Abstract
Patients with heart failure undergoing surgery that requires general anesthesia face substantial perioperative risks; however, clear guidelines are not available for anesthesia management in patients with a reduced left ventricular ejection fraction. Traditional intravenous and volatile anesthetics require careful administration to prevent severe hypotension and bradycardia in patients with heart failure. Remimazolam has emerged as a promising alternative to conventional anesthetics because of its reduced cardiovascular depressive effects. We present three cases illustrating the successful use of remimazolam to induce and maintain general anesthesia in patients with heart failure and reduced cardiac function. Our cases demonstrate the safe use of remimazolam for general anesthesia in patients with heart failure and a reduced ejection fraction.
Heart failure is a syndrome frequently encountered by anesthesiologists in the operating room. Patients with heart failure have a high risk of perioperative mortality and morbidity, and anesthesiologists may have difficulty maintaining stable hemodynamic status [1]. Owing to perioperative and postoperative adverse events, it is imperative to exercise caution when patients with heart failure undergo surgery under general anesthesia. However, there are no high-grade recommendations for anesthetic practice in patients with reduced left ventricular ejection fraction (LVEF) [2]. Commonly used intravenous and volatile anesthetics should be administered carefully because of their potential cardiovascular depressant effects, which can lead to severe hypotension and bradycardia, ultimately resulting in cardiovascular collapse in patients with heart failure [1].
Remimazolam (Byfavo; Hana Pharm Co., Ltd.) is an ultrashort-acting benzodiazepine used for sedation and general anesthesia [3]. Reportedly, remimazolam causes fewer cardiovascular depressant effects than conventional propofol induction and inhalational agent maintenance in general anesthesia. However, previous clinical trials did not include patients with severe heart failure, including those undergoing cardiac surgery [3]. Herein, we present three cases in which remimazolam was used to induce and maintain general anesthesia in patients with reduced heart function undergoing both non-cardiac and cardiac surgeries, including cardiopulmonary bypass (CPB).
Ethical statements: This study was approved by the Institutional Review Board of Pusan National University Yangsan Hospital (IRB No. 05-2023-124), which waived the requirement for informed consent given its retrospective nature.
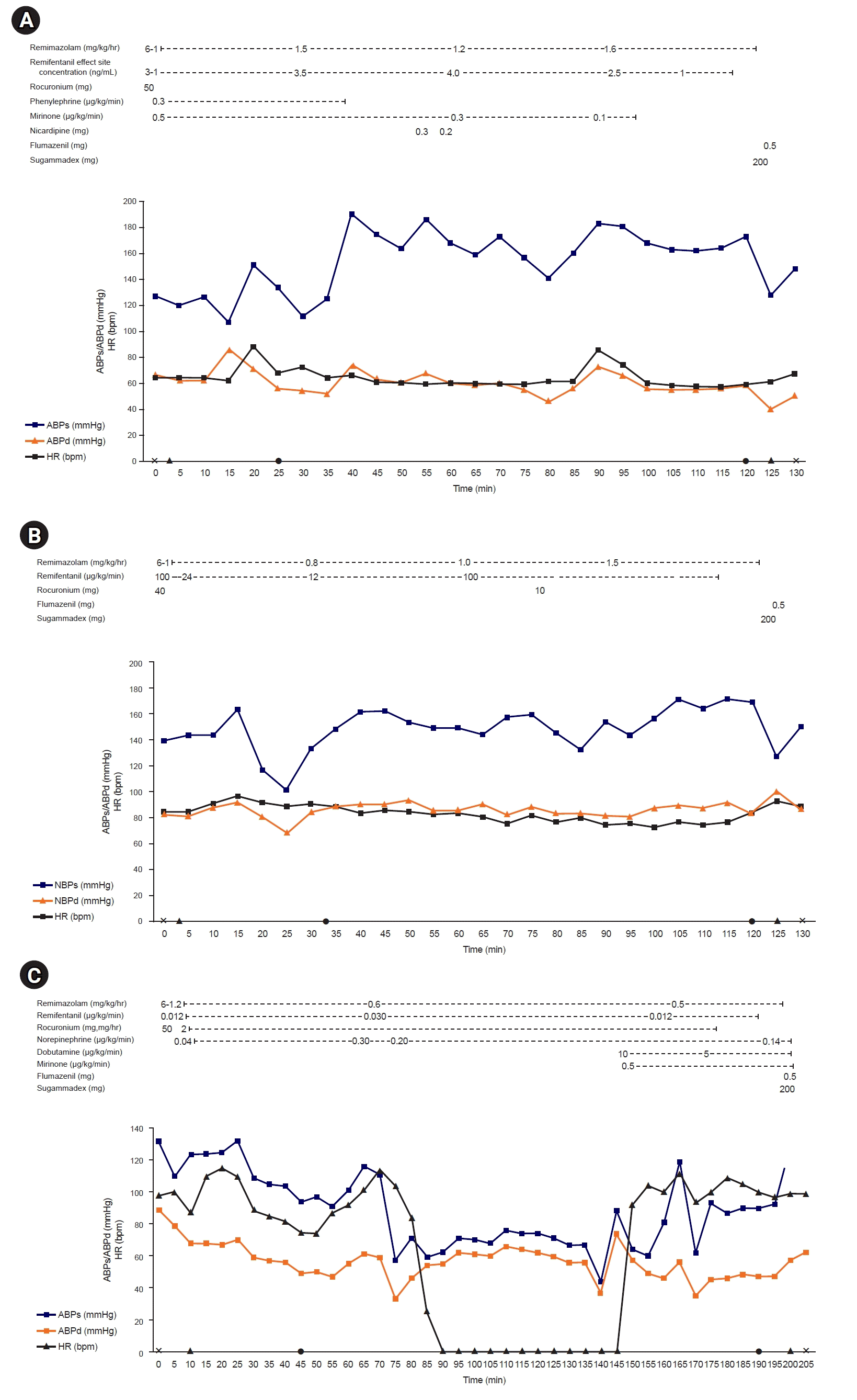
A 75-year-old man (height: 165 cm; weight: 54 kg) with a history of diabetes mellitus presented to the emergency room with closed tibia and fibula fractures caused by a slip-and-fall incident. Preoperative transthoracic echocardiography (TTE) revealed left ventricular (LV) systolic dysfunction with an ejection fraction (EF) of 35%, possibly due to an old myocardial infarction affecting the anteroseptal and lateral walls. Additionally, mild aortic stenosis was noted, with a transaortic valve maximum velocity (Vmax) of 2.25 m/sec and peak and mean pressure gradients of 20/11 mmHg. Open reduction and internal fixation of the tibia and the fibula were scheduled. After the patient entered the operating room, routine monitoring, including noninvasive blood pressure (BP), electrocardiography, percutaneous oxygen saturation (SpO2), and bispectral index (BIS), was performed. The vital signs before anesthesia induction were as follows: BP, 126/62 mmHg; heart rate (HR), 65 beats per minute; and SpO2, 100%. Remimazolam was administered at 6 mg/kg/hr with remifentanil in a target-controlled infusion mode. Once the patient lost consciousness, the infusion rate of remimazolam was adjusted to 1–2 mg/kg/hr to maintain a BIS value between 40–60. Rocuronium 1 m/kg was administered, and intubation was successfully performed. A radial artery catheter and large-bore peripheral line were inserted. Milrinone was started at a dose of 0.5 μg/kg/min to support LV systolic function and, phenylephrine was initiated at 0.3 μg/kg/min to prevent vasoplegia, but was later discontinued due to hypertension. Vital signs remained stable throughout the operation, and no additional vasopressors were required. Transient hypertension during the operation was managed with nicardipine boluses (0.3 mg and 0.2 mg). Upon the end of the operation, flumazenil (0.5 mg, Flunil; Bukwang Pharm Co., Ltd.), and sugammadex (200 mg, Bridion; Merck & Co., Inc.) were given (Fig. 1A). The patient was successfully extubated and transferred to the post-anesthesia care unit without any complications.
A 53-year-old man (height: 168.7 cm; weight: 67 kg) with a history of diabetes mellitus, end-stage renal disease on peritoneal dialysis, and three-vessel coronary artery occlusive disease with percutaneous stents inserted in the right coronary, left anterior descending, and left circumflex arteries presented to the emergency room with a bimalleolar ankle fracture. Preoperative TTE showed LV akinesia from the mid to basal posterolateral wall and middle inferior wall with moderate LV systolic dysfunction and an EF of 39%, attributed to ischemic heart failure. The patient was scheduled to undergo open reduction and internal fixation of the bimalleolar fracture. Routine monitoring of noninvasive BP, electrocardiography, percutaneous oxygen saturation, and BIS was performed. The initial vital signs before anesthesia induction were as follows: BP, 140/83 mmHg; HR, 85 beats per minute; and SpO2, 98%. Remimazolam was administered at a dose of 6 mg/kg/hr with remifentanil infusion. Once the patient became unconscious, the remimazolam dose was adjusted to 1–2 mg/kg/hr to maintain a BIS value between 40–60. Rocuronium 0.7 m/kg was administered, and intubation was successfully performed. Vital signs remained stable throughout the operation, and no additional vasopressors or inotropes were required. At the end of the surgery, flumazenil (0.5 mg) was administered along with sugammadex (100 mg) (Fig. 1B). The patient was extubated and transferred to the post-anesthesia care unit without complications.
A 64-year-old man (height: 170 cm; weight: 65 kg) with hypertension, dyslipidemia, stage 2 chronic kidney disease, and severe aortic stenosis underwent elective aortic valve replacement with thoracotomy for minimally invasive cardiac surgery. The patient presented with symptoms of dyspnea that had progressed over 3 months before the operation, reaching New York Heart Association class IV. Preoperative TTE revealed severe degenerative aortic stenosis with a transaortic Vmax of 5.3 m/sec, peak and mean pressure gradients of 111/59 mmHg, and mild aortic regurgitation. While there was no definite regional wall motion abnormality, severe LV systolic dysfunction was evident, with an EF of 30%. Moderate resting pulmonary hypertension and right ventricular dysfunction were also observed. Before the operation, a radial artery catheter was inserted for continuous arterial BP monitoring. After standard monitoring, remimazolam was initiated at 6 mg/kg/hr along with remifentanil infusion. When the patient became unconscious, the infusion rate of remimazolam was adjusted to 1–2 mg/kg/hr to maintain a patient-state index between 25–50. Rocuronium (0.8 mg/kg) was administered for intubation, and norepinephrine was administered to maintain BP. Surgery commenced, and remimazolam was continuously infused to maintain a patient-state index between 25–50, even during CPB. Dobutamine and milrinone were both started at a dose of 0.5 μg/kg/min after the aortic cross-clamp was released to support cardiac function. At the end of the surgery, sugammadex (200 mg) and flumazenil (0.5 mg) were administered, and extubation was performed in the operating room (Fig. 1C). The patient was transferred to the intensive care unit without postoperative complications. The following day, the patient was transferred to the general ward and discharged from the hospital on postoperative day 4.
Patients with reduced LVEF have risk for intraoperative hypotension and bradycardia [4]. In the presence of reduced LVEF, systolic BP, and pulse pressure are dependent on LV stroke volume, while mean arterial pressure and diastolic BP are dependent on the degree of peripheral vasodilatation [5]. Many anesthetic agents decrease myocardial contractility and systemic vascular resistance, resulting in hypotension and reduced coronary perfusion. This can be particularly risky for patients with reduced EF. While anesthetic goals in patients with advanced heart failure and reduced EF should focus on avoiding hypotension to maintain coronary perfusion and cardiac output, it is worth noting that there are no definitive anesthetic guidelines for patients with heart failure [1]. In our three cases, although cardiac output was not continuously monitored during surgery, the patients remained hemodynamically stable under remimazolam anesthesia, with the use of relatively low doses of vasopressors and inotropic agents.
Propofol is commonly used to induce and maintain anesthesia. However, through direct activation of the chloride channel of GABAA receptors, propofol rapidly inhibits cardiac contractility and reduces preload due to vasodilation. This can lead to fatal circulatory failure. However, benzodiazepines induce a mild allosteric response that increases the affinity of GABA for the receptor, and therefore have relatively less vasodilatory and myocardial depressant effects [6]. Remimazolam, whose effect can be reversed by flumazenil, causes less hypotension during general anesthesia than during conventional inhalational anesthesia [3]. This effect could be particularly valuable in patients with severe heart failure or those undergoing cardiac surgery. However, few case reports have documented the use of remimazolam in cardiac surgery and in patients with heart failure [7,8].
In a study by Nakanishi et al. [9], when remimazolam was administered at a rate of 6 mg/kg/hr to induce anesthesia, the mean arterial pressure decreased slightly until consciousness was lost. Furthermore, in a study involving healthy patients, when the administration rate of remimazolam was adjusted to 5 mg/min for 5 minutes, 3 mg/min for the next 15 minutes, and 1 mg/min for an additional 15 minutes, the average arterial pressure decreased by approximately 24% [10]. The induction dose of remimazolam is generally 12 mg/kg/hr, but successful anesthesia induction is possible with 6 mg/kg/hr; therefore, a lower dose is recommended for the elderly or patients with American Society of Anesthesiologists class 3 or higher [11]. In our cases, we used a dose of 6 mg/kg/hr for induction, and observed no adverse events, such as anesthesia recall or induction failure. Similarly, there are other case reports on the use of remimazolam in patients with heart failure and severe aortic stenosis undergoing cardiac surgery using a dose of 6 mg/kg/hr without complications [7,8]. However, further studies are required to determine a suitable induction dose for patients with reduced EF.
Despite these hemodynamic advantages, there may be concerns about the occurrence of awareness due to a slightly high BIS during general anesthesia using remimazolam. The range of the BIS index for maintaining an appropriate depth of anesthesia when using remimazolam is approximately 60–70; therefore, the appropriate range is unclear [12]. However, according to a study by Liu et al. [13], hemodynamic stability can be maintained even by maintaining a BIS of 40–60 under remimazolam anesthesiaFormatting.... Remimazolam has the advantage of being predictable in anesthesia induction and emergence due to its rapid onset of action and short contact-sensitive half-life. However, delayed awakening of more than 30 minutes has been reported to occur in approximately 8% of cases [14]. Flumazenil can be used to antagonize remimazolam; however, caution must be exercised against rapid increases in BP, as vascular resistance may rapidly increase [8].
In conclusion, our three cases demonstrate that remimazolam can be safely used for general anesthesia in patients with heart failure and reduced EF for hemodynamic stability. However, BIS monitoring for awareness prevention and careful use of flumazenil during reversal are necessary. Clinical trials involving these patient populations are also required to establish clear dosage guidelines.
Notes
References
1. Smit-Fun V, Buhre WF. The patient with chronic heart failure undergoing surgery. Curr Opin Anaesthesiol. 2016; 29:391–6.
2. Maile MD, Mathis MR, Jewell ES, Mentz GB, Engoren MC. Identification of intraoperative management strategies that have a differential effect on patients with reduced left ventricular ejection fraction: a retrospective cohort study. BMC Anesthesiol. 2022; 22:288.
3. Doi M, Hirata N, Suzuki T, Morisaki H, Morimatsu H, Sakamoto A. Safety and efficacy of remimazolam in induction and maintenance of general anesthesia in high-risk surgical patients (ASA Class III): results of a multicenter, randomized, double-blind, parallel-group comparative trial. J Anesth. 2020; 34:491–501.
4. Cheung CC, Martyn A, Campbell N, Frost S, Gilbert K, Michota F, et al. Predictors of intraoperative hypotension and bradycardia. Am J Med. 2015; 128:532–8.
5. Tartiere JM, Logeart D, Safar ME, Cohen-Solal A. Interaction between pulse wave velocity, augmentation index, pulse pressure and left ventricular function in chronic heart failure. J Hum Hypertens. 2006; 20:213–9.
6. Kanaya N, Murray PA, Damron DS. The differential effects of midazolam and diazepam on intracellular Ca2+ transients and contraction in adult rat ventricular myocytes. Anesth Analg. 2002; 95:1637–44.
7. Satoh T, Nishihara N, Sawashita Y, Ohno S, Hirata N, Yamakage M. Remimazolam anesthesia for MitraClip implantation in a patient with advanced heart failure. Case Rep Anesthesiol. 2021; 2021:5536442.
8. Furuta M, Ito H, Yamazaki M. Anaesthetic management using remimazolam in a patient with severe aortic stenosis: a case report. BMC Anesthesiol. 2021; 21:202.
9. Nakanishi T, Sento Y, Kamimura Y, Tsuji T, Kako E, Sobue K. Remimazolam for induction of anesthesia in elderly patients with severe aortic stenosis: a prospective, observational pilot study. BMC Anesthesiol. 2021; 21:306.
10. Schüttler J, Eisenried A, Lerch M, Fechner J, Jeleazcov C, Ihmsen H. Pharmacokinetics and pharmacodynamics of remimazolam (CNS 7056) after continuous infusion in healthy male volunteers: part I. pharmacokinetics and clinical pharmacodynamics. Anesthesiology. 2020; 132:636–51.
11. Lohmer LL, Schippers F, Petersen KU, Stoehr T, Schmith VD. Time-to-event modeling for remimazolam for the indication of induction and maintenance of general anesthesia. J Clin Pharmacol. 2020; 60:505–14.
12. Antonik LJ, Goldwater DR, Kilpatrick GJ, Tilbrook GS, Borkett KM. A placebo-and midazolam-controlled phase I single ascending-dose study evaluating the safety, pharmacokinetics, and pharmacodynamics of remimazolam (CNS 7056): part I. safety, efficacy, and basic pharmacokinetics. Anesth Analg. 2012; 115:274–83.
13. Liu T, Lai T, Chen J, Lu Y, He F, Chen Y, et al. Effect of remimazolam induction on hemodynamics in patients undergoing valve replacement surgery: a randomized, double-blind, controlled trial. Pharmacol Res Perspect. 2021; 9:e00851.
14. Doi M, Morita K, Takeda J, Sakamoto A, Yamakage M, Suzuki T. Efficacy and safety of remimazolam versus propofol for general anesthesia: a multicenter, single-blind, randomized, parallel-group, phase IIb/III trial. J Anesth. 2020; 34:543–53.
Fig. 1.
Changes in hemodynamic status under general anesthesia. (A) Case 1. (B) Case 2. (C) Case 3. ABPs, systolic artery blood pressure; ABPd, diastolic artery blood pressure; bpm, beats per minute; HR, heart rate; ⅹ, beginning or ending of anesthesia; ▲, endotracheal intubation or extubation; ●, start or end of surgery.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download