Abstract
Percutaneous sacroplasty is mainly used as an intervention for pain associated with sacral insufficiency fractures or sacral metastatic tumors. However, sacroplasty for managing the pain associated with direct sacral invasion of rectal cancer has been rarely reported. We present a case of a 74-year-old patient who underwent sacroplasty via the interpedicular approach under fluoroscopic guidance to relieve pain resulting from direct tumor invasion into the S3 body. After the procedure, the patient experienced immediate pain relief and did not feel worse pain with ambulation. Aside from peritumoral vascular leakage, no other significant complications occurred immediately post-procedure. Our results suggest that fluoroscopically guided interpedicular sacroplasty is a safe and effective option for relieving the pain associated with direct sacral invasion by rectal cancer.
Bone invasion of the tumor in the sacrum usually produces pain due to tumor burden, fracture, instability, and neural compression [1]. Pain is a common symptom of sacral tumor invasion and usually occurs in the rectal or perineal region. The direct sacral invasion of rectal cancer is uncommon. In a study of pelvic recurrence, direct sacral invasion accounted for less than 5% of the local recurrence [2]. The two main methods of treating sacral invasion of rectal cancer are radiotherapy and surgical resection. However, the absence of significant complications renders surgical resection difficult, and radiotherapy takes time to relieve pain.
Sacroplasty, cement augmentation of the sacrum, is a safe and effective procedure for treating pain associated with sacral insufficiency fractures and metastatic tumors [3]. Accurate needle placement within the lesion is crucial when performing sacroplasty to treat pain caused by a sacral tumor. Several approaches can be adopted to achieve accurate needle placement to the lesion without complication, but the methods for effectively treating tumor lesions in the lower sacral body are limited. The interpedcular approach is a relatively recently introduced method in which the needle is advanced from the sacral hiatus through the sacral body parallel to the anterior edge of the sacrum, providing easy access to the sacral body lesion [4].
However, there are few documented cases of sacroplasty for the treatment of pain caused by rectal cancer directly invading the lower sacral body. We describe a case of sacroplasty performed using the interpedicular approach to manage pain caused by direct invasion of rectal cancer into the S3 body under fluoroscopic guidance, which resulted in immediate pain relief.
Ethical statements: This study was exempted from review by the Institutional Review Board of Kyungpook National University Chilgok Hospital (IRB No. 2023-09-013). Written informed consent was obtained from the patients to participate in the study.
A 74-year-old woman with direct sacral invasion of rectal cancer was referred to our department due to symptoms of intractable perineal pain. She underwent transanal excision followed by neoadjuvant chemoradiotherapy. However, the patient reported escalating pain after neoadjuvant chemoradiotherapy, which scored 10 on the numeric rating scale (NRS), describing it as dull, lancinating, and similar to an electric shock. The pain worsened with ambulation.
Subsequent magnetic resonance imaging (MRI) and computed tomography (CT) scans of the lumbar spine showed rectal cancer invasion into the S3 body; no other lesions causing pain were found (Fig. 1). After consultation with the surgeon, the patient did not agree to undergo surgical tumor resection and receive additional radiotherapy. She felt that the previous radiotherapy had not been effective and thus preferred immediate pain relief. The initial intervention was a caudal epidural steroid injection, which reduced the NRS score to 6. However, the patient complained of persisting pain and wanted additional management. After consultation with her family and obtaining informed consent from her, we decided to perform percutaneous sacroplasty to relieve the pain caused by the direct sacral invasion of rectal cancer.
Prophylactic antibiotic therapy (1 g of cefazolin sodium; Yuhan Corp.) was intravenously administered 30 minutes before the procedure. Before the procedure, the patient's MRI and CT images were carefully reviewed, and the depth from the skin surface to the center of the lesion was determined for the interpedicular approach.
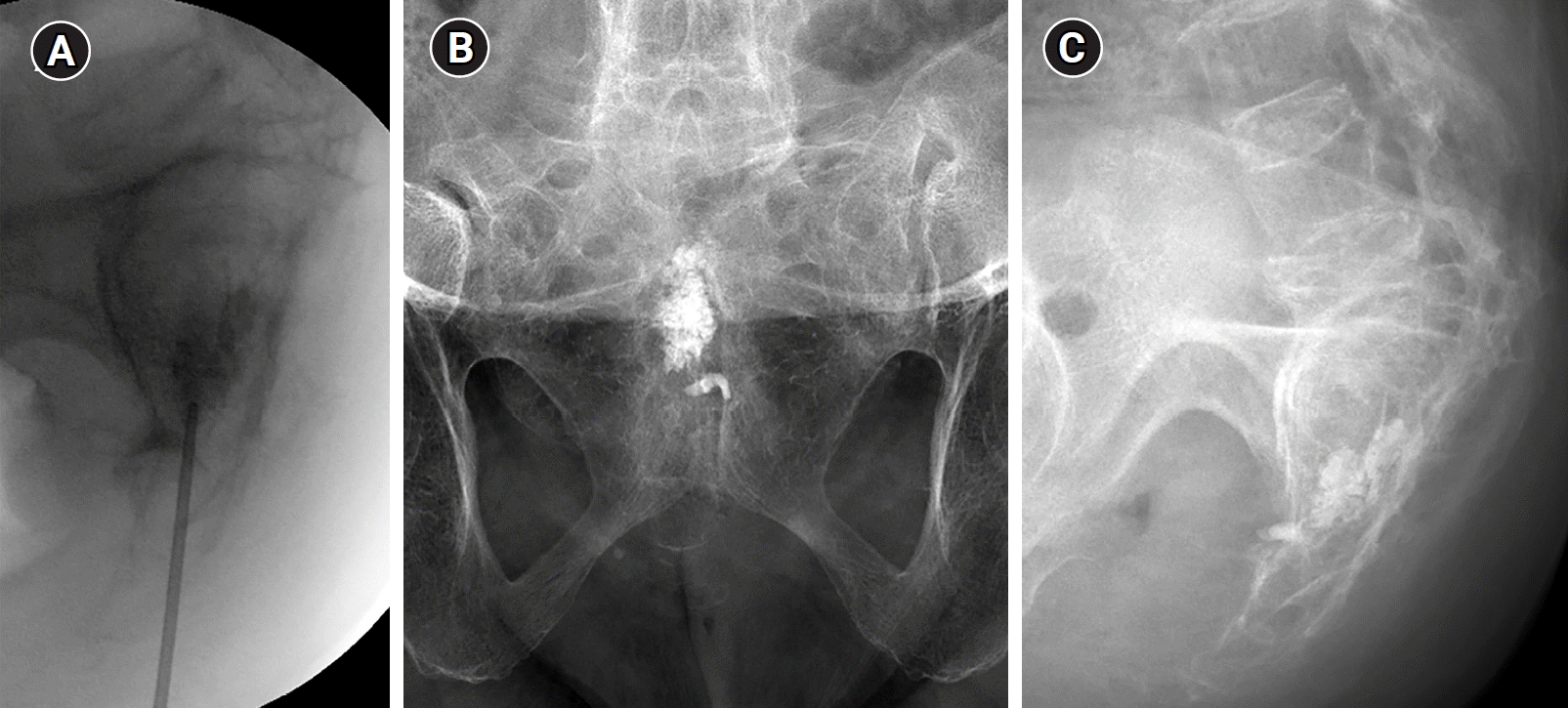
The patient was placed in the prone position to ensure relaxation. The sacrococcygeal region was carefully prepped with 10% povidone-iodine solution, and sterile draping was employed to maintain asepsis. After applying 2 mL of 1% lidocaine to the skin and subcutaneous tissue of the sacral hiatus to induce local anesthesia, a precise incision was made at the sacral hiatus using a scalpel. A 13-gauge bevel-edge needle (Manan Medical Products) was then inserted into the skin incision and subcutaneous tissue to reach the periosteum of the bone. After penetrating the posterior wall of the sacral canal, the needle was advanced to the anterior wall of the sacral canal, corresponding to the level of S4 body. Subsequently, the cortex was punctured, and the needle was carefully advanced through the bone until it reached the center of the lesion at the S3 body, maintaining an orientation parallel to the anterior edge of the sacrum. The needle was adjusted according to the sagittal and axial CT and MRI images to ensure accurate needle placement within the lesion under fluoroscopic guidance in multiple projections. After stylet removal, radiopaque contrast media was injected to obtain a tumor venogram, and the needle tip was finely adjusted to minimize peritumoral venous leakage. Polymethyl methacrylate (PMMA) (Exolent Spine, Elmdown LTD) was carefully injected into the lesion under fluoroscopic guidance in both the lateral and anterior-posterior views to ensure complete filling of the lesion and prevent PMMA leakage. Then, 2 mL of PMMA was carefully injected, and subsequent follow-up assessments confirmed optimal lesion filling without significant PMMA leakage to the sacral foramen or canal. Cement injection did not induce clinically significant complications or adverse side effects (Fig. 2).
After the procedure, the patient experienced substantial and immediate pain relief, as evidenced by an NRS score of 2, and did not have worse pain with ambulation on the following day; thus, the patient was eventually discharged. This analgesic effect was maintained until a 4-month follow-up.
Sacroplasty, a variant of vertebroplasty, was first introduced in 2000 for the treatment of pain related to sacral metastatic tumors [5]. However, sacroplasty mainly treats osteoporotic insufficiency fracture pain [6]. Several studies have reported that sacroplasty is effective in treating pain related to sacral metastatic tumors [3,7,8]. However, there is little evidence of the efficacy of sacroplasty for pain relief in cases of direct sacral invasion of rectal cancer.
Percutaneous sacroplasty is more complicated than percutaneous vertebroplasty owing to its pyramidal and convex shape and porous structure, the difficulty in visualizing the anterior sacral cortical margin with fluoroscopy, poorly defined fluoroscopic landmarks for bone needle placement, and the risk of sacral nerve root or spinal canal compromise due to cement migration. Nerve root preservation is crucial in sacroplasty. Injury to bilateral L5 nerve roots typically affects ambulatory function, whereas injury to the S2 and S3 nerve roots affects bowel and bladder function [8].
Several approaches can be used for sacroplasty, including posterior (long and short axes), transiliac (lateral), anterior-oblique, and interpedicular approaches. The majority of these techniques were initially developed to access sacral ala lesions [9].
The lateral and anterior-oblique approaches can be employed for sacral body lesions. However, the lateral approach requires passage through the sacroiliac joint and sacral neural foramina zone. It carries a potential risk for nerve root and iliac bone damage, hinders clear visualization, and makes the identification of anatomical landmarks under lateral fluoroscopy difficult.
Similarly, although capable of providing access to the sacral body, the anterior-oblique approach presents challenges when approaching the lower sacral vertebral body due to the curved ventral cortical margin of the sacrum. It also carries a risk of sacral nerve root injury [8,10].
In the present case, we opted for the interpedicular approach, which avoids passage through the sacral neural foramina zone, minimizes the likelihood of ventral cortical sacral wall breakage, and allows for adequate fluoroscopic visualization of the S3 body [7]. We performed sacroplasty without damage to the sacral roots and visceral organs and significant extravasation. After the procedure, the patient immediately experienced pain relief and could ambulate without increasing pain.
The recommended volume of PMMA filling for the cervical, thoracic, and lumbar spine is 2.5, 5.5, and 7.0 mL, respectively [11]. Despite the increasing use of sacroplasty, a recommended volume for the procedure remains undefined. Kortman et al. [9] reported an average bone cement volume of 4.1 mL for sacroplasty, Frey et al. [12] documented a range of 2–5 mL, while Moussazadeh et al. [13] used an average of 6.4 mL for sacroplasty. In a cadaver study, the volume of injected PMMA in sacroplasty does not affect the strength and stiffness of bone [14]. Therefore, injecting an appropriate amount of cement without producing cement leakage, which causes nerve root and lumbosacral plexus damage, is essential.
Pain is a manifestation of neoplastic disease of the sacrum when the lesion causes either bony destruction or neuronal invasion and compression. Bony destruction activates pain receptors of the periosteum. The neurotoxic effect of PMMA and exothermic reaction during cement polymerization are suggested to induce periosteal denervation [15]. Cement injection for internal reinforcement of the trabecular bone can potentially stabilize microfractures within the sacrum effectively [16]. Furthermore, cement injection could induce tumor necrosis and reduce tumor volume, which further contributes to pain mitigation [16].
Pain from neural involvement is typically radicular and radiates along the S1 dermatome if the upper sacrum is involved or in the perineal region if the lower sacral nerve roots are involved. Numbness and paresthesia over the perineum, sacrum, and sole may also be observed with sacral nerve root or sacral plexus compression. In the present case, the patient did not report numbness or radicular pain, and imaging studies revealed no evidence of neuronal involvement. Therefore, sacroplasty was performed to relieve pain.
The pain reduction after sacroplasty seems to be favorable. Andresen et al. [17] demonstrated a sustained reduction in pain for up to 6 months after surgery, and Moussazadeh et al. [13] reported a significant reduction in pain in 80% of patients over an average follow-up of 6.5 months. These reports suggest that sacroplasty provides a favorable reduction in pain compared to radiotherapy, where the reported efficacy in reducing pain is typically 30% to 40% [18].
CT has an advantage over fluoroscopy for accurately placing needle tips in sacroplasty. However, CT does not always provide real-time monitoring during cement injection [4]. Fluoroscopy-only guided sacroplasty has difficulty visualizing sacral foramina [8]. Zhang et al. [8] suggested combining CT and fluoroscopy is the best alternative. However, combining CT and fluoroscopy increases procedure time and radiation exposure. Previous interpedicular approaches of sacroplasty were performed only fluoroscopy guided without complications [4,8]. So, we did sacroplasty using fluoroscopic guidance only.
The two main treatment methods for sacral invasion of rectal cancer are en bloc sacrectomy and palliative radiotherapy. En bloc sacrectomy as part of pelvic exenteration [19]. This method can increase disease-free survival by up to 33 months. However, it involves longer operation time and is associated with significant blood loss (ranging from 1 to 7.5 L) and increased morbidity, with over 50% of patients reporting at least one major complication, such as pelvic abscess, sacroiliac joint instability, neurological complications of the lower body, or intractable pelvic girdle pain [19]. Owing to the high morbidity rate and significant risks of complications associated with the en bloc sacrectomy, the patient and her family decided against the procedure after consulting the surgeon.
Palliative radiotherapy is an alternative treatment for sacral tumor invasion. Radiotherapy is indicated in patients without spinal instability or acute neurological deterioration. However, it takes time for pain relief to manifest, and radiosensitivity varies among tumor types [20]. Our patient previously received radiotherapy, did not want additional radiotherapy, and complained of severe pain. Thus, we initially considered sacroplasty.
To the best of our knowledge, this is the first report to present percutaneous sacroplasty as an effective procedure for alleviating pain in the case of sacral invasion of rectal cancer. In this case, the interpedicular approach in percutaneous sacroplasty successfully relieved pain without causing significant complications.
In conclusion, sacroplasty using the interpedicular approach for managing pain related to direct sacral invasion of rectal cancer is a safe procedure and can alleviate pain without causing complications.
References
1. Raque GH Jr, Vitaz TW, Shields CB. Treatment of neoplastic diseases of the sacrum. J Surg Oncol. 2001; 76:301–7.
2. Sinaei M, Swallow C, Milot L, Moghaddam PA, Smith A, Atri M. Patterns and signal intensity characteristics of pelvic recurrence of rectal cancer at MR imaging. Radiographics. 2013; 33:E171–87.
3. Tarawneh AM, Sabou S, AlKalbani S, Pasku D, Quraishi NA. Clinical outcomes of sacroplasty for metastatic sacral tumours: a systematic review and meta-analysis. Eur Spine J. 2020; 29:3116–22.
4. Fırat AK, Gumus B, Kaya E, Kuku I, Harma A. Interpedicular approach in percutaneous sacroplasty for treatment of sacral vertebral body pathologic fractures. Cardiovasc Intervent Radiol. 2011; 34 Suppl 2:S282–7.
5. Dehdashti AR, Martin JB, Jean B, Rufenacht DA. PMMA cementoplasty in symptomatic metastatic lesions of the S1 vertebral body. Cardiovasc Intervent Radiol. 2000; 23:235–7.
6. Gupta AC, Chandra RV, Yoo AJ, Leslie-Mazwi TM, Bell DL, Mehta BP, et al. Safety and effectiveness of sacroplasty: a large single-center experience. AJNR Am J Neuroradiol. 2014; 35:2202–6.
7. Tian QH, Liu HF, Wang T, Cheng YS, Wu CG. Percutaneous sacroplasty for painful sacral metastases involving multiple sacral vertebral bodies: initial experience with an interpedicular approach. Korean J Radiol. 2019; 20:939–46.
8. Zhang J, Wu CG, Gu YF, Li MH. Percutaneous sacroplasty for sacral metastatic tumors under fluoroscopic guidance only. Korean J Radiol. 2008; 9:572–6.
9. Kortman K, Ortiz O, Miller T, Brook A, Tutton S, Mathis J, et al. Multicenter study to assess the efficacy and safety of sacroplasty in patients with osteoporotic sacral insufficiency fractures or pathologic sacral lesions. J Neurointerv Surg. 2013; 5:461–6.
10. Jayaraman MV, Chang H, Ahn SH. An easily identifiable anatomic landmark for fluoroscopically guided sacroplasty: anatomic description and validation with treatment in 13 patients. AJNR Am J Neuroradiol. 2009; 30:1070–3.
11. Cotten A, Dewatre F, Cortet B, Assaker R, Leblond D, Duquesnoy B, et al. Percutaneous vertebroplasty for osteolytic metastases and myeloma: effects of the percentage of lesion filling and the leakage of methyl methacrylate at clinical follow-up. Radiology. 1996; 200:525–30.
12. Frey ME, Warner C, Thomas SM, Johar K, Singh H, Mohammad MS, et al. Sacroplasty: a ten-year analysis of prospective patients treated with percutaneous sacroplasty: literature review and technical considerations. Pain Physician. 2017; 20:E1063–72.
13. Moussazadeh N, Laufer I, Werner T, Krol G, Boland P, Bilsky MH, et al. Sacroplasty for cancer-associated insufficiency fractures. Neurosurgery. 2015; 76:446–50.
14. Richards AM, Mears SC, Knight TA, Dinah AF, Belkoff SM. Biomechanical analysis of sacroplasty: does volume or location of cement matter? AJNR Am J Neuroradiol. 2009; 30:315–7.
15. Belkoff SM, Molloy S. Temperature measurement during polymerization of polymethylmethacrylate cement used for vertebroplasty. Spine (Phila Pa 1976). 2003; 28:1555–9.
16. O'Brien JP, Sims JT, Evans AJ. Vertebroplasty in patients with severe vertebral compression fractures: a technical report. AJNR Am J Neuroradiol. 2000; 21:1555–8.
17. Andresen R, Radmer S, Ludtke CW, Kamusella P, Wissgott C, Schober HC. Balloon sacroplasty as a palliative pain treatment in patients with metastasis-induced bone destruction and pathological fractures. Rofo. 2014; 186:881–6.
18. Chow E, Wong R, Hruby G, Connolly R, Franssen E, Fung KW, et al. Prospective patient-based assessment of effectiveness of palliative radiotherapy for bone metastases. Radiother Oncol. 2001; 61:77–82.
19. Sasikumar A, Bhan C, Jenkins JT, Antoniou A, Murphy J. Systematic review of pelvic exenteration with en bloc sacrectomy for recurrent rectal adenocarcinoma: R0 resection predicts disease-free survival. Dis Colon Rectum. 2017; 60:346–52.
20. Quraishi NA, Giannoulis KE, Edwards KL, Boszczyk BM. Management of metastatic sacral tumours. Eur Spine J. 2012; 21:1984–93.
Fig. 1.
(A) Axial computed tomography image showing direct sacral invasion of rectal cancer. (B) Sagittal magnetic resonance image showing direct tumor invasion into the S3 vertebral body. Open arrows indicate rectal cancer invasion.
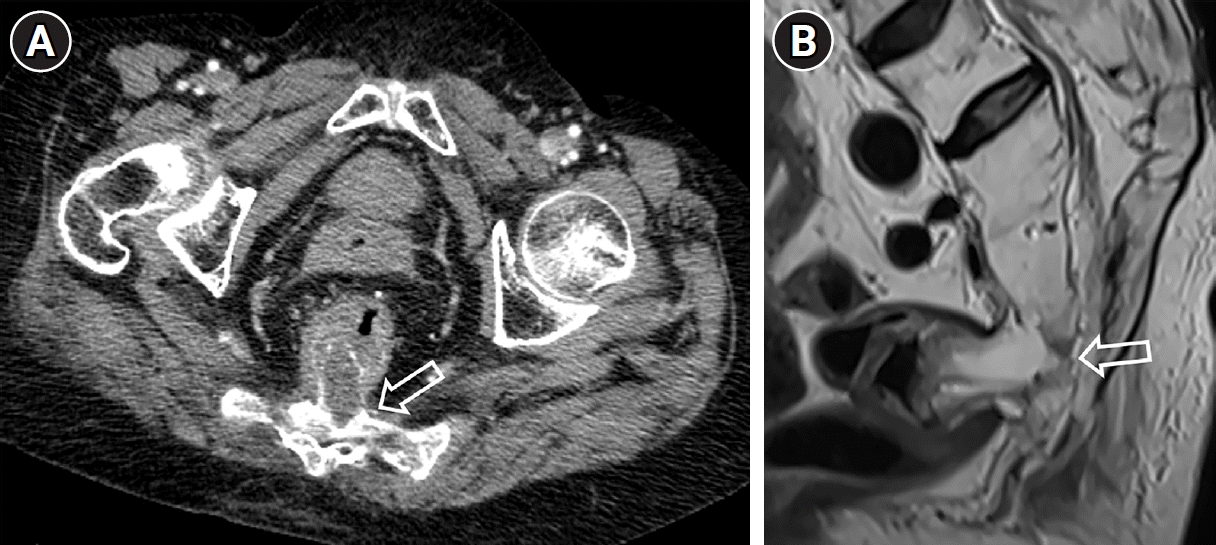
Fig. 2.
(A) Fluoroscopic image showing precise needle placement using the interpedicular approach, with the needle tip positioned within the S3 body. Posttreatment frontal (B) and lateral (C) images show the presence of polymethyl methacrylate cement within the S3 body, accompanied by minimal presacral extravasation.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download