Abstract
Dengue, a mosquito-borne viral infection, is rapidly increasing worldwide and affects over half of the world’s population in at-risk areas. Factors such as globalization, urbanization, and climate change have fueled its rapid geographical expansion. Although no indigenous dengue cases have been identified in Korea, the number of imported dengue cases has increased from travel to endemic regions. In Korea, dengue diagnosis relies mainly on detecting antidengue antibodies or viral nucleic acids using real-time polymerase chain reaction. Although specific antiviral treatments for dengue are currently unavailable, promising progress has been made in developing antiviral agents that target viral replication. Single-dose tetravalent live-attenuated dengue vaccine candidates are currently being evaluated for their safety and efficacy. Innovative vector control methods, including Wolbachia-infected and genetically modified species of Aedes mosquitos, have demonstrated promising results. Owing to the limited therapeutic options, vector control strategies remain a primary focus for preventing transmission, alongside ongoing research on antiviral drugs and vaccine development. This review provides insight into dengue fever transmission, clinical manifestations, and diagnosis. Additionally, it covers current global control measures, emerging treatment options, and the status of vaccines in development.
Dengue fever (DF) is a rapidly spreading mosquito-borne disease. Dengue virus (DENV) is primarily transmitted to humans by Aedes mosquitoes, particularly Ae. aegypti and Ae. albopictus [1,2]. DF is widespread in tropical and subtropical regions and is endemic to more than 100 countries, with approximately half of the world's population at risk [3,4]. In 2013, Bhatt et al. [5] estimated that 390 million dengue cases occur annually, of which 96 million are symptomatic. Widespread infections occurred in Southeast Asia, the Western Pacific, the Americas, and Africa, with approximately 70% occurring in Asia [5]. In Asia, rapid urbanization contributed to increased transmission and hyper-endemicity, resulting in the first major epidemic of severe and fatal forms of dengue [6,7].
DF has become a significant global health concern, largely because of global travel trends, rapid urbanization, and climate change. These factors have led to heightened viral amplification, prolonged survival and reproduction of mosquito vectors, and extended transmission seasons, exacerbating dengue in endemic areas [7,8]. Messina et al. [9] projected that 6.1 billion people will be at risk of dengue by 2080. As the number of cases and geographic distribution of DF continues to increase, further research is needed to understand its epidemiology and to implement effective prevention and control.
This review offers insights into dengue epidemiology, clinical manifestations, diagnosis, treatment, and prevention strategies.
DF is not endemic to Korea; however, there has been an increasing number of imported cases in recent years owing to international travel to dengue-endemic regions [10,11]. All dengue cases in Korea were imported from travel abroad, mainly from Southeast Asia, with no confirmed local transmission [10,11]. The number of imported dengue cases in Korea has increased since 2001, peaking at 313 in 2016 [10]; all four DENV serotypes (DENV-1 to DENV-4) were detected in imported cases. An analysis of imported dengue cases from 2018 to 2020 revealed that DENV-1 was the most commonly identified serotype, accounting for 47.1%, followed by DENV-2 (28.6%), DENV-3 (17.1%), and DENV-4 (7.1%) [12]. Most imported dengue cases in Korea present with typical manifestations such as fever, rash, and hemorrhagic symptoms; however, some studies have reported severe presentations such as acute hepatitis, colitis, acute diverticulitis, and hemophagocytic lymphohistiocytosis [13–16].
DF is caused by the DENV, which belongs to the genus Flavivirus (family Flaviviridae). DENV comprises four antigenically distinct serotypes: DENV-1, DENV-2, DENV-3, and DENV-4 [17]. The virus is primarily transmitted to humans via bites of infected female Aedes mosquitoes, especially Ae. aegypti and Ae. albopictus [18]. However, in some parts of the world, Ae. polynesiensis and Ae. niveus have been reported as secondary vectors [19]. Dengue transmission occurs when an Aedes mosquito consumes a blood meal from a DENV-infected person and bites a healthy individual, transmitting the virus into the bloodstream. Infected female mosquitoes can also transmit the virus to their offspring during egg development, contributing to viral maintenance in mosquito populations [20]. Although direct transmission of DENV from one person to another does not occur, dengue can be transmitted from mother to child during pregnancy or childbirth. In rare cases, DENV transmission can occur via blood transfusion, organ transplantation, or needlestick injuries [21].
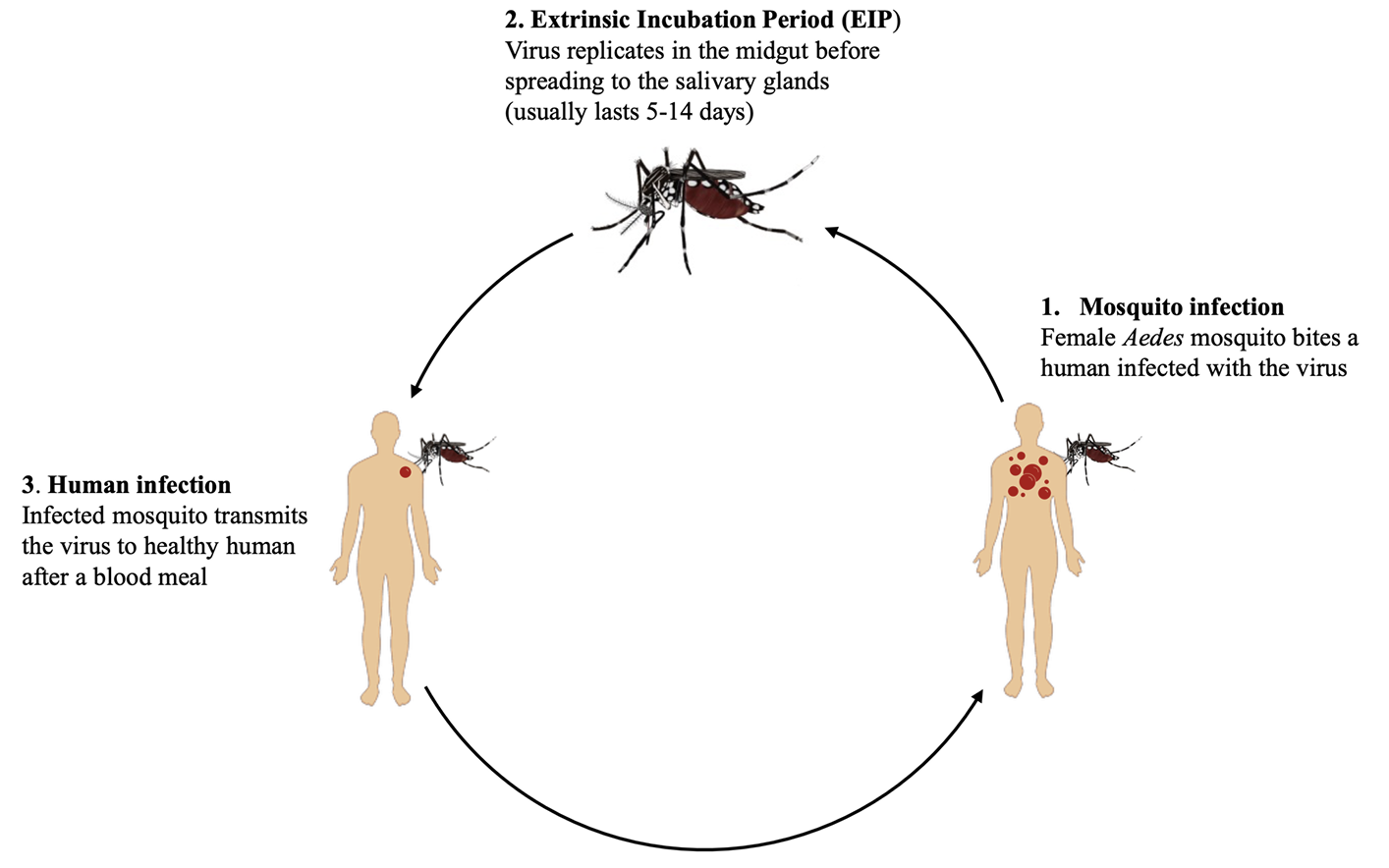
Mosquitoes are infected with DENV when they feed on viremic hosts (Fig. 1). The virus replicates in the mosquito mid-gut before disseminating into the salivary glands. The period between the ingestion of DENVinfected blood and transmission of the virus to a new host is known as the extrinsic incubation period (EIP). The duration of EIP is strongly influenced by ambient temperature (25°C to 30°C), viral strain, and mosquito competence [22,23]. Following the EIP period, which usually lasts 5–14 days, an infected mosquito can transmit the virus by biting another person [24,25]. However, spatial and temporal studies have reported that dengue clusters occur in the same household at times far below the estimated EIP (< 7 days) [26]. Results from a cluster study in Kaohsiung, Taiwan, are consistent with dengue infections occurring within the same household less than three days after the initial reported infection [27]. The mechanical transmission of DENV, where Ae. aegypti mosquitoes transmit the virus immediately after consuming an infectious blood meal without midgut incubation, as demonstrated in mouse models [27].
The incubation period for DF is approximately 4–10 days after being bitten by an infected mosquito [1], during which symptoms usually begin to appear. Dengue infections range from asymptomatic to mild or severe. DF is characterized by the sudden onset of flu-like symptoms, including fever, headache, myalgia, skin rashes, and mild mucosal bleeding. Although dengue is usually a self-limiting febrile illness, with most patients recovering 4–7 days after symptom onset, in some patients, especially those who have had dengue before, the disease may progress to severe life-threatening forms such as dengue hemorrhagic fever (DHF) or dengue shock syndrome [29].
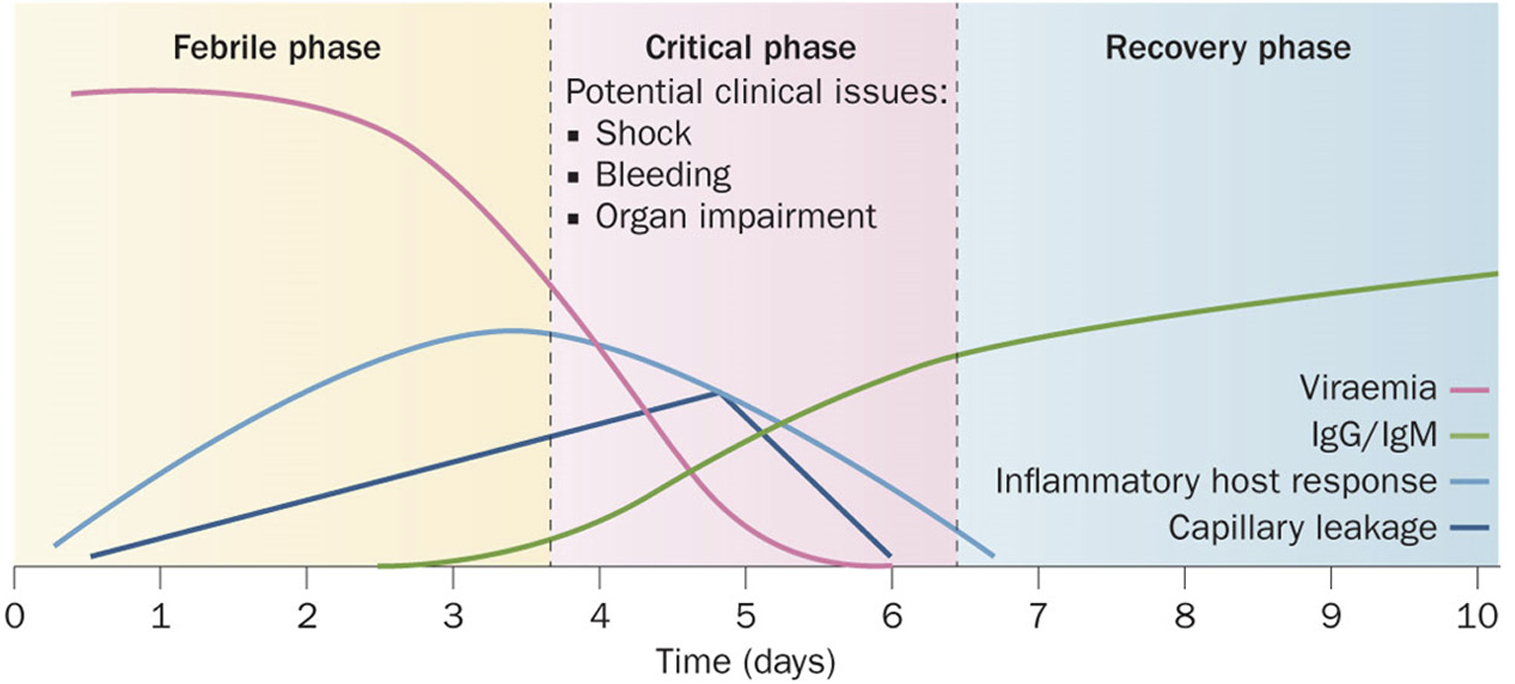
In 2009, the World Health Organization (WHO) revised the guidelines to categorize dengue infections as dengue without warning signs, dengue with warning signs, or severe dengue [1]. The warning signs include persistent vomiting, lethargy, abdominal pain, mucosal bleeding, and rapid breathing [1]. Dengue infections usually progress in three distinct phases: the febrile phase, characterized by viremia and sudden onset of high fever; the critical phase, which may begin around three days after fever onset, marked by resolution of fever and viremia, but with a high risk of shock due to plasma leakage and fluid accumulation in tissues; and the convalescent phase, when patients begin to recover, accompanied by reabsorption of accumulated fluids (Fig. 2) [1,30,31].
During the latter part of the febrile phase, minor bleeding manifestations such as petechiae and ecchymosis may occur as a result of thrombocytopenia or platelet dysfunction [32,33]. The spots can help to recognize DHF in its early stages. In most patients, minor bleeding resolves with defervescence. However, in patients with increased vascular permeability, the disease may progress to DHF, characterized by fluctuations in hematocrit levels (> 20%) [34] and gastrointestinal bleeding that is detectable 24–48 h after defervescence and before the onset of shock [23,33]. The subsequent shock can lead to disseminated intravascular coagulation, multiple organ failure, metabolic acidosis, and sometimes death [23,33].
Diagnosis of DF continues to rely on symptom-based assessment, which can be challenging during the febrile stage when it resembles other vector-borne diseases such as malaria, chikungunya, and Zika fever. Various laboratory methods have been developed to facilitate the accurate diagnosis of DF, especially in cases where the clinical presentation overlaps with other febrile illnesses. DF is diagnosed using tests that detect viral nucleic acids, antigens, or antibodies. During the febrile phase, viral isolation and nucleic acid detection by real-time polymerase chain reaction (RT-PCR), or nonstructural protein 1 (NS1) antigen using rapid immunochromatographic tests, or IgM or IgG by enzyme-linked immunosorbent assay (ELISA) are crucial for diagnosing DENV infection. In patients with no history of prior dengue infection, the sensitivity of NS1 antigen detection tests in the febrile phase was found to exceed 90%, compared with 60%–80% in secondary infections [23,35]. Recently, a meta-analysis conducted by Macedo et al. [36] evaluated the accuracy of rapid diagnostic tests for dengue. The sensitivity of IgM ranges from 13.8% to 90%, whereas that of NS1 antigen ranges from 14.7% to 100% [36]. The combination of three analytes (IgG, IgM, and NS1 antigen) showed the best overall performance, with a sensitivity of 90% and specificity of 89% [36].
During the critical phase (4–7 days after fever onset), the host initiates the production of specific IgM or IgG antibodies in response to DENV infection. Serological assays for detecting IgM and IgG antibodies typically employ ELISA with hemagglutination inhibition assay being the preferred diagnostic method [37,38]. Viral NS1 antigens have been reported to be present during the acute phase of dengue infection [39]. In primary infections, IgM can be detected as early as 3–5 days after fever onset and persists for several months after recovery [40,41]. IgG is typically not detected during the acute phase of a primary infection. However, in patients with secondary infections, IgG antibodies become the dominant immunoglobulin isotype and are detectable as early as three days after the onset of illness [40]. Consequently, IgM/IgG antibody ratios are commonly used to differentiate between primary and secondary dengue infections during the acute phase of the disease.
In Korea, the diagnosis of DF primarily relies on testing travelers returning from dengue-endemic regions because there are no local transmissions. Serological tests like IgM ELISA and RT-PCR to detect DENV among symptomatic travelers are used for diagnosis of DF by the Korea Disease Control and Prevention Agency [42]. Positive dengue results prompted further confirmatory testing of a 4-fold increase in IgG, usually 2–3 weeks after the onset of symptoms. Recently, rapid antigen tests have been made accessible at all airports and port quarantine centers in Korea for early detection of dengue among inbound travelers.
Supportive care, symptom management, close monitoring, and complication prevention are essential for managing DF. Acetaminophen (paracetamol) and other analgesics are used to reduce fever and treat joint and muscle discomfort. However, due to the high risk of bleeding, aspirin, ibuprofen, and other non-steroidal anti-inflammatory drugs should be avoided [30]. The WHO guidelines recommend that patients rest, stay hydrated, and seek immediate medical attention if they experience warning signs, such as severe abdominal pain, persistent vomiting, rapid breathing, or bleeding [1]. In most cases, patients with DF recover within 2–7 days without requiring hospitalization; however, severe cases may require hospitalization for supportive care, including blood transfusions, intravenous fluids, electrolyte replacement, and close monitoring [43]. Using insect repellents, wearing protective clothes, and eliminating mosquito breeding sites are the key strategies for preventing mosquito bites and the spread of DF, particularly in tropical regions.
There are currently no specific antiviral treatments for DF. However, a promising DENV inhibitor (JNJ-A07) has shown remarkable antiviral activity against all four DENV serotypes in 21 clinical isolates in vitro [44]. This inhibitor prevents the replication of new viral particles by blocking the interaction between the two viral proteins (NS3 and NS4B) essential for viral replication [44]. Studies on mouse models have demonstrated a significant reduction in the viral load with low oral doses of the inhibitor [44]. Additionally, natural compounds, such as flavonoids, silymarin, and baicalein have demonstrated promising potential to inhibit in vitro viral activity [45].
Global strategies to control dengue continue prioritizing vector control strategies, including mosquito surveillance and targeted insecticide application to minimize mosquito breeding sites and reduce transmission [1,8,46,47]. Public health campaigns are also underway to promote community engagement, education, and awareness, encouraging preventive measures such as using insect repellents and eliminating breeding sites. Considering the persistent increase in new dengue cases, vaccination programs for prevention are strongly recommended. Developing effective and affordable dengue vaccines remains the key focus of ongoing research.
Biological vector control methods, such as Wolbachia-infected and genetically modified mosquitoes, are being investigated to combat DF [48]. Studies on Wolbachia-infected Aedes mosquitoes are ongoing to reduce the ability of insects to transmit dengue and other viruses [49]. By releasing Wolbachiainfected mosquitoes into the environment, dengue transmission cycles are disrupted [47,49,50] through a phenomenon known as cytoplasmic incompatibility. During this process, Wolbachia-infected male mosquitoes mate with females lacking Wolbachia, resulting in nonviable eggs that reduce the mosquito population [51,52]. Introduction of the wMel strain of Wolbachia into Ae. aegypti populations in Australia have demonstrated a significant reduction in the transmission potential of DENV [53–55]. Other initiatives to genetically modify Aedes mosquitoes to prevent them from transmitting the DENV have been reported [48, 56], providing a potential long-term solution for dengue vector control.
Recent developments in dengue vaccines show promise, with two vaccines, Dengvaxia® (chimeric yellow fever virus, DENV; tetravalent dengue vaccine, CYD-TDV) and QDENGA (TAK-003), currently approved and others at various stages of research and development [57]. As the Food and Drug Administration approved in 2019, Dengvaxia® is recommended for people aged 9–45 years with previous dengue infections. Dengvaxia® has shown approximately 60% overall efficacy but has limitations owing to an increased risk of severe dengue in those not previously infected [57–59]. Another vaccine, TAK-003, is recommended by the WHO and approved for use in multiple countries. TAK-003 clinical studies have shown promising results, with an efficacy of 61.2% against symptomatic dengue, 84% against hospitalization, and 70% against the development of DHF [57,60,61]. Dengvaxia® and TAK-003 are live-attenuated vaccines using weakened viral strains to enhance immunity.
Recent advancements in dengue vaccine research have yielded promising results, including the development of a single-dose vaccine by the Butantan Institute [62]. In a phase 3 trial, the tetravalent butanDV (Butantan-DV) demonstrated 80% protection against dengue in individuals without prior exposure to the virus and an even higher efficacy of 89% in those with a history of dengue infection [62]. Another liveattenuated tetravalent candidate dengue vaccine, DengusiilTM, is undergoing clinical trials in Australia. Gunale et al. [63] reported that a single dose of the vaccine resulted in high immunogenicity, seroconversion, and seropositivity in most participants, with over 69% of the participants indicating tetravalent seroconversion and more than 15% demonstrating trivalent seroconversion [63]. However, challenges in dengue vaccine development persist, including the lack of adequate animal models for studying dengue pathogenesis, difficulty in inducing a balanced immune response against all four virus serotypes, and concerns regarding antibody-dependent enhancement [64,65].
Further research on dengue vaccines should prioritize expanding the geographical coverage of clinical trials for Butantan-DV and other potential candidates to assess vaccine performance across diverse dengueendemic regions [30,62]. Additionally, leveraging new technologies, such as virus-vectored and virus-like particle-based approaches, to enhance vaccine design and efficacy warrants further investigation to fully comprehend dengue pathogenesis and immune responses crucial for guiding rational vaccine development [64-66].
New technologies, such as stem cell therapy and wearable biosensors, have demonstrated promising results in the diagnosis and treatment of DF [67]. Stem cell therapy has the potential to alleviate complications associated with dengue by reducing inflammation and promoting tissue repair, as evidenced by the successful outcomes observed in dengue-infected mouse models [67]. Innovative technologies, such as wearable biosensors, enable the early detection of DF and real-time monitoring of symptoms and complications [68], facilitating timely intervention and improved patient management.
Dengue has been reported in more than 100 countries, and cases are projected to increase to 6.1 billion by 2080 due to global warming [9]. No specific antiviral agents have been approved for dengue, and treatment remains supportive, focusing on the close monitoring of warning signs and fluid replacement in patients with severe symptoms. The lack of a viable animal model and the inability to develop effective agents against all four DENV serotypes continue to hinder dengue antiviral and vaccine research. However, recent developments have identified several potential antiviral medications and vaccine candidates currently in clinical trials. Additionally, dengue prevention relies on effective vector control measures and communitybased interventions to reduce the risk of DENV transmission.
Ethics statement
This was not a human population study; therefore, approval by the institutional review board and informed consent were not required.
Funding
This study was supported by the Ministry of Trade, Industry, and Energy of Korea (grant number RS-202400403563). The funder had no role in the study design, data collection and interpretation, or the decision to submit the manuscript for publication.
REFERENCES
1. WHO. Dengue guidelines for diagnosis, treatment, prevention and control: new edition. Geneva: World Health Organization; 2009.
.
2. Kraemer MU, Sinka ME, Duda KA, Mylne AQ, Shearer FM, Barker CM, et al. The global distribution of the arbovirus vectors Aedes aegypti and Ae. albopictus. eLife 2015;4:e08347.
.
3. Chen LH, Marti C, Diaz Perez C, Jackson BM, Simon AM, Lu M. Epidemiology and burden of dengue fever in the United States: a systematic review. J Travel Med 2023;30:taad127.
.
4. Ferreira GL. Global dengue epidemiology trends. Rev Inst Med Trop Sao Paulo 2012;54:5-6.
.
5. Bhatt S, Gething PW, Brady OJ, Messina JP, Farlow AW, Moyes CL, et al. The global distribution and burden of dengue. Nature 2013;496:504-7.
.
6. Gubler DJ. Dengue/dengue haemorrhagic fever: history and current status. In: Bock GR, Goode JA, eds. New treatment strategies for dengue and other flaviviral diseases: Novartis foundation symposium 277. Chichester; John Wiley & Sons, 2006:3-22.
.
7. Gubler DJ. Dengue, urbanization and globalization: the unholy trinity of the 21st century. Trop Med Health 2011;39:S3-11.
.
8. Murray NEA, Quam MB, Wilder-Smith A. Epidemiology of dengue: past, present and future prospects. Clin Epidemiol 2013;299-309.
.
9. Messina JP, Brady OJ, Golding N, Kraemer MUG, Wint GRW, Ray SE, et al. The current and future global distribution and population at risk of dengue. Nat Microbiol 2019;4:1508-15.
.
10. KDCA. Infectious diseases portal. https://dportal.kdca.go.kr/pot/is/inftnsds.do [Online] (last visited on 24 May 2024).
.
11. Cho KH, Park SY, Lee WC, Lee MJ, Lee JB. International travel and exotic dengue fever in South Korea from 2006 to 2015. Jpn J Infect Dis 2018;71:378-81.
.
12. Kim JS, Kang HJ, Lim A, Lee YJ, Lee DY, Han MG. Serotypes of imported dengue fever cases in South Korea, 2018-2020. Public Health Wkly Rep 2021;14:2366-73.
.
13. Chung SM, Song JY, Kim W, Choi MJ, Jeon JH, Kang S, et al. Dengue-associated hemophagocytic lymphohistiocytosis in an adult: a case report and literature review. Medicine 2017;96:e6159.
.
14. Park S, Ryu S, Jin K, Hwang E, Han S, Kim H, et al. Acute colitis associated with dengue fever in a renal transplant recipient. Transplant Proc 2008;40:2431-2.
.
15. Song H, Jung BK, Cho J, Chang T, Huh S, Chai JY. Molecular identification of Anisakis larvae extracted by gastrointestinal endoscopy from health check-up patients in Korea. Korean J Parasitol 2019;57:207-211
.
16. Chang CY. Acute diverticulitis: a rare complication of dengue fever. Chonnam Med J 2021;57:158.
.
17. Mason PW, McAda PC, Mason TL, Fournier MJ. Sequence of the dengue-1 virus genome in the region encoding the three structural proteins and the major nonstructural protein NS1. Virol 1987;161:262-7.
.
18. Teixeira MdG, Barreto ML, Guerra Z. Epidemiologia e medidas de prevenção do dengue. Inf Epidemiol Sus 1999;8:5-33.
.
19. Zahoor MK, Rasul A, Zahoor MA, Sarfraz I, Zulhussnain M, Rasool R, et al. Dengue fever: a general perspective. In: Falcón-Lezama JA, Betancourt-Cravioto M, Tapia-Conyer R, eds. Dengue fever: a resilient threat in the face of innovation. London; IntechOpen, 2019:1.
.
20. Ferreira-de-Lima VH and Lima-Camara TN. Natural vertical transmission of dengue virus in Aedes aegypti and Aedes albopictus: a systematic review. Parasit Vectors 2018;11:1-8.
.
21. Kulkarni R, Tiraki D, Wani D, Mishra AC, Arankalle VA. Risk of transfusion-associated dengue: screening of blood donors from Pune, western India. Transfusion 2019;59:458-62.
.
22. Trejo I, Barnard M, Spencer JA, Keithley J, Martinez KM, Crooker I, et al. Changing temperature profiles and the risk of dengue outbreaks. PLoS Clim 2023;2:e0000115.
.
23. Guzman MG, Gubler DJ, Izquierdo A, Martinez E, Halstead SB. Dengue infection. Nat Rev Dis Primers 2016;2:16055.
.
24. Chouin-Carneiro T and dos Santos FB. Transmission of major arboviruses in Brazil: the role of Aedes aegypti and Aedes albopictus vectors. In: Shields VD, ed. Biological control of pest and vector insects. London; IntechOpen, 2017:2.
.
25. Tjaden NB, Thomas SM, Fischer D, Beierkuhnlein C. Extrinsic incubation period of dengue: knowledge, backlog, and applications of temperature dependence. PLoS Negl Trop Dis 2013;7:e2207.
.
26. Yoon IK, Getis A, Aldstadt J, Rothman AL, Tannitisupawong D, Koenraadt CJ, et al. Fine scale spatiotemporal clustering of dengue virus transmission in children and Aedes aegypti in rural Thai villages. PLoS Negl Trop Dis 2012;6:e1730.
.
27. Li HH, Su MP, Wu SC, Tsou HH, Chang MC, Cheng YC, et al. Mechanical transmission of dengue virus by Aedes aegypti may influence disease transmission dynamics during outbreaks. eBioMedicine 2023;94:104723.
.
28. Gómez M, Martinez D, Muñoz M, Ramírez JD. Aedes aegypti and Ae. albopictus microbiome/ virome: new strategies for controlling arboviral transmission? Parasit Vectors 2022;15:287.
.
29. Kalayanarooj S. Clinical manifestations and management of dengue/DHF/DSS. Trop Med Health 2011;39:S83-7.
.
30. Kala MP, St. John AL, Rathore AP. Dengue: update on clinically relevant therapeutic strategies and vaccines. Curr Treat Options Infect Dis 2023;15:27-52.
.
31. Yacoub S, Wertheim H, Simmons CP, Screaton G, Wills B. Cardiovascular manifestations of the emerging dengue pandemic. Nat Rev Cardiol 2014;11:335-45.
.
32. Kularatne SA and Dalugama C. Dengue infection: global importance, immunopathology and management. Clin Med 2022;22:9-13.
.
33. Tejo AM, Hamasaki DT, Menezes LM, Ho YL. Severe dengue in the intensive care unit. J Intensive Med 2024;4:16-33.
.
34. Wilder-Smith A and Schwartz E. Dengue in travelers. N Engl J Med 2005;353:924-32.
.
35. Guzman MG, Jaenisch T, Gaczkowski R, Ty Hang VT, Sekaran SD, Kroeger A, et al. Multicountry evaluation of the sensitivity and specificity of two commercially-available NS1 ELISA assays for dengue diagnosis. PLoS Negl Trop Dis 2010;4:e811.
.
36. Macêdo JV, Frias IA, Oliveira MD, Zanghelini F, Andrade CA. A systematic review and metaanalysis on the accuracy of rapid immunochromatographic tests for dengue diagnosis. Eur J Clin Microbiol Infect Dis 2022;41:1191-201.
.
37. Clarke DH and Casals J. Techniques for hemagglutination and hemagglutination-inhibition with arthropod-borne viruses. Am J Trop Med Hyg 1958;7:561-73.
.
38. Simmons CP, Farrar JJ, van Vinh Chau N, Wills B. Dengue. N Engl J Med 2012;366:1423-32.
.
39. Casenghi M, Kosack C, Li R, Bastard M, Ford N. NS1 antigen detecting assays for diagnosing acute dengue infection in people living in or returning from endemic countries. Cochrane Database Syst Rev 2014.
.
40. Sang CT, Cuzzubbo AJ, Devine PL. Evaluation of a commercial capture enzyme-linked immunosorbent assay for detection of immunoglobulin M and G antibodies produced during dengue infection. Clin Diagn Lab Immunol 1998;5:7-10.
.
41. Schwartz E, Mileguir F, Grossman Z, Mendelson E. Evaluation of ELISA-based serodiagnosis of dengue fever in travelers. J Clin Virol 2000;19:169-73.
.
42. Park JH and Lee DW. Dengue fever in South Korea, 2006–2010. Emerg Infect Dis 2012;18:1525.
.
43. Tayal A, Kabra SK, Lodha R. Management of dengue: an updated review. Indian J Pediatr 2023;90:168-77.
.
44. Kaptein SJ, Goethals O, Kiemel D, Marchand A, Kesteleyn B, Bonfanti JF, et al. A panserotype dengue virus inhibitor targeting the NS3–NS4B interaction. Nature 2021;598:504-9.
.
45. Low ZX, OuYong BM, Hassandarvish P, Poh CL, Ramanathan B. Antiviral activity of silymarin and baicalein against dengue virus. Sci Rep 2021;11:21221.
.
46. Achee NL, Gould F, Perkins TA, Reiner Jr. RC, Morrison AC, Ritchie SA, et al. A critical assessment of vector control for dengue prevention. PLoS Negl Trop Dis 2015;9:e0003655.
.
47. Jing Q and Wang M. Dengue epidemiology. Glob J Health Sci 2019;3:37-45.
.
48. Ritchie SA and Johnson BJ. Advances in vector control science: rear-and-release strategies show promise… but don’t forget the basics. J Infect Dis 2017;215:S103-8.
.
49. Fox T, Sguassero Y, Chaplin M, Rose W, Doum D, Arevalo-Rodriguez I, et al. Wolbachiacarrying Aedes mosquitoes for preventing dengue infection. Cochrane Database Syst Rev 2023.
.
50. Moreira LA, Iturbe-Ormaetxe I, Jeffery JA, Lu G, Pyke AT, Hedges LM, et al. A Wolbachia symbiont in Aedes aegypti limits infection with dengue, Chikungunya, and Plasmodium. Cell 2009;139:1268-78.
.
51. Lambrechts L, Ferguson NM, Harris E, Holmes EC, McGraw EA, O'Neill SL, et al. Assessing the epidemiological effect of Wolbachia for dengue control. Lancet Infect Dis 2015;15:862-6.
.
52. Zhang H and Lui R. Releasing Wolbachia-infected Aedes aegypti to prevent the spread of dengue virus: a mathematical study. Infect Dis Model 2020;5:142-60.
.
53. Ross PA, Robinson KL, Yang Q, Callahan AG, Schmidt TL, Axford JK, et al. A decade of stability for wMel Wolbachia in natural Aedes aegypti populations. PLoS Pathog 2022;18:e1010256.
.
54. Hoffmann AA, Montgomery BL, Popovici J, Iturbe-Ormaetxe I, Johnson PH, Muzzi F, et al. Successful establishment of Wolbachia in Aedes populations to suppress dengue transmission. Nature 2011;476:454-7.
.
55. Walker T, Johnson P, Moreira L, Iturbe-Ormaetxe I, Frentiu F, McMeniman C, et al. The wMel Wolbachia strain blocks dengue and invades caged Aedes aegypti populations. Nature 2011;476:450-3.
.
56. Ritchie S. Rear and release: a new paradigm for dengue control. Austral Entomol 2014;53:3637.
.
57. Lenharo M. Dengue is spreading. Can new vaccines and antivirals halt its rise? Nature 2023;623:470.
.
58. Hadinegoro SR, Arredondo-García JL, Capeding MR, Deseda C, Chotpitayasunondh T, Dietze R, et al. Efficacy and long-term safety of a dengue vaccine in regions of endemic disease. N Engl J Med 2015;373:1195-206.
.
59. Wilder-Smith A, Hombach J, Ferguson N, Selgelid M, O'Brien K, Vannice K, et al. Deliberations of the Strategic Advisory Group of Experts on immunization on the use of CYD-TDV dengue vaccine. Lancet Infect Dis 2019;19:e31-8.
.
60. López-Medina E, Biswal S, Saez-Llorens X, Borja-Tabora C, Bravo L, Sirivichayakul C, et al. Efficacy of a dengue vaccine candidate (TAK-003) in healthy children and adolescents 2 years after vaccination. J Infect Dis 2022;225:1521-32.
.
61. Malavige GN, Sjö P, Singh K, Piedagnel JM, Mowbray C, Estani S, et al. Facing the escalating burden of dengue: challenges and perspectives. PLoS Glob Public Health 2023;3:e0002598.
.
62. Kallás EG, Cintra MA, Moreira JA, Patiño EG, Braga PE, Tenório JC, et al. Live, attenuated, tetravalent Butantan–Dengue vaccine in children and adults. N Engl J Med 2024;390:397-408.
.
63. Gunale B, Farinola N, Yeolekar L, Shrivastava S, Girgis H, Poonawalla CS, et al. A Phase 1, double-blind, randomized, placebo-controlled study to evaluate the safety and immunogenicity of a tetravalent live attenuated dengue vaccine in adults. Vaccine 2023;41:5614-21.
.
64. Wilder-Smith A. Dengue vaccine development: challenges and prospects. Curr Opin Infect Dis 2022;35:390-6.
.
65. Thisyakorn U and Thisyakorn C. Latest developments and future directions in dengue vaccines. Ther Adv Vaccines Immunother 2014;2:3-9.
.
66. Young E, Yount B, Pantoja P, Henein S, Meganck RM, McBride J, et al. A live dengue virus vaccine carrying a chimeric envelope glycoprotein elicits dual DENV2-DENV4 serotypespecific immunity. Nat Commun 2023;14:1371.
.
67. Sakinah S, Priya SP, Mok PL, Munisvaradass R, Teh SW, Sun Z, et al. Stem cell therapy in dengue virus-infected BALB/C mice improves hepatic injury. Front Cell Dev Biol 2021;9:637270.
.
68. Rodriguez-Manzano J, Chia PY, Yeo TW, Holmes A, Georgiou P, Yacoub S. Improving dengue diagnostics and management through innovative technology. Curr Infect Dis Rep 2018;20:1-8.
.




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download