Introduction
Acanthamoeba species are ubiquitous, free-living organisms found in the environment. They can cause a sight-threatening cornea disease, termed Acanthamoeba keratitis, and are often misdiagnosed, causing delayed treatment. Although the gold standard diagnosis is amoeba isolation from corneal scraping samples, it is challenging to find evidence of Acanthamoeba in real-world clinical microbiology practice. Herein, we report a case of Acanthamoeba keratitis diagnosed without culture, and describe several diagnostic methods for Acanthamoeba that can be useful in actual clinical microbiology laboratories.
Case report
Patient information: A 12 year-old girl with a history of wearing contact lenses presented with pain, irritation, and hyperemia in the left eye visited the ophthalmology clinic at Asan Medical Center, South Korea.
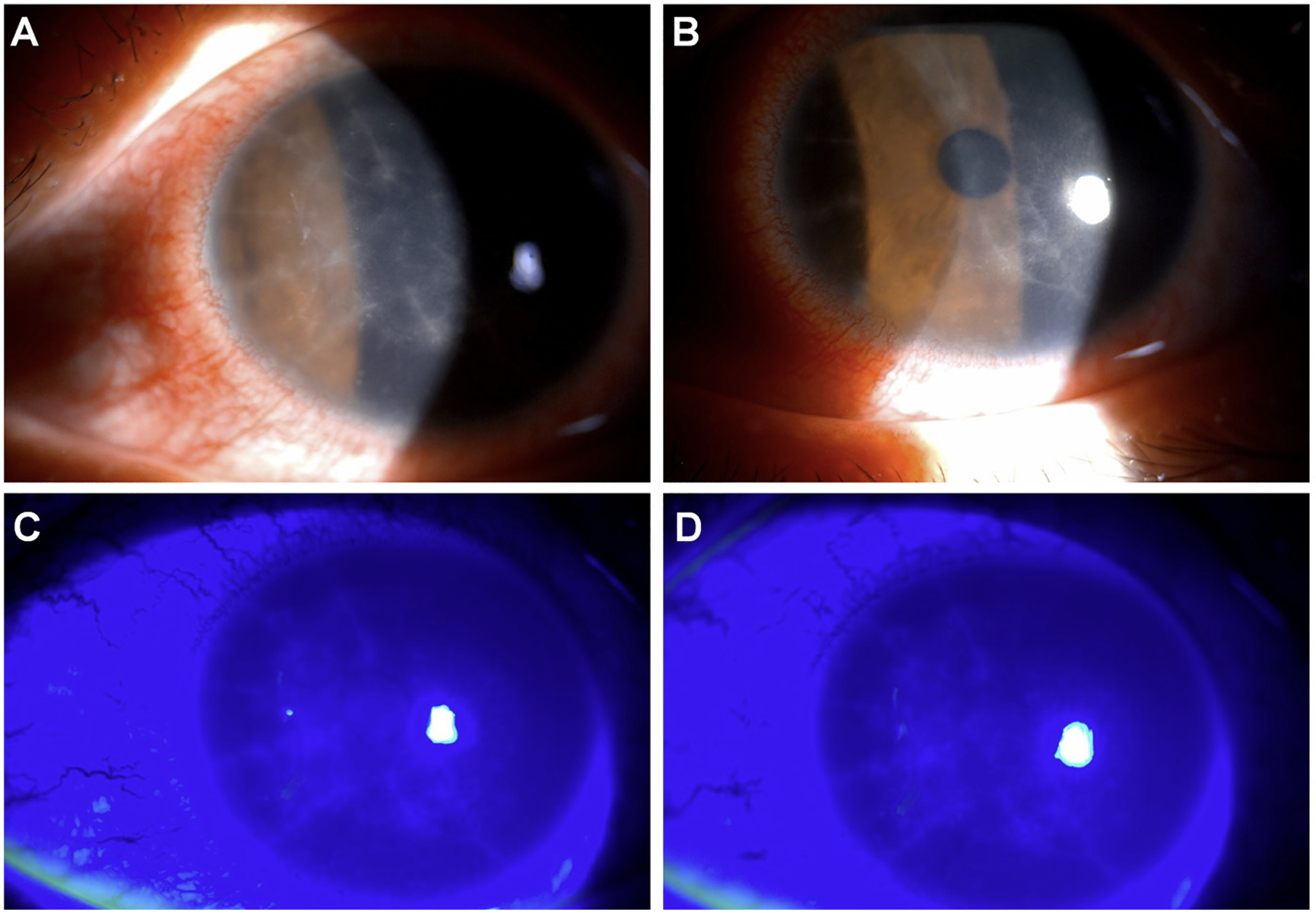
Clinical findings: During slit-lamp microscopic examination of the patient's left eye, radial keratoneuritis was observed in the central corneal stroma, whereas the vitreous and retina showed no abnormalities (Fig. 1).
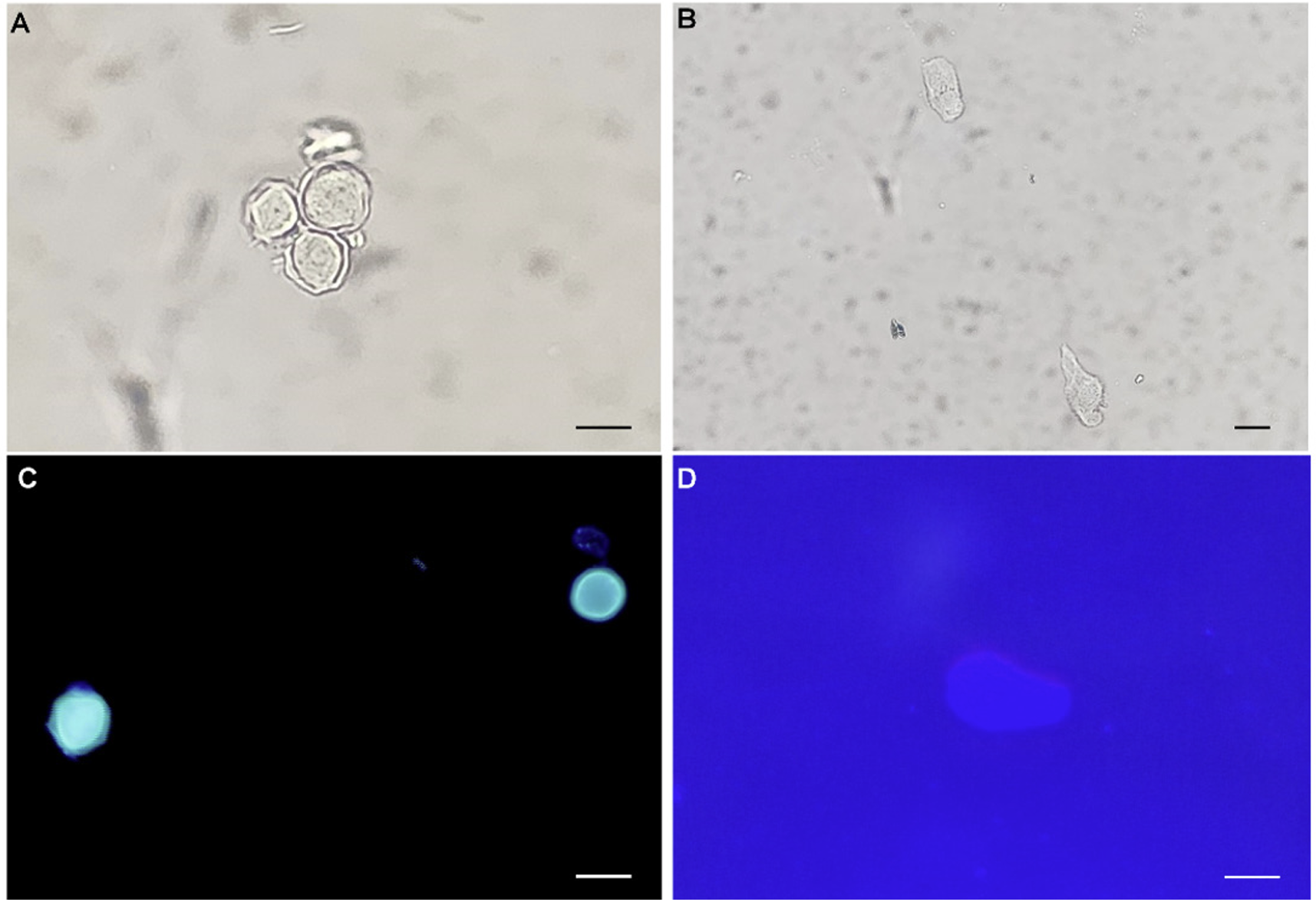
Diagnostic assessment: Corneal scraping was performed, and a direct smear slide was prepared. The slide and liquids with contact lenses were submitted to a clinical microbiology laboratory. Cultures for Acanthamoeba spp. were not available; thus, they were stained with calcofluor white as previously described [1]. Isolation of Acanthamoeba spp. from the corneal scraping and liquids allowed the detection of trophozoites and cysts under both light and fluorescence microscopes (Fig. 2). The Acanthamoeba cysts were approximately 10–15 μm in diameter, round, with a plica-like surface (Fig. 2A, 2C). The viable cysts were round or oval with double cyst walls, that is, ectocyst and endocyst walls. The ectocysts contracted into wrinkles and were separated from the thin and smooth endocysts. The trophozoites (about 20–25 μm in diameter) were pleomorphic and exhibited large lamellipodium of varying size and shape. A single large nucleus and numerous vacuoles with diverse contents in trophozoites were clearly observed by light microscopy but were unremarkable by fluorescent microscopy (Fig. 2B, 2D). The microscopic morphology was consistent with Acanthamoeba spp. reported to the ophthalmology department. To molecularly identify the microorganisms, a segment of the 18 small subunit ribosomal ribonucleic acid (rRNA) gene was amplified. Genomic DNA was extracted from the samples using the QIAamp DNA Mini Kit (Qiagen) according to the manufacturer’s instructions. Subsequently, polymerase chain reaction (PCR) was conducted employing the primer set JDP1 (5′-GGCCCAGATCGTTTACCGTGAA-3′) and JDP2 (5′-TCTCACAAG CTGCTAGGGAGTCA-3′), as documented in the literature [2]. Briefly, the PCR process involved an initial incubation at 94°C for 5 min, followed by 35 cycles of denaturation at 94°C for 1 min, primer annealing at 55°C for 1 min, and elongation at 72°C for 1 min, with a final elongation step at 72°C for 10 min to complete the amplification process. The PCR product, 436 base pairs in length, was sent to Cosmo Genetech for direct sequencing. The chromatograms of sequences were trimmed manually and assembled using the SeqMan software (DNASTAR). Basic local alignment search tool searches of the assembled sequences revealed 99% identity with Acanthamoeba spp. (GenBank accession no. MG825460.1) and conclusively identified the microorganism as Acanthamoeba spp.
Therapeutic intervention: Based on the morphological characteristics observed under the microscope, the patient was prescribed 0.02% polyhexamethylene biguanide eyedrops as a treatment on the day of the visit to our hospital.
Follow-up and outcomes: The patient underwent five months of pharmacological treatment, and the initially observed radial keratoneuritis showed significant improvement.
Discussion
Since the first report of Acanthamoeba keratitis in 1975, its incidence has increased, particularly among contact lens users [3]. In Korea, most published reports on the clinical patterns and treatments of Acanthamoeba keratitis have been case reports [4,5]; however, the incidence is perceived to be low, and the exact prevalence remains unclear [6]. The gold standard for diagnosing Acanthamoeba keratitis involves scraping the corneal epithelium, particularly from areas with severe epithelial damage, and culturing on Escherichia coli-seeded non-nutrient agar plates. This method allows the observation of Acanthamoeba predation paths on E. coli, demonstrating a detection rate of 60%–76% [7]. Several additional tests have been suggested to ascertain the growth rate, temperature tolerance, or pathogenicity of isolated amoebae, and axenization using proteose peptone-yeast extract-glucose medium is required [8]. Nonetheless, this culture method is time-consuming, taking a 1 week or more, which delays diagnosis and treatment. Although culture has been recognized as the gold standard for Acanthamoeba laboratory diagnosis [1], it has not been wellestablished in clinical laboratories. Instead of culture, several staining methods, such as lactophenol-cotton blue, acridine orange, calcofluor white, silver, and hematoxylin and eosin, can be useful for the identification of Acanthamoeba spp. from clinical specimens [1,9]. In this study, we found that Acanthamoeba cysts appeared light green on staining with calcofluor white. Direct microscopy of the original sample can detect characteristic cysts and trophozoites [1]. In this case, the causative agent was diagnosed through observation of Acanthamoeba morphology via a direct smear of the original sample without performing an axenic culture. However, relying solely on morphology for Acanthamoeba keratitis diagnosis through microscopic examination can lead to a misdiagnosis without experienced inspectors. This necessitates quality control in clinical laboratories, and continuous education regarding this uncommon pathogen. In addition to direct observation, we performed PCR using JDP1 and JDP2 primer sets, as previously described. The PCR method is well-established for detecting the 18S rRNA gene, facilitating genotyping in most cases through sequencing [2]. In addition to sequencing, real-time PCR has been preferred in clinical laboratories because there is no need for postamplification handling, leading to faster analysis and reduced risk of amplicon contamination [10]. Several real-time PCR-based assays have been developed and evaluated [11], but no commercial real-time PCR kits for the diagnosis of Acanthamoeba keratitis are available in Korea. Financial and procedural hurdles due to the non-reimbursable status of axenic cultures and PCR in certain diagnostic frameworks should be further overcome. Although it is challenging to find real-world evidence of Acanthamoeba in clinical microbiology without using culture methods, this case underscores the need for clinical microbiology laboratories to maintain their inspection capabilities. Furthermore, this study highlights the need for insights into Acanthamoeba diagnosis in clinical laboratories using various diagnostic tools.




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download