1. Bloomgarden ZT. Diabetes complications. Diabetes Care. 2004; 27(6):1506–1514. PMID:
15161810.

2. Knapp S. Diabetes and infection: is there a link?--A mini-review. Gerontology. 2013; 59(2):99–104. PMID:
23182884.
3. Casanova L, Hughes FJ, Preshaw PM. Diabetes and periodontal disease: a two-way relationship. Br Dent J. 2014; 217(8):433–437. PMID:
25342350.

4. Holt RI, de Groot M, Golden SH. Diabetes and depression. Curr Diab Rep. 2014; 14(6):491. PMID:
24743941.

5. Stöhr J, Barbaresko J, Neuenschwander M, Schlesinger S. Bidirectional association between periodontal disease and diabetes mellitus: a systematic review and meta-analysis of cohort studies. Sci Rep. 2021; 11(1):13686. PMID:
34211029.

6. Graves DT, Ding Z, Yang Y. The impact of diabetes on periodontal diseases. Periodontol 2000. 2020; 82(1):214–224. PMID:
31850631.

7. Genco RJ, Graziani F, Hasturk H. Effects of periodontal disease on glycemic control, complications, and incidence of diabetes mellitus. Periodontol 2000. 2020; 83(1):59–65. PMID:
32385875.

8. Khader YS, Dauod AS, El-Qaderi SS, Alkafajei A, Batayha WQ. Periodontal status of diabetics compared with nondiabetics: a meta-analysis. J Diabetes Complications. 2006; 20(1):59–68. PMID:
16389170.

9. ElSayed NA, Aleppo G, Bannuru RR, Bruemmer D, Collins BS, Cusi K, et al. American Diabetes Association Professional Practice C. 4. comprehensive medical evaluation and assessment of comorbidities: standards of care in diabetes-2024. Diabetes Care. 2024; 47(Suppl 1):S52–S76. PMID:
38078591.
10. Han K, Park JB. Clinical implications of age and sex in the prevalence of periodontitis in Korean adults with diabetes. Exp Ther Med. 2018; 15(4):3865–3873. PMID:
29556264.

11. ElSayed NA, Aleppo G, Aroda VR, Bannuru RR, Brown FM, Bruemmer D, et al. 2. Classification and diagnosis of diabetes: standards of care in diabetes-2023. Diabetes Care. 2023; 46(Suppl 1):S19–S40. PMID:
36507649.
12. Lee DH, Jung KY, Park KS, Kim KM, Moon JH, Lim S, et al. Characterization of patients with type 2 diabetes according to body mass index: Korea National Health and Nutrition Examination Survey from 2007 to 2011. Endocrinol Metab (Seoul). 2015; 30(4):514–521. PMID:
26354494.

13. Choi JH, Lee KA, Moon JH, Chon S, Kim DJ, Kim HJ, et al. 2023 Clinical practice guidelines for diabetes mellitus of the Korean Diabetes Association. Diabetes Metab J. 2023; 47(5):575–594. PMID:
37793979.

14. World Health Organization. Oral Health Surveys: Basic Methods. Geneva, Switzerland: World Health Organization;2013.
15. Hancocks S. Federation Dentaire Internationale. The FDI’s first ten years, 1900–1910. Fédération Dentaire Internationale. Int Dent J. 2000; 50(4):175–183. PMID:
11042817.
16. Cappelli DP. Prevention in Clinical Oral Health Care. Philadelphia, PA, USA: Elsevier Health Sciences;2007.
17. Klein HP, Knutson JW. Dental status and dental needs of elementary school children. Public Health Rep. 1938; 53:751–765.
18. A guide to the UK Adult Dental Health Survey 1998. Br Dent J. 2001; (Spec No):1–56.
19. Sheiham A, Steele JG, Marcenes W, Finch S, Walls AW. The impact of oral health on stated ability to eat certain foods; findings from the National Diet and Nutrition Survey of Older People in Great Britain. Gerodontology. 1999; 16(1):11–20. PMID:
10687504.

20. Kim SW, Han K, Kim SY, Park CK, Rhee CK, Yoon HK. The relationship between the number of natural teeth and airflow obstruction: a cross-sectional study using data from the Korean National Health and Nutrition Examination Survey. Int J Chron Obstruct Pulmon Dis. 2015; 11:13–21. PMID:
26730184.

21. Kim MK, Lee WY, Kang JH, Kang JH, Kim BT, Kim SM, et al. 2014 clinical practice guidelines for overweight and obesity in Korea. Endocrinol Metab (Seoul). 2014; 29(4):405–409. PMID:
25559568.

22. Shin SH, Park J, Cho J, Sin DD, Lee H, Park HY. Severity of airflow obstruction and work loss in a nationwide population of working age. Sci Rep. 2018; 8(1):9674. PMID:
29946117.

23. Azarpazhooh A, Leake JL. Systematic review of the association between respiratory diseases and oral health. J Periodontol. 2006; 77(9):1465–1482. PMID:
16945022.

24. Sacks DB, Arnold M, Bakris GL, Bruns DE, Horvath AR, Lernmark Å, et al. Guidelines and recommendations for laboratory analysis in the diagnosis and management of diabetes mellitus. Diabetes Care. 2023; 46(10):e151–e199. PMID:
37471273.

25. Kim HL, Lee EM, Ahn SY, Kim KI, Kim HC, Kim JH, et al. The 2022 focused update of the 2018 Korean Hypertension Society Guidelines for the management of hypertension. Clin Hypertens. 2023; 29(1):11. PMID:
36788612.

26. Jellinger PS, Handelsman Y, Rosenblit PD, Bloomgarden ZT, Fonseca VA, Garber AJ, et al. American Association of Clinical Endocrinologists and American College of Endocrinology Guidelines for Management of Dyslipidemia and Prevention of Cardiovascular Disease. Endocr Pract. 2017; 23(Suppl 2):1–87.

27. Cekici A, Kantarci A, Hasturk H, Van Dyke TE. Inflammatory and immune pathways in the pathogenesis of periodontal disease. Periodontol 2000. 2014; 64(1):57–80. PMID:
24320956.

28. Latti BR, Kalburge JV, Birajdar SB, Latti RG. Evaluation of relationship between dental caries, diabetes mellitus and oral microbiota in diabetics. J Oral Maxillofac Pathol. 2018; 22(2):282.

29. Lontchi-Yimagou E, Sobngwi E, Matsha TE, Kengne AP. Diabetes mellitus and inflammation. Curr Diab Rep. 2013; 13(3):435–444. PMID:
23494755.

30. Kudiyirickal MG, Pappachan JM. Diabetes mellitus and oral health. Endocrine. 2015; 49(1):27–34. PMID:
25487035.

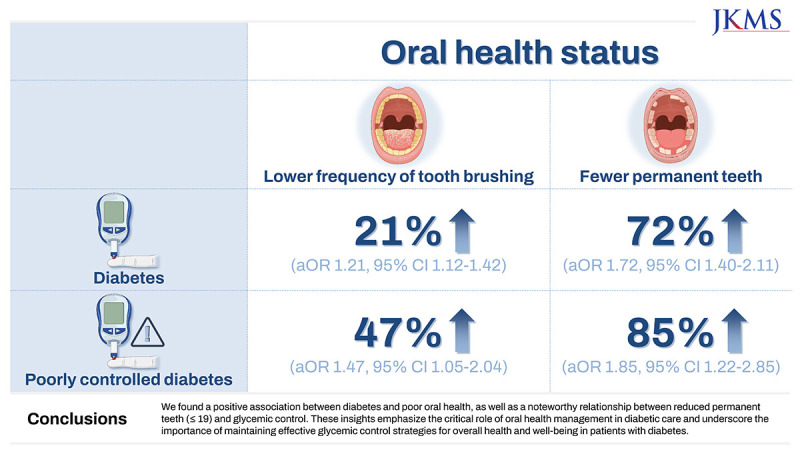
31. Taboza ZA, Costa KL, Silveira VR, Furlaneto FA, Montenegro R Jr, Russell S, et al. Periodontitis, edentulism and glycemic control in patients with type 2 diabetes: a cross-sectional study. BMJ Open Diabetes Res Care. 2018; 6(1):e000453.

32. Zhang Y, Leveille SG, Camhi SM, Shi L. Association of oral care with periodontitis and glycemic control among US adults with diabetes. BMC Oral Health. 2023; 23(1):903. PMID:
37990177.

33. de Lima AK, Amorim Dos Santos J, Stefani CM, Almeida de Lima A, Damé-Teixeira N. Diabetes mellitus and poor glycemic control increase the occurrence of coronal and root caries: a systematic review and meta-analysis. Clin Oral Investig. 2020; 24(11):3801–3812.

34. Chang Y, Lee JS, Lee KJ, Woo HG, Song TJ. Improved oral hygiene is associated with decreased risk of new-onset diabetes: a nationwide population-based cohort study. Diabetologia. 2020; 63(5):924–933. PMID:
32128623.

35. Flemmig TF. Periodontitis. Ann Periodontol. 1999; 4(1):32–38. PMID:
10863373.

36. Newman MG, Takei H, Klokkevold PR, Carranza FA. Carranza’s Clinical Periodontology. Philadelphia, PA, USA: Elsevier Health Sciences;2011.




 PDF
PDF Citation
Citation Print
Print



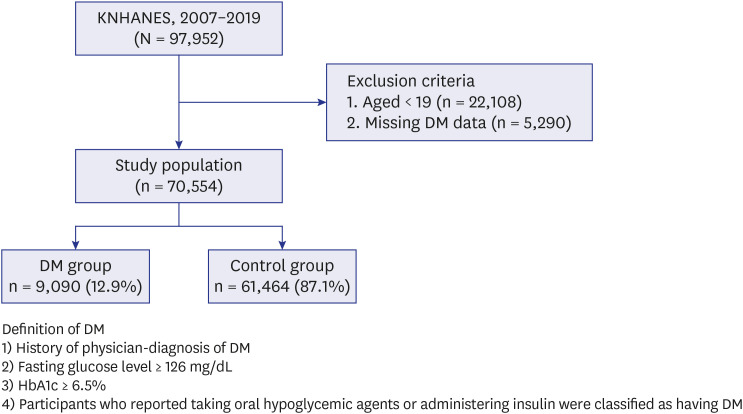
 XML Download
XML Download