis. We confirmed the diagnosis of disseminated herpes zoster after herpes zoster laryngitis based on positive blood tests and polymerase chain reaction for varicella zoster virus antibodies. The skin rashes and laryngeal ulcers rapidly resolved after treatment with intravenous acyclovir and high-dose steroids. The patient still had persistent dysphagia and microaspiration as assessed by a video fluoroscopic swallowing study, but showed improvement in dysphagia in response to swallowing rehabilitation therapy. This case provides valuable insights into the presenting symptoms of disseminated herpes zoster, which can cause acute vagus neuritis in solid organ transplantation recipients.
Varicella zoster virus (VZV) can cause a primary infection known as varicella (chicken pox), which later reactivates into herpes zoster (HZ, shingles) within the sensory neuron body in the dorsal ganglion [1]. HZ is more common in immunocompromised individuals, particularly in those who have undergone solid organ transplantation (SOT), with an incidence of 8% to 11% in the first 4 years [2]. HZ typically presents with prodromal symptoms such as fever, malaise, and burning pain around the unilateral dermatome, followed by erythematous vesicular rashes over several days. The most commonly affected dermatomes in patients with HZ are in the thoracic, trigeminal, cervical, and lumbar regions [3]. Although less common, HZ can also involve the cranial nerves (CN). A retrospective study demonstrated that the trigeminal nerve (CN V, 57.9%), facial nerve (CN VII, 52.1%), and vestibulocochlear nerve (CN VIII, 20%) are commonly affected; however, involvement of the vagus nerve (CN X, 0.9%) is very rare [4].
Disseminated HZ is a serious complication that occurs more frequently in SOT recipients than in the general population. Previous studies have reported a global mortality rate of approximately 30% in cases of disseminated HZ and a high incidence in SOT recipients [5,6]. HZ typically presents with unilateral dermatomal involvement and subsequently spreads to affect three or more dermatomes, which can lead to serious complications involving the central nervous system or internal organs, such as the gastrointestinal tract, liver, lung, and pancreas [2]. Herein, we present a rare case of disseminated HZ following acute vagus neuritis in a kidney transplant recipient.
Ethical statements: This study was approved by the Institutional Review Board of Pusan National University Hospital (IRB No. 2311-008-133). Written informed consent was waived because the retrospective design of the study.
A 62-year-old man with a history of end-stage kidney disease of unknown cause underwent deceased donor kidney transplantation (KT) 3 years ago. He had no other comorbidity such as hypertension or diabetes, and had been receiving hemodialysis for 6 years before KT. At the time of KT, the patient had no donor-specific antibodies and received induction therapy with basiliximab. Two months after KT, he was treated for T-cell-mediated rejection with methylprednisolone pulse therapy. He had to reduce his immunosuppressive agents because of subsequent cytomegalovirus viremia. The patient was taking prednisolone (2.5 mg daily), mycophenolic acid (180 mg twice daily), and tacrolimus (2 mg twice daily) with trough levels of 4–5 ng/mL. His serum creatinine level has been maintained within the range of 0.9–1.0 mg/dL.
He presented to our clinic with sore throat and hoarseness, followed by multiple vesicular-pustular rashes on the face and trunk (Fig. 1A, 1B). Flexible laryngoscopy revealed left paramedian vocal cord paralysis, with multiple ulcerative lesions extending from the left pyriform sinus to the epiglottis (Fig. 1C). Computed tomography of the neck and chest revealed no significant abnormalities that could have caused vocal cord paralysis. Laryngeal electromyography (EMG) revealed a fibrillation potential in the left thyroarytenoid muscle innervated by the left recurrent laryngeal nerve (RLN). Laboratory data showed a white blood cell of 5,570/μL, C-reactive protein of 0.68 mg/dL, serum creatinine of 1.05 mg/dL, VZV immunoglobulin G of 2.25 (reference, positive: ≤0.90 ), and immunoglobulin M of 1.4 (reference, positive: ≤1.10 ), as well as a positive blood test for VZV polymerase chain reaction. Serologies for other viruses that could cause laryngitis were negative (Table 1).
Based on these findings, we diagnosed the patient with disseminated HZ following acute vagus neuritis. We administered intravenous acyclovir (10 mg/kg every 8 hours) for 14 days along with 1,000 mL of intravenous normal saline to prevent acyclovir-related nephropathy, and high-dose prednisolone (0.5 mg/kg/day) was given for 7 days, then tapered to 2.5 mg/day to reduce the risk of neurological complications. We also reduced the immunosuppressive agents, including mycophenolic acid (180 mg daily) and tacrolimus (1.5 mg twice daily) with trough levels of 3–5 ng/mL. During hospitalization, his serum creatinine was carefully monitored and the dose of tacrolimus was adjusted according to trough levels (Fig. 2). As a result, there was rapid resolution of skin rashes and laryngeal ulcers (Fig. 3A). However, 2 weeks after the completion of treatment, the patient still experienced persistent hoarseness, dysphagia, and evidence of microaspiration, as assessed by video fluoroscopic swallowing study (Fig. 3B). We injected hyaluronic acid into the paralyzed vocal cord and the patient showed improvement in dysphagia and hoarseness with continuous swallowing and speech rehabilitation therapy.
SOT recipients on lifelong immunosuppressive therapy are at high risk of VZV reactivation, which can subsequently lead to disseminated disease or internal organ involvement. A meta-analysis of 6,560 SOT recipients showed that the overall incidence of HZ was 9.1% and that heart transplantation had the highest incidence of HZ (15.2%), followed by lung transplantation (11.0%) and KT (6.7%) [7]. They further demonstrated that a higher incidence of post-transplant HZ was associated with a higher proportion of previous graft rejection.
A previous study reported atypical cases of HZ in KT recipients, including the involvement of CN V (V1), multiple thoracic dermatomes (T2–T5), and disseminated disease with septic shock as primary manifestations [8]. These cases demonstrated that the presentation of HZ may be atypical, and the severity of HZ may vary depending on the degree of immunosuppression in SOT recipients.
In this case, the patient presented with vagus nerve involvement, which is a rare manifestation of HZ. Flexible laryngoscopy revealed unilateral ulcerative lesions on the laryngeal mucosa, suggesting involvement of the internal branch of the superior laryngeal nerve, which originates from the vagus nerve. Additionally, we observed left paramedian vocal cord paralysis, suggesting injury to the RLN, which innervates the laryngeal muscles and originates from the vagus nerve [9].
Several viral etiologies have been reported to cause vocal cord paralysis, including cytomegalovirus, influenza virus, Epstein-Barr virus, herpes simplex virus, and VZV. Of these, VZV and herpes simplex viruses can cause permanent damage [10]. A previous study has revealed that the full recovery rate of HZ-associated vocal cord paralysis is 60% to 62.5% [11].
Laryngeal EMG is a useful tool for evaluating the neurological prognosis of RLN injury. The presence of spontaneous activity, such as fibrillation potentials or positive sharp waves on EMG, indicates a poor prognosis [12]. In this case, 2 weeks after the completion of treatment, the laryngeal EMG revealed a fibrillation potential in the left thyroarytenoid muscle innervated by the RLN, and a subsequent flexible laryngoscopy still showed paramedian vocal cord paralysis. Despite the poor prognosis indicated by the laryngeal EMG, his dysphagia and hoarseness improved with conservative treatments, including injection of hyaluronic acid into the paralyzed vocal cord to correct the incomplete closure of the glottis, and continuous swallowing and speech rehabilitation therapy. Therefore, these conservative treatments are essential for preventing permanent neurological complications in patients with HZ complicated by acute vagus neuritis.
The preferred treatment for HZ in immunocompromised patients is intravenous acyclovir (10 mg/kg every 8 hours) for 7 to 10 days. Administration of intravenous acyclovir within 72 hours of rash onset effectively suppresses disease progression by inhibiting viral replication and prevents the spread of viral infections to other organs or dermatomes [13]. Of note, a synergistic nephrotoxicity should be considered when intravenous acyclovir is used in combination with a calcineurin inhibitor in SOT recipients [14]. Adequate intravenous hydration is required to prevent the formation of acyclovir crystals, along with careful monitoring of serum creatinine and trough levels of calcineurin inhibitors.
When patients are complicated by VZV-associated neuritis, combination therapy with intravenous acyclovir and high-dose steroids may be helpful. Steroids are known to reduce neural edema through their anti-inflammatory effects, thereby preventing permanent damage. Early initiation of steroid therapy during the course of the disease may improve neurological complications [15,16]. Furthermore, it is necessary to reduce immunosuppressive agents, particularly mycophenolate mofetil or mycophenolic acid, in order to reduce severe complications and mortality [17,18].
To reduce the risk of HZ in SOT recipients, it is ideal to administer the HZ vaccination before organ transplantation, but it is not always guaranteed. Currently, a non-live recombinant zoster vaccine has been demonstrated to be immunogenic and safe, even when administered 4 to 18 months after transplantation [19]. This provides an opportunity to overcome the limitation of being unable to administer a live vaccine to unvaccinated patients after transplantation, thus reducing the risk of HZ.
In conclusion, we encountered a rare case of HZ involving the vagus nerve in a kidney transplant recipient, which subsequently progressed to disseminated disease and may have resulted in serious neurological complications. However, this was minimized by early administration of an antiviral agent with high-dose steroids, and intensive rehabilitation therapy. This case provides valuable insights into the presenting symptoms of disseminated HZ, which can cause acute vagus neuritis in SOT recipients.
Notes
References
1. Zerboni L, Sen N, Oliver SL, Arvin AM. Molecular mechanisms of varicella zoster virus pathogenesis. Nat Rev Microbiol. 2014; 12:197–210.
2. Pergam SA, Limaye AP; AST Infectious Diseases Community of Practice. Varicella zoster virus in solid organ transplantation. Am J Transplant. 2013; 13 Suppl 4(Suppl 4):138–46.
3. Purwoko MI, Darmawan H. Herpes zoster: clinical manifestation, treatment, and prevention. Biosci Med Biosci Med J Biomed Transl Res. 2020; 4:34–44.
4. Tsau PW, Liao MF, Hsu JL, Hsu HC, Peng CH, Lin YC, et al. Clinical presentations and outcome studies of cranial nerve involvement in herpes zoster infection: a retrospective single-center analysis. J Clin Med. 2020; 9:946.
5. Rommelaere M, Marechal C, Yombi JC, Goffin E, Kanaan N. Disseminated varicella zoster virus infection in adult renal transplant recipients: outcome and risk factors. Transplant Proc. 2012; 44:2814–7.
6. Kho MM, Roest S, Bovee DM, Metselaar HJ, Hoek RA, van der Eijk AA, et al. Herpes zoster in solid organ transplantation: incidence and risk factors. Front Immunol. 2021; 12:645718.
7. Kwon DE, Lee HS, Lee KH, La Y, Han SH, Song YG. Incidence of herpes zoster in adult solid organ transplant recipients: a meta-analysis and comprehensive review. Transpl Infect Dis. 2021; 23:e13674.
8. Hanna RM, Abd-El-Malak F, Alnaser A, Cader R, Yabu JM. Herpes zoster in kidney transplant recipients: a series of three cases. Case Rep Nephrol Dial. 2020; 10:139–46.
9. Orestes MI, Chhetri DK. Superior laryngeal nerve injury: effects, clinical findings, prognosis, and management options. Curr Opin Otolaryngol Head Neck Surg. 2014; 22:439–43.
10. Myssiorek D. Recurrent laryngeal nerve paralysis: anatomy and etiology. Otolaryngol Clin North Am. 2004; 37:25–44.
11. Kim YH, Chang MY, Jung HH, Park YS, Lee SH, Lee JH, et al. Prognosis of Ramsay Hunt syndrome presenting as cranial polyneuropathy. Laryngoscope. 2010; 120:2270–6.
12. Wang CC, Chang MH, Wang CP, Liu SA. Prognostic indicators of unilateral vocal fold paralysis. Arch Otolaryngol Head Neck Surg. 2008; 134:380–8.
13. Dworkin RH, Johnson RW, Breuer J, Gnann JW, Levin MJ, Backonja M, et al. Recommendations for the management of herpes zoster. Clin Infect Dis. 2007; 44 Suppl 1:S1–26.
14. Bhagat V, Pandit RA, Ambapurkar S, Sengar M, Kulkarni AP. Drug interactions between antimicrobial and immunosuppressive agents in solid organ transplant recipients. Indian J Crit Care Med. 2021; 25:67–76.
15. Kim Y, Doo JG, Chon J, Lee JH, Jung J, Lee JM, et al. Steroids plus antiviral agents are more effective than steroids alone in the treatment of severe Bell’s palsy patients over 40 years of age. Int J Immunopathol Pharmacol. 2021; 35:20587384211042124.
16. Monsanto RD, Bittencourt AG, Bobato Neto NJ, Beilke SC, Lorenzetti FT, Salomone R. Treatment and prognosis of facial palsy on Ramsay Hunt syndrome: results based on a review of the literature. Int Arch Otorhinolaryngol. 2016; 20:394–400.
17. Fehr T, Bossart W, Wahl C, Binswanger U. Disseminated varicella infection in adult renal allograft recipients: four cases and a review of the literature. Transplantation. 2002; 73:608–11.
18. Koo S, Gagne LS, Lee P, Pratibhu PP, James LM, Givertz MM, et al. Incidence and risk factors for herpes zoster following heart transplantation. Transpl Infect Dis. 2014; 16:17–25.
19. Vink P, Ramon Torrell JM, Sanchez Fructuoso A, Kim SJ, Kim SI, Zaltzman J, et al. Immunogenicity and safety of the adjuvanted recombinant zoster vaccine in chronically immunosuppressed adults following renal transplant: a phase 3, randomized clinical trial. Clin Infect Dis. 2020; 70:181–90.
Fig. 1.
(A, B) Multiple vesicular-pustular rashes on the face and trunk. (C) Left paramedian vocal cord paralysis with multiple ulcerative lesions extending from the left part of the pyriform sinus to the epiglottis.
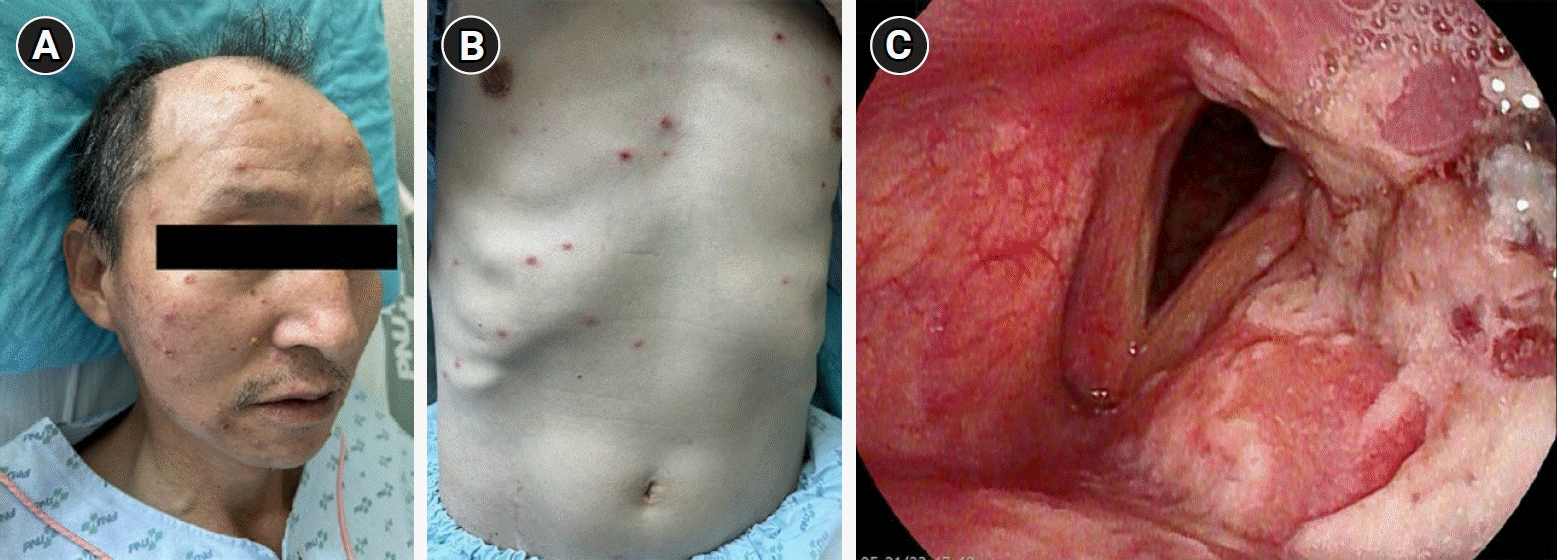
Fig. 2.
Changes in serum creatinine, trough levels of tacrolimus, and dose adjustment of immunosuppressive agents during hospitalization. IV, intravenous.
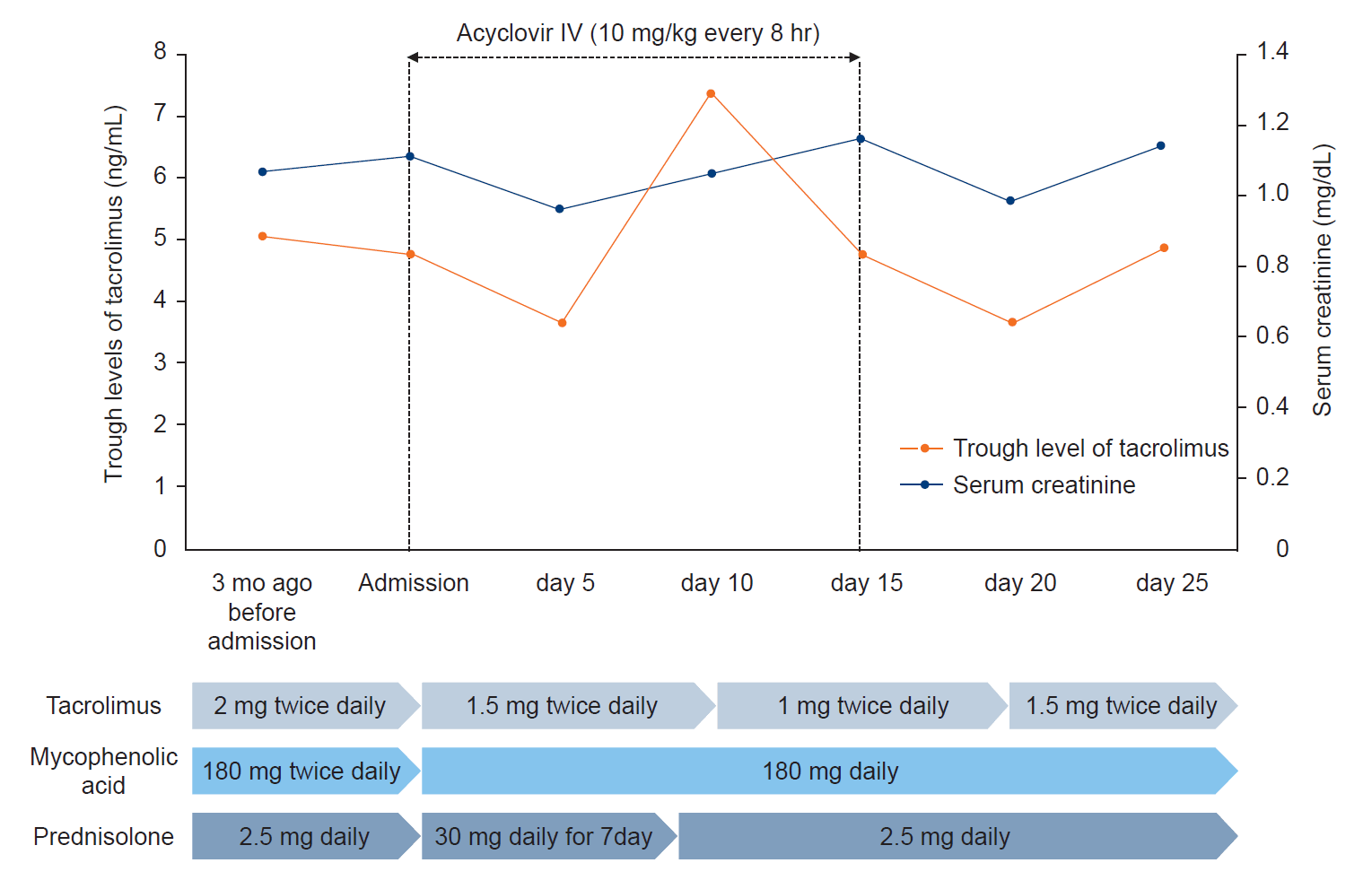
Fig. 3.
(A) Persistent vocal cord paralysis despite resolution of laryngeal ulcers (white arrow) 2 weeks after the completion of treatment. (B) Video fluoroscopic swallowing study showing poor pharyngeal constriction with moderate liquid retention and delayed aspiration (black arrow) in the pharyngeal phase.
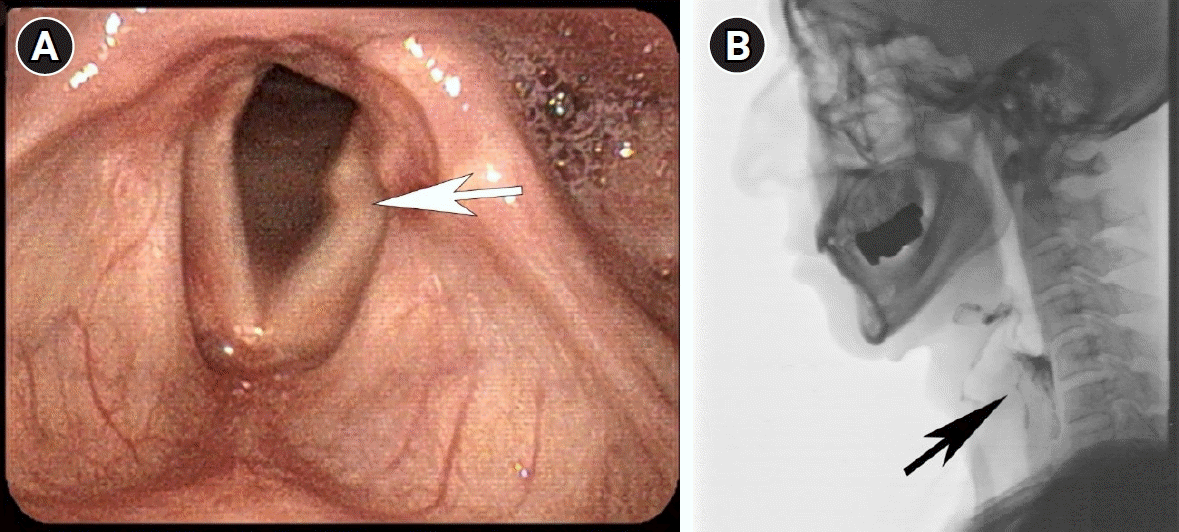
Table 1.
Serological findings associated with vocal cord paralysis




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download