Abstract
Human anisakiasis (or anisakidosis) is a disease caused by the ingestion of marine fish or cephalopods infected with anisakid nematode larvae of the genera Anisakis, Pseudoterranova, Contracaecum, and Hysterothylacium. Anisakiasis is a clinically important disease that often manifests as an acute abdominal syndrome requiring emergency medical attention and care. In Korea, at least several thousand clinical cases have been diagnosed to date; however, only a small proportion of them have been reported in the literature (1971-2022). The most common etiological agents were Anisakis pegreffii (reported as Anisakis sp., Anisakis type I, or erroneously Anisakis simplex), followed by Pseudoterranova decipiens, Contracaecum sp., and Anisakis simplex sensu stricto (s.s.). Most cases involved the stomach and small or large intestine, with a few involving the oral cavity (oral mucosa, pharynx, and tonsils), esophagus, omentum, and mesocolic lymph nodes. Anisakis allergies and host immune responses have been studied in humans and experimental animals. Marine fish and cephalopods, including sea eel (Astroconger myriaster), squid (Todarodes pacificus), yellow corvina (Pseudosciaena manchurica), Japanese flounder (Paralichthys olivaceus), codfish (Gadus macrocephalus), yellowtail (Seriola quinquaradiata), and rockfish (Sebastes spp.), are the most common infection sources. Surveys were performed on anisakid nematode larvae in marine fish and cephalopods caught in the western, eastern, and southern seas of Korea. The larvae recovered from fish or cephalopods caught from the western and southern seas were predominantly A. pegreffii larvae; however, the larvae from the eastern sea were either A. pegreffii larvae (in the chub mackerel, Japanese flounder, and rockfish) or A. simplex s.s. (in the salmon and pollock; these fish migrate through the northern North Pacific Ocean and Bering Sea and come to Korea). Health education to avoid eating raw or improperly cooked marine fish and cephalopods (particularly the viscera) is crucial for preventing human anisakidosis in Korea.
Go to : 
Anisakiasis, or more broadly anisakidosis (infection with anisakid nematode larvae; Anisakidae and Raphidascarididae), is a typical example of fishborne parasitic zoonoses in localities where marine fish or cephalopods are consumed raw or improperly cooked [1]. The etiological agents include species of Anisakis (A. pegreffii, A. simplex s.s., and A. simplex), Pseudoterranova (P. decipiens s.s., P. azarasi, and P. cattani), Contracaecum (C. osculatum), and Hysterothylacium (H. aduncum) [2,3]. However, 4 species, A. pegreffii, A. simplex, P. decipiens, and P. azarasi, are most frequently involved in highly endemic countries, especially Korea and Japan [3,4]. Natural definitive hosts include dolphins, porpoises, whales, seals, and rarely, large marine fish [2]. Animal or human (accidental host) infection is caused by third-stage larvae found in the viscera or muscles of various fish and cephalopod (mollusks) species [1].
The concept of this zoonotic disease (initially called ‘herring worm disease’ or ‘codworm disease’) was f irst introduced in 1960 by van Thiel et al. [5], who reported 11 patients complaining of acute abdominal syndrome in the Netherlands, followed by Ashby et al. [6] in England in 1964, who reported 2 such patients and reviewed 89 cases possibly due to this disease. However, the diagnosis of the causative worms at this time was erroneously Eustoma rotundatum [5,6]. In 1965, Asami et al. [7] and Yokogawa and Yoshimura [8] reported similar human infections in Japan and assigned the causative worms to Anisakis-like larvae. Since then, human anisakidosis has been reported in various locations worldwide, including Japan, Korea, the Netherlands, Spain, France, Italy, Germany, England, and the USA [1,2]. To date, at least 50,000 clinical cases have been reported in these countries [1,2]. However, this is a significant underestimation of the actual situation. For example, in Japan, an average annual incidence of 19,737 anisakidosis cases was estimated from 2018 to 2019 [4], and in Spain, an annual incidence of 7,700-8,320 anisakidosis cases was estimated [9].
The most common site of anisakidosis in humans is the stomach, followed by the intestines (small and large intestines), and rarely the oral cavity, pharynx, tonsils, and esophagus [1,2]. Extra-gastrointestinal infections may also occur in rare instances, in the peritoneum, liver, lymph nodes, omentum, mesentery, pancreas, ovaries, and lungs [2]. In gastrointestinal anisakiasis, acute abdomen, nausea, vomiting, and mucosal bleeding are the most common clinical manifestations, although some cases reveal no special clinical problems, and the larvae can be incidentally found during surgery or endoscopy [2]. Diagnosis is mainly based on the recovery of anisakid larvae from the affected lesions through gastrointestinal endoscopy or surgery. In some cases, the diagnosis is made based on sectional morphologies of the larvae in histopathological specimens [2]. Serology to detect serum antibodies, for example, enzyme-linked immunosorbent assay (ELISA), and radiography, are other diagnostic methods used to diagnose acute and/or chronic anisakidosis [2]. No anthelmintic drugs have been proven to be effective in anisakidosis patients.
In Korea, human anisakidosis is common because Koreans generally prefer to eat the raw or undercooked f lesh of marine fish or cephalopods (cuttlefish, squid, and octopus). Since the first case was reported in 1971 [10], over a hundred articles have been published on clinical anisakidosis cases until 2022. The status was briefly reviewed by Sohn et al. [11] in 2015, in which 64 articles were analyzed concerning the number of cases, locality of patient occurrence, gender characteristics, source of infection (fish or cephalopods), morphological types of larvae, and predilection sites. However, since then, there has been no further review of the anisakidosis status in Korea.
Besides clinical case reports, there have been reports on the detection of anisakid larvae from marine fish and cephalopods, the occurrence of animal anisakidosis, and immunologic studies on experimental anisakidosis in animals. Thus, the present study focused on reviewing the Korean literature on human and animal anisakidosis (occurrence of clinical cases, clinicopathologic characteristics, immunity, diagnosis, and natural and experimental animal infections), as well as the status of anisakid larval infections in fish and cephalopods, larval morphology, molecular analyses, and resistance of anisakid larvae to physicochemical agents.
Go to : 
We searched and analyzed Korean literature on Anisakis, Pseudoterranova, anisakiasis, and anisakidosis from sources, including Google Scholar, PubMed Central, and KoreaMed. International literature to refer was also obtained from the same data sources.
Go to : 
In Korea, human anisakidosis was first reported in 1971 [10] in a patient living in Seoul complaining of a foreign body sensation and dull pain in the palatine tonsil. It was evident that these symptoms were due to infection with a larva of Anisakis sp. penetrating the palatine tonsil, which was extracted using forceps. The second case was reported in 1980 by Cho et al. [12] in a patient living in Seoul with an acute abdomen due to duodenal ulcer perforation. An anisakid larva section was incidentally found in the ileum of this patient.
By the end of 2022, at least 108 articles (including several abstracts) had been published on human anisakidosis cases in Korea (Tables 1-4) [3,10-116]. The total number of cases reported was 851, including 131 cases during 1971-1990, 225 cases during 1991-2000, 229 cases during 2001-2010, and 268 cases during 2011-2022, which demonstrated a chronologically increasing trend (Fig. 1). Seropositive cases were excluded if anisakid larvae were not parasitologically confirmed [117-120]. In addition, an increasing number of anisakiasis cases were reported from 2011 to 2018 in Korea based on big data analysis of Health Insurance Review Assessment claims [121]. According to this article, the annual number of anisakiasis cases registered in the Korean Health Insurance Database (2011-2018) was 333-818, including 409 cases in 2011, 333 cases in 2012, 359 cases in 2013, 409 cases in 2014, 450 cases in 2015, 491 cases in 2016, 685 cases in 2017, and 818 cases in 2018, totaling 3,954 cases during the 8 years (avg. 494 cases/year) [121]. Therefore, at least 800 cases of human anisakidosis occur annually in the Korean population. However, most of these cases were not officially reported in scientific publications (it is also uncertain whether these cases were parasitologically proven) and thus were not included in the clinical case analyses of this review.
Go to : 
The geographic locations where anisakidosis cases were reported in Korea were variable, including Jeju (229 cases; 26.9%), Seoul (182; 21.4%), Jinju (159; 18.7%), Busan (93; 10.9%), Incheon (42; 4.9%), Gwangju (21; 2.5%), Daegu (21; 2.5%), Pohang (20; 2.4%) and other cities (Fig. 2). A strong trend was observed for cases to be more commonly diagnosed in coastal areas than in inland areas. In addition, the frequency of reports seemed to depend on the number of medical facilities available in each area.
Go to : 
The possible sources of infection (fish or cephalopod dishes) were mentioned in most articles (Table 5). Of the 851 anisakidosis patients, approximately a quarter (25.4%) stated that they had eaten undefined or mixed f ish dishes (over 2 kinds of fish). The next most common source of infection was sea eel (19.9%), followed by squid (5.2%), yellow corvina (4.2%), Japanese flounder or flatfish (3.4%), codfish (2.6%), yellowtail (1.6%), and rockfish (1.4%) (Table 5). Anchovy, seabream, skate, and shad were the next most common dishes consumed by patients with anisakidosis in Korea (Table 5). It is of special note that approximately 1/5 of the cases involved the consumption of raw or undercooked sea eels. Considering that mixed fish dishes frequently include sea eel, the number of cases associated with sea eel consumption would increase to over 1/3 of all cases. A significant concern related to this is that anisakid larvae are commonly found in the muscles of sea eel, whereas larvae are generally found in the viscera and abdominal cavity of most other types of marine fish [122-126]. Therefore, if the sea eel muscle is consumed raw, it could be possible to be infected with anisakid larvae that are frequently found alive in the muscles of this fish.
Go to : 
Among the 851 cases analyzed in this review, the most frequently involved organs were the stomach (698 cases; 82.0%) followed by the small and large intestines (88 and 40 cases, respectively, 128 cases in total; 15.0%), esophagus (8 cases; 0.9%), tonsils (2 cases; 0.2%), oral cavity/oral mucosa (2 cases; 0.2%), mesocolic lymph nodes (1 case; 0.1%), greater omentum (1 case; 0.1%), peritoneum (1 case; 0.1%), and unknown location (15 cases; 1.8%) (Fig. 3). The involvement of multiple organs, more than 2 of the stomach, ileum, and colon, has been reported in 5 cases [73,80,89,98,103].
Go to : 
Regarding the types (or species) of anisakid larvae detected, most of the reports simply described that the worms were anisakid larvae or Anisakis sp. larvae (514; 60.4%). The larvae were designated as Anisakis type I (A. pegreffii or A. simplex larvae) in 80 cases (9.4%), A. simplex larvae in 88 cases (10.3%), and Anisakis type II (probably A. simplex) in 8 cases (0.9%). Thus, the overall number of cases reported as Anisakis spp. (including type I, type II, A. pegreff ii, and A. simplex) was 724 (85.2%). However, molecular analyses of larvae extracted from human infections using the internal transcribed spacer (ITS) region sequences were first reported by Lim et al. [107], and 25 of 26 larvae extracted from 15 cases were confirmed to be A. pegreffii, and only 1 larva (1 case) was A. simplex s.s. Subsequently, Song et al. [113] identified 20 additional human cases of A. pegreffii infection through molecular analyses. Thereby, a total of 35 (4.1%) patients were confirmed to be infected with A. pegreff ii. Based on these molecular findings, it is highly likely that the species of anisakid larvae responsible for human infections in Korea is predominantly A. pegreffii. Regarding P. decipiens s.s. (or Terranova type A larvae) infections, 44 cases (5.2%) were reported through morphological and/or molecular analyses. Three cases (0.4%) were caused by Contracaecum spp. or Contracaecum type A larvae [34,46]. The larvae were not identified in 79 patients (9.3%).
Different types of larvae appear to have distinct clinical features, such as affected organs or sites of infection. Of the 725 patients infected with Anisakis sp. larvae (including Anisakis sp., Anisakis type I, Anisakis type II, A. pegreffii, or A. simplex s.s.), 591 (81.5%) had infections in the stomach, 88 (12.1%) in the small intestine (duodenum, jejunum, ileum, or cecum), and 38 (5.2%) in the large intestine (ascending, transverse, descending colon or rectum). However, out of 44 cases infected with P. decipiens s.s. (or Terranova type A) larvae, 42 (95.5%) developed gastric infections, and the remaining 2 (4.5%) had 1 ileal and 1 colonic infection. These results agree with the higher potential of P. decipiens larvae to cause gastric infections than Anisakis spp. larvae. [1].
In Korea, most previous anisakidosis cases in which the extracted larvae were assigned to Anisakis sp. or Anisakis type I larvae might have been A. pegreff ii infections; and only a small proportion might have been A. simplex s.s. infections. This trend is different from that in Japan, where 168 (88.9%) of 189 larvae obtained from human patients were molecularly confirmed to be A. simplex s.s., and only a few were A. pegreffii (10 larvae; 5.3%) and P. azarasi (11 larvae; 5.8%) [4]. However, the relative proportion of causative larval species differed according to the localities in Japan; for example, on Kyushu Island (adjacent to South Korea), 33.3% of the patients were infected with A. pegreff ii larvae (other 66.7% were infected with A. simplex s.s. larvae), whereas in Hokkaido Island, Honshu, and Shikoku Islands (distant from South Korea), only 4.1%-6.7% of the patients were infected with A. pegreffii larvae [4]. This difference was presumed to be due to differences in larval anisakid distribution in fish caught from the Pacific Ocean, East Sea (Sea of Japan), and Yellow Sea (East China Sea) [4,107,122]. A. simplex s.s. larvae were more commonly detected in fish caught from Hokkaido and eastern Japan than A. pegreff ii larvae, whereas in fish from Kyushu and Fukuoka (close to South Korea), A. pegreff ii larvae were more frequently detected than A. simplex s.s. larvae [122].
The number of anisakid larvae detected in each patient was 1 in most cases, but rarely 2-4 larvae were detected [34,56,90,99]. However, more worms were detected on rare occasions. For example, 9 specimens were detected in 2 gastric patients from Jinju [107], and 15 worms in 1 gastric patient from Seoul [107]. Of special note were 2 patients with oral cavity infections, in which over 8 and 20 larvae, respectively, were extracted using forceps; both patients had eaten raw squids [79,108]. Moreover, in an extreme case, 51 larvae were collected from the stomach of a single patient, and 3 months after the removal of these larvae by endoscopy, submucosal tumors were again found in the stomach and transverse colon which were suggested to be due to anisakid larval infections that were not removed at the time of surgery [98].
The chief complaint or major clinical feature of anisakidosis patients (n = 851) in Korea was acute abdomen (including acute epigastric pain) or acute abdominal syndrome, which was observed in 59.9% of all patients (Table 6). The next frequent clinical symptom was nausea and vomiting which was recorded in 11.6% of patients, followed by chronic gastritis or gastric pain (3.6%), intestinal obstruction, ileus, or adhesion (2.6%), diarrhea (1.9%), urticaria and/or edema (1.2%), hematemesis and/or melena (1.1%), esophageal symptoms (0.9%), and intestinal inflammation, including appendicitis (0.6%) (Table 6). Extraintestinal anisakidosis was very rare, having been reported in 7 cases, involving the oral mucosa (2 cases), tonsils (2cases), greater omentum (1 case), mesocolic lymph nodes (1 case), and peritoneum (1 case). Notably, a considerable proportion of cases (5.3%) were asymptomatic or found incidentally during health check-ups, including endoscopy, in people who had mild gastrointestinal discomfort (Table 6). Cases with over 2 chief complaints comprised 2.4%, and symptoms and signs were not described in 7.9% of the patients.
The clinical features of Korean gastric anisakidosis cases have been reviewed several times [11,34,46,56,90]. Im et al. [34] reported that the most common clinical complaint of gastric anisakidosis among the 39 cases analyzed was sudden onset or intermittent epigastric pain (31; 79.5%), followed by nausea and vomiting (8; 20.5%). Song et al. [56] analyzed the clinical symptoms of 39 gastric anisakidosis patients and found that the most frequent symptoms were epigastric pain (34 cases; 87.2%) and epigastric discomfort (31; 79.5%), followed by nausea and vomiting (18; 46.2%), diarrhea (1; 2.6%), headache (1; 2.6%), and chest pain (1; 2.6%). Lee et al. [90] reported that the most common symptoms in 141 anisakidosis patients were acute epigastric pain (121 cases; 85.8%), followed by nausea and vomiting (40; 28.4%), mild epigastric discomfort (7; 5.0%), and hematemesis/melena (2; 1.4%). Sohn et al. [11] analyzed 15 gastric anisakidosis cases and reported that epigastric pain (14 cases; 93.3%) was the most common followed by nausea and vomiting (5; 33.3%), hematemesis (1; 6.7%), and hemoptysis (1; 6.7%).
Among intestinal anisakidosis cases (n = 128), the most common symptom was acute abdominal pain (including acute epigastric pain) (60 cases; 46.9%), followed by intestinal obstruction, adhesion, or intussusception (23; 18.0%), nausea and vomiting (19; 14.8%), diarrhea (14; 10.9%), asymptomatic or incidental finding (12; 9.4%), intestinal inflammation (5; 3.9%), and others (3; 2.3%). Esophageal anisakidosis was 8 cases [60,65,80,85,114], and the most common clinical complaint was acute epigastric pain (7 cases; 87.5%), followed by nausea and vomiting (2; 25.0%), chest pain (1; 12.5%), and epigastric fullness (1; 12.5%). In 2 oral mucosa cases [79,108], oral or substernal pain was the chief complaint, and in 2 tonsil cases, sore throat, foreign body sensation, and/or dull pain around the pharynx were the major clinical complaints.
Anisakiasis is often associated with a strong allergic response involving an elevated immunoglobulin (Ig), particularly IgE, in response to A. simplex or A. pegreffii, with clinical symptoms ranging from isolated swelling to generalized urticaria and life-threatening anaphylactic reactions several hours (or 24-36 hours) after ingestion of raw or undercooked fish [1,127-129]. Skin symptoms (especially urticaria) were the most frequent (almost 100% of patients with allergies), followed by digestive symptoms (74%) [129]. Anisakisassociated hypersensitivity reactions have been reported particularly in northern Spain [128] but are also known in France, Italy, Portugal, and Japan [128,130]. Genetic predisposition (HLA class II alleles) has been observed in patients with Anisakis allergies [128]. A. simplex is the dominant species implicated in human allergic responses in Spain and other countries; however, A. pegreff ii has also been identified as a causative agent in Italy [131,132]. The allergenic potential of P. decipiens has been suggested by several researchers and several P. decipiens allergens have been shown to have proteins homologous to A. simplex; however, further studies are required [133].
In Korea, at least 4 articles have reported allergic reactions due to anisakidosis [83,92,99,134]. The first allergic case was a 33-year-old woman residing in Jeju who complained of repeated episodes of urticaria with severe itching, stomachache, nausea, and vomiting a few hours after eating raw fish. The skin prick test and specific IgE test were positive for Anisakis somatic and excretory-secretory antigens [83]. Subsequently, 10 allergic patients complaining of urticaria (100%), abdominal pain (30%), and anaphylactic reactions (30%) were reported. High serum IgE levels were detected against A. simplex antigens in these patients [92]. On Jeju Island, a 47-year-old male patient complained of generalized urticaria, angioedema, abdominal pain, and dyspnea, and his serum revealed a strongly positive level of Anisakis-specific IgE [99]. Another study on Jeju Island included 15 additional gastroallergic patients positive for Anisakis-specific IgE with clinical complaints of urticaria, abdominal pain, angioedema, nausea, and vomiting [134]. We believe that most of these allergic cases involved infections with A. pegreffii, although the specific diagnosis of the involved larvae was not confirmed in these cases.
Go to : 
Recurrent anaphylaxis due to A. simplex larva infection was first reported by Audicana et al. [135] in Spain. Now it has been well established that acute allergic symptoms, such as urticaria, angioedema, or anaphylaxis, accompanied by epigastric pain, nausea, and vomiting, called gastroallergic anisakiasis, are elicited by live (not dead or heat-inactivated) third-stage larvae of A. simplex and A. pegreff ii [136]. This hypersensitivity reaction is often accompanied by an acute IgE-mediated generalized reaction with cutaneous (urticaria to angioedema) and/or respiratory (asthma and rhinitis) symptoms [136]. IgE bound to mast cells or basophils recognizes specific allergens, and degranulation of these cells leads to local and/or systemic allergic reactions [136]. IgE-producing parasite-derived antigens have been isolated and characterized, including Ani s 1 through Ani s 14, which are somatic antigens, excretory-secretory products (ESP), or of unknown origin [137]. Major allergens, as defined by the recognition of > 50% of sensitized individuals, were 5 kinds, including Anis 1 (ESP; similar to thermostable serine protease inhibitors), Ani s 2 (somatic antigen; paramyosin), Ani s 7 (ESP; function unknown), Ani s 12 (origin and function unknown), and Ani s 13 (ESP; hemoglobin) [136,137]. Ani s 1 and Ani s 7 have high allergenic potential and no cross-reactivity with other allergens, whereas Ani s 2 is a pan-allergen with cross-reactivity with other foods or inhalant allergens [137].
In Korea, Cho and coworkers [138-142] performed immunological and serological studies on anisakiasis in experimental animals and humans. Kim et al. [138] found that the ESP of A. simplex third-stage larvae (L3) contained important allergens (12 low-molecular-weight bands ranging from 10 to 186 kDa by immunoblotting) necessary to induce Anisakis allergy in rats. In human anisakiasis, the binding patterns of L3-specific antibodies varied depending on the different L3-ESP preparations, and low-molecular-weight proteins appeared to be strong and specific antigens [139]. Choi et al. [140] reported high serum titers of IgG4 and IgE antibodies in 4 of 9 Anisakis allergy patients in Korea. Cho et al. [141] demonstrated that allergic responses induced by L3 oral infection were predominantly caused by reinfection rather than primary infection, accompanied by elevated IgE and IgM levels. The role of specific IgG, especially IgG4, has also been suggested in immune responses to intraperitoneally injected L3, as IgG4 binds to epitopes recognized by specific IgE [143]. In human anisakidosis, the importance of specific IgA has been suggested; however, its role has not been demonstrated in experimental mice and rats [139,142].
Choi et al. [143] reported that A. pegreffii larval extract induced airway inflammation and asthma 4 weeks after the challenge, enhancing the expression of Th2-type immune responses (IL-4, IL-5, and IL-13) in the lungs of mice. Conversely, Park et al. [144] cloned a macrophage migration inhibitory factor (MIF)-like protein from L3 of A. simplex. This MIF-like protein was shown to reduce Th2-related cytokines in the bronchoalveolar lavage fluid and allergen-specific IgG2a in the sera of mice, which is probably associated with host immune modulation. Treg cells were recruited to the spleen and lungs of these MIF-treated mice [144]. Park et al. [145] identified a 24 kDa protein from the ESP of A. simplex larvae as a possible allergen, homologous to the 22U protein of filarial nematodes and distinct from the Ani s 1 antigen, although having the same molecular weight. Park et al. [146] performed repeated intranasal applications of recombinant 22U of A. simplex 6 times and found that this antigen induces airway allergic inflammation accompanied by enhanced lung Th2 and Th17 responses. Kim et al. [147] isolated and characterized an α-methylacetyl CoA racemase originating from the ventriculus of A. simplex larvae, which may have a function in the growth and development of the larvae. Jeon et al. [148] compared the hydrolase activities (esterases, peptidases, proteases, and glycosidases) of ESP and the somatic proteins of A. simplex and A. pegreff ii larvae collected from salmon in Yangyang (Namdae Stream) and mackerels in the southern sea of Korea, respectively. Cho et al. [149] studied the allergenicity of Ani s 1 (major antigen) and Ani s 9 (minor antigen) by repeated intranasal inoculation into mice and observed airway inflammation in mice with significantly elevated lung Th2 and Th17 cytokine responses. An interesting study by Cha and Ock [150] demonstrated that antigenic proteins from A. simplex larvae could change cytokine profiles and prevent drug-induced Crohn’s disease in experimental mice.
Go to : 
A relationship between chronic anisakid nematode infection and stomach or colon cancers has been suggested [151-154]. Some clinical cases have shown the co-localization of anisakidosis and cancer in the gastrointestinal tract [155,156]. Garcia-Perez et al. [151] provided data showing an epidemiological link between previous Anisakis infections and gastrointestinal cancer. In addition, products from Anisakis were shown to cause inflammation and DNA damage in the host [152], and the complete extract of Anisakis worms could induce changes in epithelial cells in vitro and in vivo (rats) [153]. However, in Korea, only 1 case of the co-existence of ascending colon anisakiasis and sigmoid colon cancer has been reported [86]. It is noteworthy that whereas carcinogenic stimuli should continue for a long time to undergo a neoplastic transformation of the mucosal tissues of the stomach or colon, most anisakidosis cases are detected in the acute stage, and the disease does not continue for a long time. Notably, in experimental anisakiasis in rabbits, the pathological lesions almost completely resolved in the submucosa of the stomach within 5 months after infection, and the worms were calcified, became tiny, and encircled by fibrous tissues infiltrated by inflammatory cells [157]. However, attention should be paid to patients who are repeatedly infected with anisakid nematodes because in such cases mucosal changes in the stomach and/or colon may persist for a considerably long time.
Go to : 
Studies of experimental anisakiasis in laboratory animals (regarding susceptibility, habitat, and pathology/ histopathology) have been reported in at least 3 articles in Korea. Kwon and Chyu [158] observed the distribution of anisakid larvae in 2 experimental rabbits after oral inoculation with 30 L3 from day 2 to 14 post-infection (PI). On days 2-3 PI, a total of 42 larvae (live) were recovered in the stomach (33 larvae), omentum (8), and mesentery (1), and on days 7-14 PI, only 7 larvae (dead) were detected in the abscess cavity (6) and fundus submucosa (1) of the stomach wall [158]. Choi and Kim [159] infected rats and rabbits with Anisakis L3 and chronologically observed the distribution of the larvae within 1 day (rats) and during days 1-14 PI (rabbits); in rats, 1 larva penetrated the stomach wall within 1 hour PI, and a total of 4 larvae were found in the stomach wall by 8 hours PI, while some larvae were found in the omentum, intestinal wall, abdominal cavity, and liver within 24 hours PI. In rabbits, 22 larvae penetrated the stomach wall within 1 day, and some larvae were found in the omentum, intestinal wall, abdominal cavity, and mesentery during days 3-14 PI [159]. Hong and Lee [157] observed larval locations and gross and histopathologic findings in the stomach, small intestine, colon, and mesentery from day 13 to day 150 PI; on day 13 PI, larvae were found in the stomach (15 of 60 larvae inoculated), omentum (1), intestine (2), mesentery (5), and abdominal wall (2), and on day 90 PI, larvae were found in the stomach (12 of 60 larvae inoculated), omentum (3), intestine (2), mesentery (1), and abdominal wall (1). Only 1 larva was detected on day 150 PI in the stomach wall of a rabbit [157]. The involved tissues showed severe histopathological changes, including granuloma formation, inflammatory cell infiltrations, fibrosis, and calcification until day 90 PI, and only a few sections of tiny, calcified larvae were detected in the submucosal layer of the stomach on day 150 PI [157]. Jeon and Kim [160] performed in vitro (agar block plates) and in vivo (rats) experiments on the pathogenic potential of 2 sibling species, A. simplex and A. pegreffii, and found that A. pegreff ii had higher penetration ability (in vitro) and longer survival time in rats (in vivo) than A. simplex.
Go to : 
A finless porpoise was stranded on the coast of Jeju Island with adhesive bowel disease (ABD), which is life-threatening, and nematode parasites, including Anisakis sp. larvae and Crassicauda sp., were found at autopsy. The primary cause of ABD is presumed to be a reaction related to these parasitic infections [167]. The first detection of A. simplex s.s. adult nematodes (n = 2) containing eggs was reported by Kim et al. [168] in the gastrointestinal tract of a common minke whale (Balaenoptera acutorostrata Lacépède, 1804) that died in the sea between Namhae and Bangeojin, Korea. The diagnosis was molecularly confirmed using the gene sequences of the mitochondrial cytochrome c oxidase 2 (cox2) [168].
Go to : 
The first detection of anisakid larvae in marine fish in Korea was reported by Chun et al. [169] in 1968 (Table 7). A total of 313 fish specimens (17 species) caught from the western (Yellow Sea) and southern seas were examined, and 312 (99.7%) were found to be infected with anisakid larvae (1-334 larvae per fish) [169]. The next study was reported by Lim [170]; a total of 1,940 fish specimens (15 species) were purchased from 5 localities (caught from western and southern seas) in Korea, and the average number of anisakid larvae per f ish ranged from 0.2 (damselfish; Chromis notata) to 156.0 (yellow corvina; P. manchurica). The third study was a morphological analysis of anisakid larvae collected from the yellow corvina caught in the western sea; a total of 1,026 identifiable anisakid larvae were collected from 30 fish, and Anisakis type I was the most common (859 larvae; 80.4%) followed by Contracaecum type Dʹ (77; 7.2%), Contracaecum type C′ (55; 5.1%), Contracaecum type D (18; 1.7%), Contracaecum type A (13; 1.2%), Contracaecum type V (3; 0.28%), and Raphidascaris sp. (1; 0.09%) [123].
Thereafter, at least 19 studies reported anisakid larval infections in fish and cephalopods in the vicinity of Korean seawater [171-189]. Eight studies that examined more than 7 species of fish or cephalopods are presented in Table 7 [125,169,170,171,177,178,182,183]. Among the 99 species of fish/cephalopods examined, 82 were infected with anisakid larvae (mostly Anisakis spp.) (Table 7). The major (positive in over 3 studies) infected fish/cephalopods were 15 species, including sea eel (C. myriaster), chub mackerel (Scomber japonicus), hairtail (Trichiurus lepturus), anchovy (Engraulis japonicus), silver pomfret (Pampus argenteus), yellow corvina (P. manchurica), yellowtail (Seriola quinqueradiata), jack mackerel (Trachurus japonicus), white croaker (Argyrosomus argentatus), herring (Clupea palassii), red seabream (Pagrus major), Japanese flounder (P. olivaceus), flathead (Platycephalus indicus), rockfish (Sebastes inermis), and Japanese common squid (T. pacificus) (Table 7). The reason why chub mackerels are not ranked among the 12 most common fish consumed by human patients in Korea (Table 5) seems to be that mackerels are less frequently consumed raw by the Korean people.
In addition, chum salmon (Oncorhynchus keta) purchased from the eastern coastal areas (Taep’o Port in Sokcho City and Namdae Stream in Yangyang, Gangwon-do) were examined in 3 studies; the infection rate was 100%, with the mean number of larvae per fish ranging from 28.9 to 108.0 [172,179,181]. Masu salmon (Oncorhynchus masou masou) purchased from the eastern part (Joomunjin) of Gangwon-do were also examined, and the recovered larvae were molecularly identified as A. simplex s.s. L3 by polymerase chain reaction-based restriction fragment length polymorphism (PCR-RFLP) analysis [180]. Sea eels (C. myriaster) were studied 5 more times in Korea; Chai et al. [124] examined 26 sea eels purchased from the Noryangjin Fish Market in Seoul, and among them, 15 (57.5%) were positive for anisakid larvae (90.1 larvae/fish), including Anisakis type I (564/1,351 larvae; 41.7%) followed by Contracaecum spp. (787/1,351; 58.3%). Song and Hwang [126] examined 382 sea eels purchased from Busan, of which 259 (67.8%) were infected with 1,768 anisakid larvae (Anisakis spp. and Contracaecum spp.). Cho et al. [183] examined 20 sea eels caught in Tongyeong City, Gyeongsangnam-do, and recovered 129 Anisakis type I larvae; these larvae were molecularly analyzed using PCR-RFLP and sequencing of the ITS region and cox2 genes, and 112 (86.8%) were confirmed to be A. pegreff ii, 10 (7.8%) were Anisakis typica, and 7 (5.4%) were unidentified [183]. Lee et al. [186] examined sea eels (C. myriaster), chub mackerels (S. japonicus), and hairtails (T. lepturus) from the seawater of Korea and performed molecular analyses (PCR-RFLP) on the larvae; 136 (97.1%) of 140 larvae were A. pegreffii, and the remaining 4 (2.9%) were A. typica. Neves [188] also examined sea eels (C. myriaster), chub mackerels (S. japonicus), and yellow croakers (Larimichthys polyactis) caught from the western, southern, and eastern coasts of Korea to detect anisakid larvae. Bak et al. [184] caught chub mackerels from the western, southern, and eastern coasts of Korea and examined them for anisakid larvae; 231 (55.4%) of the 417 mackerels were positive, with a total number of larvae of 1,628 (7.0 larvae/fish). Molecular studies (PCR-RFLP of ITS and sequencing of cox2) of these larvae revealed that most of them were A. pegreff ii (94.3%), and small proportions were A. simplex s.s. (0.4%), a hybrid of A. pegreffii and A. simplex (2.5%), or Hysterothylacium sp. (2.8%) [184].
Jeong and Song [176] examined yellow corvina (P. manchurica) (n = 551) purchased from seawater around Jeju Island and recovered 4,386 larvae (8.5 larvae/fish) comprising 3,428 Anisakis type I larvae and 958 Contracaecum spp. larvae. Anchovies were examined in 2 more studies [174,189] in addition to the 4 studies in Table 7 [170,171,177,182]; Song et al. [174] purchased a total of 2,180 anchovies (E. japonica) from the eastern and southern seas and 171 (7.8%) were found to be infected with anisakid larvae (Anisakis type I or II, Anisakis sp., and Contracaecum type B or type C). Chang et al. [189] purchased anchovies from the southern sea and found anisakid larvae in 39 (19.5%) of 200 specimens (51 larvae in total; 1.3/ fish); molecular analyses using ITS gene sequences revealed that 28 larvae were A. pegreffii, 12 were Hysterothylacium sinense, and 11 were H. aduncum. The large-head hairtail (T. japonicus) (n = 9) purchased from Jeju Island was examined for anisakid larvae (1,259; 139.9/fish) using PCR-RFLP of ITS and cox2 sequencing; A. pegreff ii larvae (1,243/1,259; 98.7%) were most commonly detected, followed by a hybrid of A. pegreffii and A. simplex s.s. (6/1,259; 0.5%), Hysterothylacium sp. larvae (2/1,259; 0.2%), and unknown species (8/1,259; 0.5%) [187]. Regarding a squid species (T. pacificus), 1 more study was available [175] in addition to 3 studies shown in Table 7 [125,177,182]; 272 squids purchased from Busan (southern sea) were examined, and Anisakis type I, type II, Contracaecum types A and D larvae were recovered.
There may be questions about the possibility of anisakid larval infection in cultured marine fish. As fish farms are usually located near coastal areas, remote from the sea where whales and other sea mammals thrive, and within the farm there seems to be no infected first intermediate host (shrimp-like crustaceans) with anisakid larvae, cultured fish can be regarded to be safe from anisakid larval infection.
Go to : 
Various morphological types of anisakid larvae have been reported since the reports by Yamaguti [190], Berland [191], and Koyama et al. [192]. Anisakis type I (A. pegreffii, A. simplex, and A. typica) and type II larvae (A. simplex) were named by Berland [191]; Contracaecum types I through V by Yamaguti [190]; and Terranova types A (P. decipiens), B (P. decipiens), Contracaecum types A through D (C. osculatum), and Raphidascaris sp. by Koyama et al. [192]. In Korea, Chai et al. [123,124] found variants of Contracaecum types A, C, and D larvae in sea eel and yellow corvina, which were named Contracaecum types A′, C′, and D′, respectively.
Studies on anisakid larvae in fish from Korea started in 1966 but were officially reported in 1968 by Chun et al. [169]. Anisakis type I larvae (as Anisakis sp.) were detected in marine fish from the western and southern seas. Since then, several articles have been published regarding the morphology of anisakid larvae found in Korea. Rim [193] described the morphology of Anisakis type I larvae obtained from marine f ish and swine in Gwangju, South Korea. Kim et al. [171,172] detected Anisakis type I; Terranova type B; Contracaecum types A, B, and D; and Raphidascaris sp. larvae in marine fish purchased from fish markets in Seoul and Sokcho (Gangwon-do). Woo and Kim [125] detected Anisakis types I and II, Terranova types A and B, Contracaecum types A, B, C, and D, and Raphidascaris sp. larvae in marine fish purchased from Jeju Island. Sohn and Lee [194] and Sohn [195] observed the sectional morphologies of Anisakis type I, Contracaecum type A, and type D’ larvae by light and transmission electron microscopies. Chai et al. [196] studied the morphologies (light microscopic, sectional, and scanning electron microscopic) of P. decipiens larvae detected from the codfish (Notothenia neglecta) caught in the Antarctic Ocean near the South Pole.
Go to : 
Hong and Lee [157] studied the changing patterns of IgG in experimental rabbit anisakiasis for 150 days PI. IgG levels started to increase from day 3 PI, peaked on day 30 PI, maintained a plateau during days 30-90 PI, and then decreased to almost control levels on day 150 PI [157]. The serological diagnosis of human anisakidosis patients in Korea was first reported by Lee et al. [197], who used ELISA on 12 parasiteconfirmed anisakidosis cases and observed significantly elevated optical density values in 11 of the 12 patients (0.229-1.547 in 12 patients vs 0.103-0.353 in 5 controls). They also performed immunoblotting on sera from humans and experimental rabbits and found that the 46 kDa L3 antigen was highly sensitive and specific for Anisakis infection [197]. Quan et al. [198] compared different types of antigens used in ELISA for anisakiasis in rabbits and found that ESP was the most suitable antigen. Yang et al. [199] performed ELISA and immunoblotting to observe the changing patterns of serum IgM and IgG in experimental rabbits and detected at least 41 reactive bands; IgM reacted to 7 bands and IgG reacted to 8 bands. Choi et al. [200] observed the antibody responses of experimental rabbits infected with A. simplex L3 using ELISA and immunoblotting; serum IgG began to increase at days 7-9 PI, reached their maximum levels by days 1215 PI, and then decreased, while serum IgM began to increase from day 5 to 7 PI, reached a peak at day 12 PI, and then decreased. Significantly elevated levels of total and L3-specific IgE were observed in 10 gastroallergic patients [92]. However, serum IgM levels have not been studied in human anisakiasis patients in Korea and require further investigation.
Rim et al. [201] performed a seroepidemiological study using ELISA for 6 major parasitic diseases in 6,074 individuals selected from several localities in Korea. The number of anisakiasis-seropositive cases was 495 (8.1%) and varied by locality; it was higher in the southern areas of Jeollanam-do (16.9%) and some parts of Gyeongsangbuk-do (10.6%) than in other localities. Kim et al. [202] detected 33 seropositive cases by ELISA against A. simplex L3 ESP antigen in 498 health-examined people in 3 hospitals from southern parts of Korea; the allergen of 130 kDa could be a candidate for the serodiagnosis of anisakiasis. Chung and Lee [134] reported that 14 out of 17 patients with suspected gastroallergic anisakiasis on Jeju Island were strongly positive for Anisakis-specific IgE.
Go to : 
In Korea, molecular studies of anisakid larvae were initiated by Kim et al. [203] in 2006 who analyzed the complete mitochondrial genome of A. simplex L3 from sea eel (C. myriaster). Thereafter, at least 23 studies were performed on the molecular genetic characteristics of anisakid larvae from Korean seawater [147,178,180,181,184-189,203-215]. Yu et al. [204] analyzed the cDNA library of A. simplex L3 and detected 493 expressed sequence tags (ESTs), including 21 ESTs that matched previously reported A. simplex genes or proteins in addition to many other EST clones related to cell functions and worm growth and development.
Subsequently, the PCR-RFLP technique was applied to detect anisakid larvae in several fish species caught in Korean seawater [205-207]. Kang et al. [205] molecularly analyzed anisakid larvae from marine fish and squids caught in Korean seawater using PCR-RFLP of the ITS gene and found that they were larvae of A. pegreffii. Lee et al. [206] also molecularly confirmed that most larvae from Korean sea-fish were A. pegreffii (47 of 60 larvae), and small proportions were A. typica (10 larvae), A. simplex s.s. (1 larva), and a hybrid of A. pegreffii and A. simplex (2 larvae). Choi et al. [178] performed molecular studies (18S ribosomal DNA; 18S rDNA) on 10 Anisakis type I larvae recovered from marine fish in Korean seawater, and the larvae demonstrated high identity with A. pegreff ii. Park et al. [208] molecularly analyzed 45 anisakid larvae collected from rockfish (S. schlegeli) from the western sea and identified 3 species, which included A. simplex (17 larvae), A. pegreffii (4 larvae), and Hysterothylacium sp. (24 larvae). Kim et al. [209] molecularly analyzed 26 anisakid larvae from fish in the southern sea and found that A. pegreffii was the most common (17 larvae) followed by H. aduncum (2 larvae), Hysterothylacium fabri (1 larva), Raphidascaris lophii (1 larva), and hybrid forms (5 larvae). However, Jeon et al. [180] and Setyobudi et al. [181] reported that the anisakid larvae from masou salmon (O. masou masou) and chum salmon (O. keta), respectively, from the eastern sea analyzed by PCR-RFLP of the ITS region and cox2 gene were A. simplex s.s. Subsequently, this discrepancy was well explained by Setyobudi et al. [210] that A. pegreff ii larvae were distributed mainly in the southern (90.7% of all anisakid larvae detected) and western seas (98.9% of all larvae detected) but less in the eastern sea of Korea based on molecular analyses of larvae collected from common squids (T. pacificus). A similar but interesting finding was observed by Sohn et al. [211]; all 48 larvae from 3 species of fish from the Yellow Sea (western sea) were A. pegreffii, whereas 79 of 80 anisakid larvae from 7 species of fish from the southern sea were A. pegreff ii, and only 1 larva was A. simplex s.s. In f ish from the eastern sea, the species of anisakid larvae were different depending on the species of fish; the larvae detected in Japanese flounder (P. olivaceus) and rockfish (S. schlegeli) were all A. pegreffii, whereas those found in 2 species of salmon (O. masou masou and O. keta) were determined to be A. simplex s.s. [211]. Salmon fish are considered to have been infected by anisakid larvae when they migrate from the east coast of Korea through Hokkaido (Japan), the northern North Pacific Ocean, and the Bering Sea. Similar features were observed in the distribution of A. pegreff ii and A. simplex s.s. larvae in chub mackerel (S. japonicus) caught from the eastern, southern, and western seas [184], and it seemed that A. pegreffii is the dominant species in Korean chub mackerels which are mainly caught from the Tsushima Current stock, whereas Japanese chub mackerels are 2 stocks and harbor 2 kinds of larvae; A. pegreffii in the Tsushima Current stock and A. simplex s.s. in the Pacific Current stock.
The predominance of A. pegreff ii larvae was repeatedly observed in different fish species collected from the western and southern seawater of Korea (Fig. 4); sea eel (C. myriaster) [185,186,188], Pacific cod (Gadus macrocephalus) [212], chub mackerel (S. japonicus) [186,188], hairtail (T. lepturus) [186], large-head hairtail (Trichiurus japonicus) [187], yellow croaker (L. polyactis) [188], and anchovy (E. japonica) [189]. Nurhidayat et al. [213] examined walleye pollock (G. chalcogrammus) caught from the East Sea of Korea and found that 73.5% of the anisakids were A. simplex; only 1.4% were A. pegreffii (Fig. 4). Walleye pollock inhabits cold and deep-water columns and is abundant in the northern North Pacific Ocean (Pacific Current) and the Bering Sea [213]. In addition, the predominance of A. simplex larvae in chum salmon caught from the East Sea (Namdae Stream, Yangyang) of Korea was confirmed by Kim et al. [214], who performed comparative transcriptome analyses of the larvae. Kim et al. [215] developed a new genetic technique to discriminate the species of anisakid larvae collected from Korean seawater and the amplification-refractory mutation system (ARMS) was found to be effective in discriminating A. pegreffii, A. simplex, and their hybrids.
In human infections in Korea, specific molecular diagnosis of anisakid larvae has been performed in 3 studies [3,107,113]. Lim et al. [107] performed molecular analyses of 26 larvae extracted from human infections using ITS region sequences, and the larvae from 15 (25 larvae) of 16 cases were confirmed to be A. pegreffii, while the larva from only 1 case (1 larva) was A. simplex s.s. Song et al. [113] performed molecular analyses of anisakid larvae from 20 human cases (health check-up patients) and confirmed that all of them were infected with A. pegreffii. Molecular studies of Pseudoterranova sp. larvae were performed in only 1 study by Song et al. [3]; 12 larvae collected from 12 human cases in Korea were analyzed using sequences of the mitochondrial cox1 and nd1 genes, all of which were confirmed to be P. decipiens s.s. larvae.
Go to : 
In Korea, it has been shown that anisakid larvae are highly resistant to various physicochemical stimuli, including temperature (high and low), chemical substance (salinity, acidity, alcohol, and others), irradiation, ultrasound, hydrostatic pressure, and anthelmintic drugs [170,216-224]. Lee and Chyu [216] reported that anisakid larvae survived 1, 4, 6, 3, and > 30 hours at -20℃, -15℃, -10℃, -5℃, and 0℃, respectively, and were killed when stored at 30% saline for 17 hours, 20% saline for 2 days, and 10% saline for 5 days; however, some (17%-19%) larvae lived > 10 days at 0.85% or 5% saline. In addition, anisakid larvae survived < 1 hour at 20% acetic acid, < 24 hours at 10% acetic acid, < 10 days at 5% acetic acid, < 2 days at 40% ethanol, < 3 days at 20% ethanol, and < 6 days at 10% ethanol. The larvae were highly resistant to variable concentrations of soy sauce, Japanese soy sauce, mustard, and Japanese mustard (wasabi) surviving 1 day or longer [216]. Lim [170] reported similar results. Chai et al. [217] observed high radioresistance of anisakid larvae; they demonstrated active movements even after irradiation with a surprisingly high dose of 10,000 Gy, although their infectivity to experimental rats and rabbits decreased considerably. This radioresistance was suggested to be due to the role of superoxide dismutase [218]. Jeon and Jee [219] studied the inhibitory effects of ivermectin, doramectin, and ethanol in vitro and found that ivermectin and ethanol had some effect on the migration and movement of anisakid larvae, whereas doramectin had no effect. However, the inhibitory effects of ivermectin were lower in vivo in experimental rats [220]. Herbal extracts (Meliae ezadarach, Dryopteris crassirhizoma, and Quisqualis indica var. villosa) have been tested for their inhibitory effects in vitro against anisakid larvae [221]; however, the in vivo effects need to be determined. Oh et al. [222] studied the effects of freezing, salting, and combined treatment with chlorine and ultrasound on anisakid larvae inoculated in salt-fermented squid and pollock tripe; all larvae were inactivated after 48 hours at -20℃ and 24 hours at -40℃. The viability of the larvae was 81.7% and 26.7% in 5% and 10% saline solutions, respectively, after 7 days of storage, and all larvae were inactivated when submerged in 15% saline for 7 days or in 20% saline for 6 days of storage [222]. The viability of the larvae (isolated from the f ish) was significantly reduced after combined treatment with low concentrations (< 2,000 ppm) of chlorine and ultrasound for 5-30 min; however, this combined treatment exerted no effects on the viability of the larvae located within the viscera of heavily infected sea eels [222]. Lee et al. [223] examined the effect of high hydrostatic pressure (HHP) on the viability of anisakid larvae in the flesh of sea eels and found that HHP at 200 MPa for 5 min could be used as a potential measure for the inactivation of anisakid larvae in f ish without changes in color and sensory quality of the fish. Nam et al. [224] observed the larvicidal effects of various food condiments, including soybean sauce, Japanese mustard, vinegar, red pepper paste, saline solution, ethanol, and soju (Korean liquor); all these exhibited significant effects after several hours, but L3 is exposed to these condiments only for seconds before ingestion, so these conditions cannot be used to prevent anisakid larval infections.
Go to : 
Ethics statement
This was not a human population study; therefore, approval by the institutional review board and informed consent were not required.
Acknowledgments
We acknowledge all Korean authors who reported the clinical cases of human and animal anisakidosis between 1971 and 2022. We also appreciate the dedicated works, including molecular studies, on the detection of anisakid larvae in marine fish and cephalopods caught near the western, southern, and eastern seas of Korea.
REFERENCES
1. Chai JY, Murrell KD, Lymbery AJ. Fish-borne parasitic zoonoses: status and issues. Int J Parasitol 2005;35:1233-54.
.
2. Sohn WM and Chai JY. Anisakiosis (Anisakidosis). In Palmer SR, Soulsby L, Torgerson PR, Brown DWG, eds. Oxford textbook of zoonoses. 2nd ed. Oxford; Oxford university press. 2011:774-86.
.
3. Song H, Ryoo S, Jung BK, Cho J, Chang T, Hong S, et al. Molecular diagnosis of Pseudoterranova decipiens sensu stricto infections, South Korea, 2002-2020. Emerg Infect Dis 2022;28:1283-5.
.
4. Sugiyama H, Shiroyama M, Yamamoto I, Ishikawa T, Morishima Y. Anisakiasis annual incidence and causative species, Japan, 2018-2019. Emerg Infect Dis 2022;28:2105-8.
.
5. Van Thiel PH, Kuipers FC, Roskam RT. A nematode parasitic to herring, causing acute abdominal syndrome in man. Trop Geogr Med 1960;2:97-113.
.
6. Ashby BS, Appleton PJ, Dawson I. Eosinophilic granuloma of gastro-intestinal tract caused by herring parasite Eustoma rotundatum. Br Med J 1964;1:1141-5.
.
7. Asami K, Watanuki T, Sakal H, Imano H, Okamoto R. Two cases of stomach granuloma caused by Anisakis-like larval nematodes in Japan. Am J Trop Med Hyg 1965;14:119-23.
.
8. Yokogawa M and Yoshimura H. Anisakis-like larvae causing eosinophilic granuloma in the stomach of man. Am J Trop Med Hyg 1965;14:770-3.
.
9. Bao M, Pierce GJ, Pascual S, González-Muñoz M, Mattiucci S, Mladineo I, et al. Assessing the risk of an emerging zoonosis of worldwide concern: anisakiasis. Sci Rep 2017;7:43699.
.
10. Kim CH, Chung BS, Moon YI, Chun SH. A case report on human infection with Anisakis sp. in Korea. Korean J Parasitol 1971;9:39-43 .
.
11. Sohn WM, Na BK, Kim TH, Park TJ. Anisakiasis: report of 15 gastric cases caused by Anisakis type I larvae and a brief review of Korean anisakiasis cases. Korean J Parasitol 2015;53:465-70.
.
12. Cho SY, Chi JG, Kim IS, Min YY, Chun WC, Son JH, et al. A case of human anisakiasis in Korea. Seoul J Med 1980;21:203-8.
.
13. Lee KH, Koo JT, Song JH, Hyun MS, Jhi CJ. Acute gastric anisakiasis -endoscopic, radiologic diagnosis and its management. Korean J Intern Med 1981;24:1220-8.
.
14. Chung WC, Oh MY, Chun SW, Kang SB, Chung YK. Clinical observation of gastric anisakiasis. Korean J Intern Med 1983;26:1394-9 .
.
15. Paik AL, Hong SR, Paik IK, Ko IH, Lee J, Paik IW, et al. Anisakiasis in terminal ileum. Korean J Pathol 1984;18:453-6 .
.
16. Seo BS, Chai JY, Lee SH, Hong ST, Seo JW, Noh SH. A human case infected by the larva of Terranova type A in Korea. Korean J Parasitol 1984;22:248-52.
.
17. Jeong JS and Suk DS. A case of human gastric anisakiasis in Korea. Inje Med J 1984;3:359-67.
.
18. Lee AH, Kim SM, Choi KY. A case of human infection with the larva of Terranova type A. Korean J Pathol 1985;19:463-7 .
.
19. Lee CY, Lee MH, Hong KW. Four cases of gastric anisakiasis found in Taegu, Korea. Korean J Med Technologists 1986;18:171-6 .
.
20. Yun YK and Whang IW. Eosinophilic gastroenteritis associated with a larval anisakine infestation. Kyungpook Univ Med J 1987;28:224-7 .
.
21. Kim PY, Chung MK, Lee HJ, Kim CS. Clinical review of acute gastric anisakiasis. Youngnam Univ Med J 1987;4:43-7 .
.
22. Chi JG, Sung RH, Cho SY. Tissue parasitic diseases in Korea. J Korean Med Sci 1988;3:51-62.
.
23. Yang DH, Cho JK, Yoon CM, Suh SP, Kim SJ, Lim YK, et al. Anisakis. Korean J Gastrointest Endosc 1988;8:133-6 .
.
24. Ko GH, Park CK, Kong HJ, Choi CS, Lee SH, Hong SJ. Intestinal anisakiasis. Korean J Pathol 1988;22:154-8 .
.
25. Han DS, Han YB, Park MI, Kim SH, Kim SS. Clinical study of anisakiasis. J Korean Med Assoc 1988;31:645-50 .
.
26. Ahn MH, Min DY, Jung HC, Lee GY. Human infection caused by a larva of Anisakis sp. Korean J Parasitol 1988;26:309.
.
27. Jang GL, Chung JY, Kim WK, Kim KS, Kim JG. Clinical observation of 12 cases of gastric anisakiasis. Korean J Intern Med 1989;37:403-10 .
.
28. Jin SY, Jung SH, Kim TS. Acute gastric anisakiasis. Korean J Pathol 1989;23:149-53 .
.
29. Lee HS, Kim SJ, Rim KS, Suh JE, Kang JO. Three cases of acute gastric anisakiasis. Human Sci 1989;13:413-6 .
.
30. Lee MS, Cho SW, Kim JH, Cho SW, Shim CS, Moon C, et al. Ileal anisakiasis: a case report. Korean J Gastroenterol 1989;21:639-44 .
.
31. Choi SH, Ahn BM, Kim JK, Han SW, Shim GS, Baek NJ, et al. Five cases of acute gastric anisakiasis. Korean J Gastroenterol 1989;21:593-9 .
.
32. Jung JY, Kim WK, Song JH, Kim KS, Kim JG, Park HC, et al. Eosinophilic enteritis caused by Anisakis infestation. Korean J Gastroenterol 1990;22:434-40 .
.
33. Park SH, Suh JM, Shim KS,Baeg NJ, Kim BS, Moon IS. A case report of intestinal anisakiasis. Korean J Gastrointest Endosc 1990;10:373-5 .
.
34. Im KI, Yong TS, Shin HJ, Kim BH, Moon SI. Gastric anisakiasis in Korea - with review of 47 cases. Yonsei Rep Trop Med 1990;21:1-7.
.
35. Im KI and Shin HJ. Morphological observation of Terranova sp. larvae found in human stomach wall. Yonsei Rep Trop Med 1991;22:35-41.
.
36. Kim SE, Kim SL, Lee KS, Kim JM, Jang MY, Cho JG, et al. Nine cases of acute gastric anisakiasis. Korean J Gastrointest Endosc 1991;23:866-72 .
.
37. Kim LS, Lee YH, Kim S, Park HR, Cho SY. A case of anisakiasis causing intestinal obstruction. Korean J Parasitol 1991;29:93-6.
.
38. Sohn WM, Kho WG, Seol SY, Chung JM. Two cases of gastric anisakiasis caused by Anisakis type I larva. Inje Med J 1991;12:421-5 .
.
39. Shin JT, Oh SJ, Park SM, Kim YH, Park YK. A case of ileal anisakiasis. Ann Surg Treat Res1992;43:152-6 .
.
40. Lee SH, Sin HG, Seol SY, Chung JM. A case of gastric anisakiasis causing gastric ulcer bleeding. Korean J Gastrointest Endosc 1993;13:693-6 .
.
41. Lee HS, Park KS, Jung KT, Yoo SJ, Kho JH, Park PS, et al. A case of chronic gastric anisakiasis with massive bleeding. Korean J Gastrointest Endosc 1993;13:697-700 .
.
42. Kim J, Chung WS, Cho KH. Status of parasitic infection diagnosed by surgical biopsy in Kwangju and Chollanam-do. Korean J Parasitol 1994;32:93-100 .
.
43. Sohn WM and Seol SY. A human case of gastric anisakiasis by Pseudoterranova decipiens larva. Korean J Parasitol 1994;32:53-6.
.
44. Sohn WM and Seol SY. A case of acute intestinal anisakiasis caused by Anisakis type I larva. Inje Med J 1994;15:355-9 .
.
45. Seol SY, Ok SC, Pyo JS, Kim IH, Lee SH, Chung JM, et al. Twenty cases of gastric anisakiasis caused by Anisakis type I larva. Korean J Gastroenterol 1994;26:17-24 .
.
46. Im KI, Shin HJ, Kim BH, Moon SI. Gastric anisakiasis cases in Cheju-do, Korea. Korean J Parasitol 1995;33:179-86 .
.
47. Shim YR, Kim DS, Lee TS. Ileal anisakiasis-report of two cases. Korean J Pathol 1995;29:915 .
.
48. Cho BS, Moon JW, Ahn JG, Lee BC, Jeon HY, Shin KC, et al. Report on 7 cases of anisakiasis involving the upper gastrointestinal tract. Korean J Gastrointest Endosc 1996;16:242-5 .
.
49. Geum MS, Cho CM, Kim DH, Choi SG, Lee CH, Kweon YO, et al. Six cases of acute anisakiasis. Korean J Gastrointest Endosc 1997;17:680-3 .
.
50. Kim HJ, Park C, Cho SY. A case of extraintestinal anisakiasis involving a mesocolic lymph node. Korean J Parasitol 1997;35:63-6.
.
51. Cho YJ, Chu JP, Lee JH, Jeong GS, Yang MH. Analysis of parasitic diseases by biopsy in Kyunghee Medical Center (1972-1983). Infect Chemother 1998;30:173-9 .
.
52. Guahk JY, Kim YK, Lee MK, Cho YH, Kim KS, Lee YR, et al. A case of gastric anisakiasis causing Mallory-Weiss syndrome. Korean J Gastrointest Endosc 1998;18:727-31 .
.
53. Yoon BH, Kim WY, Cho CH, Lee SW, Kim KH, Kang MW, et al. Endoscopic ligation therapy for upper gastrointestinal bleeding. Korean J Gastrointest Endosc 1998;18:345-50 .
.
54. Lee IH, Jang S, Lee CY, Park JS, Lee HJ, Kim HJ, et al. A case of human infection of the larvae from Pseudoterranova decipiens. Korean J Gastrointest Endosc 1998;18:732-6 .
.
55. Koh MS, Huh S, Sohn WM. A case of gastric pseudoterranoviasis in a 43-year-old man in Korea. Korean J Parasitol 1999;37:47-9.
.
56. Song TJ, Cho SW, Joo KH. Endoscopic findings of acute gastric anisakiasis. Korean J Gastrointest Endosc 1999;19:878-84 .
.
57. Ahn SH, Chon JY, Lee SK, Park BK, Choi BH, Lee YC, et al. A case with gastric anisakiasis presented with a submucosal tumor. Korean J Gastrointest Endosc 1999;19:449-53 .
.
4. Park CY, Chang YW, Kim HJ, Kang SW, Hwang IS, Kim KJ, et al. A case of acute gastric anisakiasis causing massive hematemesis. Korean J Gastrointest Endosc 1999;19:445-8 .
.
59. Kang JH, Park EJ, Cho YB, Kim YS, Lee MS, Shim CS. Two cases of submucosal tumors caused by gastric anisakiasis. Korean J Gastrointest Endosc 1999;19:67-72 .
.
60. Park TG, Lee DH, Lee JS, Kim HS, Sung YH, Choi DH. A case of esophageal anisakiasis. Korean J Gastrointest Endosc 1999;19:597-600 .
.
61. Choi CS, Choi HS, Byun JW, Yoon CO, Kang MS, Jun DW, et al. Two cases of gastric anisakiasis caused by Pseudoterranova decipiens with acute abdominal pain and upper gastrointestinal bleeding. Korean J Gastroenterol 2000;35:501-6 .
.
62. Chung TW, Kang HK, Jeong YY, Jeong GW, Seo JJ, Kim YH, et al. Radiographic findings of gastric anisakiasis. J Korean Radiol Soc 2000;43:209-13.
.
63. Yu JR, Seo M, Kim YW, Oh MH, Sohn WM. A human case of gastric infection by Pseudoterranova decipiens larva. Korean J Parasitol 2001;39:193-6.
.
64. Yeum CH, Ma SK, Kim SW, Kim NH, Kim J, Choi KC. Incidental detection of an Anisakis larva in continuous ambulatory peritoneal dialysis effluent. Nephrol Dial Transplant 2002;17:1522-3.
.
65. Hwang MG, Kim JH, Lee CK, Lee JK. The three cases of anisakiasis in esophagus or gastroesophageal junction. Korean J Gastroenterol 2002;40(suppl):313A.
.
66. Choi P, Hur JW, Lim HJ, Lee JY, Kim DW, Park MI, et al. A case of gastric submucosal tumor suspected to be caused by Anisakis. Korean J Gastrointest Endosc 2003;27:26-30 .
.
67. Lim SR, Hong SN, Kim DS. An investigation of instance infected after eating a slice of raw f ish of Astroconger myriaster. Korean J Clin Lab Sci 2003;35:86-9 .
.
68. Noh JH, Kim BJ, Kim SM, Ock MS, Park MI, Goo JY. A case of acute gastric anisakiasis provoking severe clinical problems by multiple infection. Korean J Parasitol 2003;41:97-100.
.
69. Yoon SW, Yu JS, Park MS, Shim JY, Kim HJ, Kim KW. CT findings of surgically verified acute invasive small bowel anisakiasis resulting in small bowel obstruction. Yonsei Med J 2004;45:739-45.
.
70. Suh YA, Jang HJ, Eun CS, Jang WY, Lee JJ, Kae SH, et al. A case of a submucosal tumor in the ascending colon probably caused by Anisakis. Korean J Gastrointest Endosc 2004;28:2027 .
.
71. Woo DH, Kim HJ, Kim HS, Chang JY, Nam KD, Kim NH, et al. A case of anisakiasis invading the ascending colon. Intest Res 2004;2:120-3 .
.
72. Kwon JH, Uhm JH, Chung KS. A case of gastric anisakiasis with recurrent abdominal pain in a child. Korean J Pediatr Gastroenterol Nutr 2004;7:74-7 .
.
73. Kim BH, Park CU, Lee JH, Yeom SM, Chae DY, Kim SP, et al. A case of anisakiasis concurrently invading the stomach, ileocecal valve and transverse colon. Korean J Gastrointest Endosc 2004;28:43-6 .
.
74. Song HO, Ahn MH, Choi HK, Ryu JS, Min DY, Ree HI. Analysis of 205 cases of parasite infection confirmed in clinical specimens. Korean J Clin Microbiol 2004;7:66-71 .
.
75. Suh SB, Suh BJ, Yu HJ, Lim SD, Kim JP. Gastrointestinal anisakiasis. J Korean Surg Soc 2005;69:417-9 .
.
76. Shin CM, Myung SJ, Cho SJ, Kim JW, Lee KL, Park JH, et al. A case of colonic anisakiasis diagnosed by colonoscopy. Korean J Med 2006;70:S137-40 .
.
77. Park SW, Joo YE, Jung PJ, Park MH, Lee NH, Jhu IK, et al. Three cases of gastric anisakiasis mimicking submucosal tumor. Korean J Gastrointest Endosc 2006;32:381-6 .
.
78. Kim YH, Choi WB, Lee SC, Choi HW. Three cases of colonic anisakiasis. Korean J Gastrointest Endosc 2006;33:239-43 .
.
79. Park JY, Park YJ, Ko HS, Song YJ. Anisakiasis in oral cavity: a case report. Korean J Otolaryngol 2006;49:763-5 .
.
80. Kim JH, Hwang JU, Kim SH, Kim KH, Kang SJ, Kim KS, et al. A case of anisakiasis concurrently invading esophagus and stomach, and another case of esophageal anisakiasis. Korean J Gastrointest Endosc 2006;32:116-9 .
.
81. Cho EY, Song WK, Ahn YH, Oh HJ, Seo GS, Kim TH, et al. Anisakiasis of the colon: report of two cases. Korean J Gastrointest Endosc 2006;32:298-301 .
.
82. Jang ST, Choi IJ, Kim WT, Lee H, Lee SW, Kang SB, et al. A case of rectal anisakiasis. Korean J Gastrointest Endosc 2006;32:156-9 .
.
83. Kim SH, Kim HU, Lee J. A case of gastroallergic anisakiasis. Korean J Med 2006;70:111-6 .
.
84. Kim SG, Jo YJ, Park YS, Kim SH, Song MS, Lee HH, et al. Four cases of gastric submucosal mass suspected as anisakiasis. Korean J Parasitol 2006;44;61-6.
.
85. Shin KD, An CM, Nam SW, Kim SK, Kim SH, Kim IH, et al. A case of esophageal anisakiasis presenting as chest pain mimicking angina. Korean J Gastrointest Endosc 2007;35:19-22.
.
86. Yoo HJ, Kim SH, Lee JM, Kim MA, Han JK, Choi BI. The association of anisakiasis in the ascending colon with sigmoid colon cancer: CT sonographic findings. Korean J Radiol 2008;9(suppl):S56-60.
.
87. Lee CM, Choi JY, Kim JH. Intestinal obstruction caused by anisakiasis. J Korean Surg Soc 2008;74:154-6 .
.
88. Choi SC, Kim K, Lee KR, Cho JH, Park SW, Hong KY, et al. A case of an eosinophilic granuloma mimicking a submucosal tumor in the ascending colon probably caused by Anisakis. Korean J Gastrointest Endosc 2008;37:127-31 .
.
89. Cho MH, Lee SJ, Joung HJ, Lee HS, Kim YD, Jeong WJ, et al. A case of anisakiasis on stomach and colon mimicking submucosal tumor. Int J Infect Dis 2009;13:S93.
.
90. Lee EJ, Kim YC, Jeong HG, Lee OJ. The mucosal changes and influencing factors in upper gastrointestinal anisakiasis: analysis of 141 cases. Korean J Gastroenterol 2009;53:90-7.
.
91. Hong SS, Kim JH, Park ST, Chang YW, Kim HJ, Kwon KH, et al. Duodenal anisakiasis presenting as bowel obstruction and fistula formation: a case report. J Korean Soc Radiol 2009;60:419-22 .
.
92. Choi SJ, Lee JC, Kim MJ, Hur GY, Shin SY, Park HS. The clinical characteristics of Anisakis allergy in Korea. Korean J Intern Med 2009;24:160-3.
.
93. Choi WH, Chu JP, Jiang M, Lee YS, Kim BS, Kim DG, et al. Analysis of parasitic diseases diagnosed by tissue biopsy specimens at Kyunghee Medical Center (1984-2005) in Seoul, Korea. Korean J Parasitol 2010;48:85-8.
.
94. Do KR, Cho YS, Kim HK, Hwang BH, Shin EJ, Jeong HB, et al. Intestinal helminthic infections diagnosed by colonoscopy in a regional hospital during 2001-2008. Korean J Parasitol 2010;48:75-8.
.
95. Kang DB, Oh JT, Park WC, Lee JK. Small bowel obstruction caused by acute invasive enteric anisakiasis. Korean J Gastroenterol 2010;56:192-5.
.
96. Kim WK, Song SY, Cho OK, Koh BH, Kim Y, Jung WK, et al. CT findings of small bowel anisakiasis: analysis of four cases. J Korean J Radiol 2011;64:167-71.
.
97. Hwang D, Park SI, Pack SC, Lee KS, Choi SK, Kang H, et al. A case of duodenal anisakiasis with duodenal ulcer. Chonnam Med J 2012;48:73-5.
.
98. Cho MH, Lee SJ, Joung HJ, Kang JW, Lee KW, et al. A case of gastric and colonic submucosal tumors after the removal of 51 Anisakis larvae. Korean J Med 2012;82:453-8 .
.
99. Kim SH, Beom JW, Kim SH, Jo J, Song HJ, Lee J. A case of anaphylaxis caused by acute gastric anisakiasis. Korean J Asthma Clin Immunol 2012;32:276-9 .
.
100. Na HK, Seo M, Chai JY, Lee EK, Jeon SM. A case of anisakidosis caused by Pseudoterranova decipiens larva. Korean J Parasitol 2013;51:115-7.
.
101. Yoon DH, Lee HB, Seon KH, Cho SH. A case of small bowel obstruction and perforation by anisakiasis. J Korean Soc Emergency Med 2013;24:246-9 .
.
102. Kim T, Song HJ, Jeong SU, Choi EK, Cho YK, Kim HU, et al. Comparison of the clinical characteristics of patients with small bowel and gastric anisakiasis in Jeju Island. Gut Liver 2013;7:23-9.
.
103. Kim SH, Park CW, Kim SK, Won S, Park WK, Kim HR, et al. A case of anisakiasis invading the stomach and the colon at the same time after eating anchovies. Clin Endosc 2013;46:293-6.
.
104. Choi SC, Lee SY, Song HO, Ryu JS, Ahn MH. Parasitic infections based on 320 clinical samples submitted to Hanyang University, Korea (2004-2011). Korean J Parasitol 2014;52:215-20.
.
105. Lee JS, Kim BS, Kim SH, Park JK, Choi G, Hwang IK, et al. Acute invasive small-bowel anisakiasis: clinical and CT findings in 19 patients. Abdom Imaging 2014;39:452-8.
.
106. Kang DB, Park WC, Lee JK. Chronic gastric anisakiasis provoking a bleeding gastric ulcer. Ann Surg Treat Res 2014;86:270-3.
.
107. Lim H, Jung BK, Cho J, Yooyen T, Shin EH, Chai JY. Molecular diagnosis of cause of anisakiasis in humans, South Korea. Emerg Infect Dis 2015;21:342-4.
.
108. Choi SK, Kim CK, Kim SH, Jo DI. Anisakiasis involving the oral mucosa. Arch Craniofac Surg 2017;18:261-3.
.
109. Park EY, Baek DH, Kim GH, Lee BE, Lee SJ, Park DY. Endosonographic findings and the natural course of chronic gastric anisakiasis: a single-center experience. Gastroenterol Res Pract 2018;8562792.
.
110. Kim BH, Park HU, Park SK, Jeon SM, Jung CW, Son CM, et al. Anisakiasis induced segmental jejunum obstruction. Korean j Gastroenterol 2018;72:33-6 .
.
111. Choi YI, Park DK, Cho HY, Choi SJ, Chung JW, Kim KO, et al. Adult intussusception caused by colonic Anisakis: a case report. World J Clin Cases 2019;7:2536-41.
.
112. Joo SK, Kim JW, Kim BG, Kim W, Lee JK, Lee KL. Clinical and endoscopic features of colonic anisakiasis. Korean J Parasitol 2019;57:411-5.
.
113. Song H, Jung BK, Cho J, Chang T, Huh S, Chai JY. Molecular identification of Anisakis larvae extracted by gastrointestinal endoscopy from health check-up patients in Korea. Korean J Parasitol 2019;57:2070211.
.
114. Jeong IS and Ahn JY. A case of anisakiasis involving esophageal mucosa. Korean J Med 2020;suppl:S078.
.
115. Joo DC, Kim GH, Lee MW. Acute anisakiasis at the esophagogastric junction mimicking angina pectoris. Korean J Helicobacter Up Gastrointest Res 2021;21:161-4.
.
116. Cha EJ, Kim JS, Heo ST. Anisakiasis in palatine tonsil. J Craniofac Surg 2022;33:e692-4.
.
117. Lee YJ, Joo KH, Chung MS, Rim HJ. Studies on the serodiagnosis of anisakiasis using immunoblot. Korea Univ Med J 1990;27:149-56 .
.
118. Rim HJ, Lee JS, Joo KH, Chung MS. Studies on the seroepidemiology of helminthic diseases in Korea. Korean J Rural Med 1991;16:48-60 .
.
119. Kim J, Jo JO, Choi SH, Cho MK, Yu HS, Cha HJ, et al. Seroprevalence of antibodies against Anisakis simplex larvae among health-examined residents in three hospitals of southern parts of Korea. Korean J Parasitol 2011;49:139-44.
.
120. Chung YB and Lee J. Clinical characteristics of gastroallergic anisakiasis and diagnostic implications of immunologic tests. Allergy Asthma Immunol Res 2014;6:228-33.
.
121. Kim JY, Yi MH, Yong TS. Parasitic infections and medical expenses according to Health Insurance Review Assessment claims data in South Korea, 2011-2018. PLoS One 2019;14:e0225508.
.
122. Quiazon KMA, Yoshinaga T, Ogawa K. Distribution of Anisakis species larvae from fishes of the Japanese waters. Parasitol Int 2011;60:223-6.
.
123. Chai JY, Chu YM, Sohn WM, Lee SH. Larval anisakids collected from the yellow corvina in Korea. Korean J Parasitol 1986;24:1-11.
.
124. Chai JY, Cho SR, Kook J, Lee SH. Infection status of the sea eel (Astroconger myriaster) purchased from the Noryangjin fish market with anisakid larvae. Korean J Parasitol 1992;30:157-62 .
.
125. Woo HC and Kim JA. The infection status and identification of anisakid larvae in marine fish caught from the sea near Cheju Island. Korean J Vet Public Health 2000;24:307-17 .
.
126. Song SB and Hwang EG. Infection status of larval anisakids in Astroconger myriaster collected from the Southern Sea near Pusan. Korean J Parasitol 1992;30:263-7 .
.
127. Alonso A, Daschner A, Moreno-Ancillo A. Anaphylaxis with Anisakis simplex in the gastric mucosa. N Engl J Med 1997;337:350-1.
.
128. Audicana MT and Kennedy MW. Anisakis simplex: from obscure infectious worm to inducer of immune hypersensitivity. Clin Microbiol Rev 2008;21:360-79.
.
129. Audicana MT. Anisakis, something is moving inside the fish. Pathogens 2022;11:326.
.
130. Kimura S, Takagi Y, Gomi K. IgE response to Anisakis compared to seafood. Allergy 1999;54:1225-6.
.
131. Mattiucci S, Fazii P, Rosa AD, Paoletti M, Megna AS, Glielmo A, et al. Anisakiasis and gastroallergic reactions associated with Anisakis pegreff ii infection, Italy. Emerg Infect Dis 2013;19:496-9.
.
132. Rahmati AR, Kiani B, Afshari A, Moghaddas E, Williams M, Shamsi S. Word-wide prevalence of Anisakis larvae in fish and its relationship to human allergic anisakiasis: a systematic review. Parasitol Res 2020;119:3585-94.
.
133. Saelens G, Plankaert S, Nartinez-Sernández V, Ubeira FM, Devresse B, et al. Targeted proteomics and specific immunoassays reveal the presence of shared allergens between the zoonotic nematodes Anisakis simplex and Pseudoterranova decipiens. Sci Rep 2022;12:4127.
.
134. Chung YB and Lee J. Clinical characteristics of gastroallergic anisakiasis and diagnostic implications of immunologic tests. Allergy Asthma Immunol Res 2014;56:228-33.
.
135. Audicana MT, de Corres LF, Muñoz D, Fernández E, Navarro JA, del Pozo MD. Recurrent anaphylaxis caused by Anisakis simplex parasitizing fish. J Allergy Clin Immunol 1995;96:558-60.
.
136. Daschner A and Cuéllar C. Progress in Anisakis allergy research: milestones and reversals. Curr Treat Options Allergy 2020;7:457-70.
.
137. Moneo I, Carballeda-Sangiao N, González-Muñoz M. New perspectives on the diagnosis of allergy to Anisakis spp. Curr Allergy Asthma Resp 2017;17:27.
.
138. Kim JS, Kim KH, Cho S, Park HY, Cho SW, Kim YT, et al. Immunochemical and biological analysis of allergenicity with excretory-secretory products of Anisakis simplex third-stage larvae. Int Arch Allergy Immunol 2005;136:320-8.
.
139. Hwang YK, Kim JS, Lee JB, Song TJ, Joo KH, Lee JS, et al. Human anisakiasis: diversity in antibody response profiles to the changing antigens in larval excretions/secretions. Parasite Immunol 2003;25:1-7.
.
140. Choi SJ, Hur GY, Um SJ, Choi GS, Park HJ, Ye YM, et al. Identification of IgE binding components and allergenic relationships of the somatic antigens of Anisakis simplex. Korean J Asthma Clin Immunol 2008;28:277-83 .
.
141. Cho TH, Park HY, Cho S, Sohn J, Yoon YW, Cho JE, et al. The time course of biological and immunochemical allergy states induced by Anisakis simplex larvae in rats. Clin Exp Immunol 2006;143:203-8.
.
142. Cho SW and Lee HN. Immune reactions and allergy in experimental anisakiasis. Korean J Parasitol 2006;44:271-83.
.
143. Choi JH, Kim JY, Yi MH, Kim M, Yong TS. Anisakis pegreffii extracts induces airway inflammation with airway remodeling in a murine model system. BioMed Res Int 2021;2021:2522302.
.
144. Park SK, Cho MK, Park HK, Lee KH, Lee SJ, Choi SH, et al. Macrophage migration inhibitory factor homologs of Anisakis simplex suppresses Th2 response in allergic airway inflammation model via CD4+CD25+Foxp3+ T cell recruitment. J Immunol 2009;182:690714.
.
145. Park JS, Cho MK, Yu HS, Ahn SC. Identification of a 24 kDa excretory secretory protein in Anisakis simplex. Exp parasitol 2012;130;69-72.
.
146. Park HK, Cho MK, Park MK, Kang SA, Kim YS, Kim KU, et al. A 24 kDa excretorysecretory protein of Anisakis simplex larvae could elicit allergic airway inflammation in mice. Korean J Parasitol 2011;49:373-80.
.
147. Kim BJ, Kim SM, Cho MK, Yu HS, Lee YS, Cha HJ, et al. Expression and characterization of a α-methyacyl CoA racemase from Anisakis simplex larvae. Korean J Parasitol 2012;50:65171.
.
148. Jeon CH, Wi S, Kim JH. A comparison of the hydrolase activities of excretory-secretory product and somatic extracts from fish parasitic nematodes, Anisakis simplex sensu stricto and Anisakis pegreffii larvae. J Fish Pathol 2014;27:25-33 .
.
149. Cho MK, Park MK, Kang SA, Caballero ML, Perez-Pinar T, Rodriguez-Perez R, et al. Allergenicity of two Anisakis simplex allergens evaluated in vivo using an experimental mouse model. Exp Parasitol 2014;146:71-7.
.
150. Cha HJ and Ock MS. Preventive and therapeutic effects of Anisakis simplex larval proteins in a mouse model of Crohn’s disease. Kosin Med J 2013;28:107-13.
.
151. Garcia-Perez JC, Rodriguez-Perez R, Ballestero A, Zuloaga J, Fernandez-Puntero B, AriasDíaz J, et al. Previous exposure to the fish parasite Anisakis as a potential risk factor for gastric or colon adenocarcinoma. Medicine 2015;94:107.
.
152. Messina CM, Pizzo F, Santulli A, Bušellic I, Boban M, Orhanovic S, et al. Anisakis pegreff ii (Nematoda: Anisakidae) products module oxidative stress and apoptosis-related biomarkers in human cell lines. Parasit Vectors 2016;9:607.
.
153. Corcuera MT, Rodriguez-Bobada C, Zuloaga J, Gómez-Aguado F, Rodriguez-Perez R, Mendizabal A, et al. Exploring tumourigenic potential of the parasite Anisakis: a pilot study. Parasitol Res 2018;117:3127-36.
.
154. Adroher-Auroux FJ and Benítez-Rodríguez R. Anisakiasis and Anisakis: an underdiagnosed emerging disease and its main etiological agents. Res Vet Sci 2020;132:535-45.
.
155. Mineta S, Shimanuki K, Sugiura A, Tsuchiya Y, Kaneko M, Sugiyama Y, et al. Chronic anisakiasis of the ascending colon associated with carcinoma. J Nippon Med Sci 2006;73:16974.
.
156. Sakurai E, Okubo M, Tsutsumi Y, Shibata T, Tahara T, Kiriyama Y, et al. A case of chronic gastric anisakiasis coexisting with early gastric cancer. Fujita Med J 2023;9:163-9.
.
157. Hong ST and Lee SH. Histopathological and serological observations I experimental anisakiasis of rabbits. Korean J Parasitol 1987;25:168-80.
.
158. Kwon YP and Chyu I. Studies on the host factors influencing the infection of Anisakis larvae in rabbits. J Catholic Med Coll 1968;15:103-16.
.
159. Choi WJ and Kim JP. Experimental anisakiasis in albino rats and rabbits. Seoul J Med 1984;25:569-78 .
.
160. Jeon CH and Kim JH. Pathogenic potential of two sibling species, Anisakis simplex (s.s.) and A. pegreffii (Nematoda: Anisakidae): in vitro and in vivo studies. BioMed Res Int 2015;2015:983656.
.
161. Moon MH and Kwak SD. Natural cases of pig anisakiasis. Korean J Vet Res 1980;21:45-50 .
.
162. Kang MI, Rim BH, Lee JG. Pathological studies on the anisakiasis in swine. Korean J vet Res 1981;21:7-10 .
.
163. Moon MH and Choi WP. Studies on the serodiagnosis for swine anisakiasis. J Korean Vet Med Assoc 1982;18:51-63 .
.
164. Huh S, Sohn WM, Chai JY. Intestinal parasites of cats purchased in Seoul. Korean J Parasitol 1993;31:371-3.
.
165. Sohn WM and Chai JY. Infection status with helminthes in feral cats purchased from a market in Busan, Republic of Korea. Korean J Parasitol 2005;43:93-100.
.
166. Kim HC, Kim MS, Son HY, Kwon BK, Jin H, Cho JG, et al. Morphological study of Contracaecum rudolphii (Nematoda: Anisakidae) from white pelican. J Vet Clin 2010;27:11-6.
.
167. Lee SB, Yuen AHL, Lee YM, Kim SW, Kim S, Poon CTC, et al. Adhesive bowel obstruction (ABO) in a stranded narrow-ridged finless porpoise (Neophocaena asiaeorientalis sumameri). Animals 2023;13:3767.
.
168. Kim S, Lee BS, Choe S. Phylogenetic and phylogeographic analyses of Anisakis simplex sensu stricto (Nematoda: Anisakidae) from the common minke whale in Korean waters. Parasites Hosts Dis 2023;61:240-50.
.
169. Chun SK, Chung BK, Ryu BS. On the infection rate of Anisakis-like larvae isolated from various marine fishes. Bull Korean Fisher Soc 1968;1:7.
.
170. Lim JT. Studies on Anisakis type larvae. Korean J Vet Res 1975;15:293-307 .
.
171. Kim KH, Joo KH, Lee JS, Rim HJ. Morphological classification and infection rate of anisakid larvae in marine fishes. Korean J Rural Med 1988;13:32-40.
.
172. Kim KH, Joo KH, Quan FS, Rim HJ. Infection state and classification of anisakid larvae in salmon (Oncorhynchus keta) caught from Taep’o Port, Kangwon-do. Korean J Rural Med 1990;15:3-8.
.
173. Sun S, Koyama T, Kagei N. Anisakidae larvae found in marine fishes and squids from the gulf of Tongking, the East China Sea and the Yellow Sea. Jpn J Med Sci Biol 1991;44:99-108.
.
174. Song SB, Lee SR, Chung HH, Han NS. Infection status of anisakid larvae in anchovies purchased from local fishery market near southern and eastern sea in Korea. Korean J Parasitol 1995;33:95-9.
.
175. Chun KS and Kim SW. Infection status of Tadarodes pacif icus (Mollusca: Cephalopoda) with anisakid larvae in the South Sea, Korea. J Korean Soc Oceanogr 1995;30:197-202.
.
176. Jeong HJ and Song SB. Infection status on anisakids of yellow corvina caught from southern sea in Korea. J Pusan Med Assoc 1999;35:5-13 .
.
177. Song SB, Jeong HJ, Han NS, Hwang EG, Kim C, Huh D, et al. Infection status on nematodes of human parasites in marine fish. J Pusan Med Assoc 2001;37:8-22 .
.
178. Choi HJ, Kim EJ, Lee DC, Cho MY, Lee BY, Im YS, et al. Survey of Anisakis spp. infection in wild populations of marine fish caught from coastal areas of Korea. J Fish Pathol 2009;22:201-10 .
.
179. Seo JS, Jun EJ, Jung SH, Kim MS, Park MA, Lee CH, et al. Prevalence of anisakid larvae in chum salmon Oncorhynchus keta in Korea. J Fish Pathol 2010;23:123-9 .
.
180. Jeon CH, Setyobudi E, Kim JH. Molecular identification of anisakid worm third stage larvae isolated from masou salmon Oncorhynchus masou. J Fish Pathol 2010;23:421-7 .
.
181. Setyobudi E, Jeon CH, Lee CH, Seong KB, Kim JH. Occurrence and identification of Anisakis spp. (Nematoda: Anisakidae) isolated from chum salmon (Oncorhynchus keta) in Korea. Parasitol Res 2011;108:585-92.
.
182. Choi SH, Kim J, Jo JO, Cho MK, Yu HS, Cha HJ, et al. Anisakis simplex larvae: infection status in marine fish and cephalopods purchased from the Cooperative Fish Market in Busan, Korea. Korean J Parasitol 2011;49:39-44.
.
183. Cho SH, Lee SE, Park OH, Na BK, Sohn WM. Larval anisakid infections in marine fish from three sea areas of the Republic of Korea. Korean J Parasitol 2012;50:295-9.
.
184. Bak TJ, Jeon CH, Kim JH. Occurrence of anisakid nematode larvae in chub mackerel (Scomber japonicus) caught off Korea. Int J Food Microbiol 2014;191:149-56.
.
185. Cho J, Lim H, Jung BK, Shin EH, Chai JY. Anisakis pegreffii larvae in sea eels (Astroconger myriaster) from the South Sea, Republic of Korea. Korean J Parasitol 2015;53:349-53.
.
186. Lee WJ, Seo DJ, Oh H, Jeon SB, Jung D, Choi C. Simultaneous detection and prevalence of allergens in Anisakis species isolated from marine fishes. J Food Protect 2016;79:789-94.
.
187. Kim JH, Nam WH, Jeon CH. Genetic identification of anisakid nematodes isolated from largehead hairtail (Trichiurus japonicus) in Korea. Fisher Aquat Sci 2016;19:26.
.
188. Neves MAAM. Molecular identification of Anisakis larvae (Nematoda: Anisakidae) from marine fishes collected from three Korean seawaters. [Master’s thesis]. Yonsei University; 2019. p 1-33.
.
189. Chang T, Jung BK, Hong S, Shin H, Lee J, Patarwut L, et al. Anisakid larvae from anchovies in the South Coast of Korea. Korean J Parasitol 2019;57:699-704.
.
190. Yamaguti S. Studies on the helminth fauna of Japan. Part. 33. Nematodes of fish (II). Jpn J Zool 1941;9:343-96.
.
191. Berland B. Nematodes from some Norwegian marine fishes. Sarsia 2:1-50.
.
192. Koyama T, Kobayashi A, Kumada M, Komiya Y, Oshima T, Kagei N, et al. Morphological and taxonomical studies on anisakidae larvae found in marine fishes and squids. Jpn J Parasitol 1969;18:466-87.
.
193. Rim BH. Studies on anisakiasis. Especially morphological studies on the Anisakinae larva. Korean J Vet Res 1981;21:105-12.
.
194. Sohn WM and Lee SH. Cross sectional observation of Anisakis and Contracaecum larvae. Inje Med J 1989;10:463-79.
.
195. Sohn WM. Ultrastructural changes on the cuticular surface, excretory and digestive organs of Anisakis simplex larvae chronologically recovered from experimental cats. Korean J Electron Microsc 1999;29:211-21.
.
196. Chai JY, Guk SM, Sung JJ, Kim HC, Park YM. Recovery of Pseudoterranova decipiens (Anisakidae) larvae from codfish of the Antarctic Ocean. Korean J Parasitol 1995;33:231-4.
.
197. Lee YJ, Joo KH, Chung MS, Rim HJ. Studies on the serodiagnosis of anisakiasis using immunoblot. Korea Univ Med J 1990;27:149-56.
.
198. Quan FS, Chung MS, Joo KH, Rim HJ. Application of various antigens on the detection of antibody in rabbits infected with anisakid larvae. Korean J Rural Med 1991;16:70-8.
.
199. Yang HJ, Cho YJ, Paik YH. Changes of IgM and IgG antibody levels in experimental rabbit anisakiasis as observed by ELISA and SDS-PAGE/immunoblot. Korean J Parasitol 1991;29:389-96.
.
200. Choi KH, Chung MS, Joo KH, Lee JS. Characterization of Anisakis larval antigen after gel f iltration using immunoblot. Korea Univ Med J 1994;31:365-76.
.
201. Rim HJ, Lee JS, Joo KH, Chung MS. Studies on the seroepidemiology of helminthic diseases in Korea. Korean J Rural Med 1991;16:48-60.
.
202. Kim J, Jo JO, Choi SH, Cho MK, Yu HS, Cha HJ, et al. Seroprevalence of antibodies against Anisakissimplex larvae among health-examined residents in three hospitals of southern parts of Korea. Korean J Parasitol 2011;49:139-44.
.
203. Kim KH, Eom KS, Park JK. The complete mitochondrial genome of Anisakissimplex (Ascaridida: Nematoda) and phylogenetic implications. Int J Parasitol 2006;36:319-28.
.
204. Yu HS, Park SK, Lee KH, Lee SJ, Choi SH, Ock MS, et al. Anisakissimplex: analysis of expressed sequence tags (ESTs) of third-stage larva. Exp Parasitol 2007;117:51-6.
.
205. Kang JH, Lee MH, Lee KB, Choi C. Comparison of macroscopic inspection and polymerase chain reaction-restriction fragment length polymorphism (PCR-RFPL) for the detection of Anisakis simplex complex. J Food Hyg Safety 2008;23:314-8.
.
206. Lee MH, Cheon DS, Choi C. Molecular genotyping of Anisakis species from Korean sea fish by polymerase chain reaction-restriction fragment length polymorphism (PCR-RFLP). Food Contr 2009;20:623-6.
.
207. Kim SM, Cho MK, Yu HS, Cha HJ, Ock MS. Application of the 18S ribosomal DNA (rDNA) PCR-RFLP technique for the differential diagnosis of anisakidosis. J Life Sci 2009;19:132832 .
.
208. Park JJ, Park MA, Choi HS, Kim SR. Morphological comparison of Hysterothylacium sp. and Anisakis simplex (Nematoda: Anisakidae) from wild black rockfish, Sebastes schlegeli, and histopathological host reaction. Korean J Microsc 2011;41:205-13.
.
209. Kim WS, Jeon CH, Kim JH, Kim DH, Oh MJ. Current status of anisakid nematode larvae infection in marine fishes caught from the coastal area of Korea between 2010 and 2012. J Fish Pathol 2012;25:189-97.
.
210. Setyobudi E, Jeon CH, Choi K, Lee SI, Lee CI, Kim JH. Molecular identification of anisakid nematodes third stage larvae isolated from common squid (Todarodes pacificus) in Korea. Ocean Sci J 2013;48:197-205.
.
211. Sohn WM, Kang JM, Na BK. Molecular analysis of Anisakis type I larvae in marine fish from three different sea areas in Korea. Korean j Parasitol 2014;52:383-9.
.
212. Jeon CH, Setyobudi E, Kim JH. Occurrence and molecular identification of anisakid nematodes isolated from pacific cod (Gadus microcephalus) caught off Korea. Food Contr 2016;69:100-7.
.
213. Nurhidayat SW, Nam UH, Kim JH. Occurrence of anisakid nematodes in Walleye pollock (Gadus chalcogrammus) caught off the East Sea of Korea: their molecular identification and biological implication. Ocean Sci J 2018;53:679-89.
.
214. Kim JH, Kim JO, Jeon CH, Nam UH, Subramaniyam S, Yoo SI, et al. Comparative transcriptome analyses of the third and fourth stage larvae of Anisakis simplex (Nematoda: Anisakidae). Mol Biochem Parasitol 2018;226:24-33.
.
215. Kim HS, Baek KW, Park MK, Jeon KY, Ko EJ, Cha HJ, et al. Establishment and validation of ARMS (amplification-refractory mutation system) for identification of Anisakis species collected from Korean waters. Gene 2019;691:125-31.
.
216. Lee WH, Chyu I. Experiments on the resistance and infectivity of Anisakis larvae. J Catholic Med Coll 1970;18:229-37.
.
217. Chai JY, Hong ST, Lee SH. Effects of gamma irradiation on the survival and infectivity of Anisakis larvae. Final research co-ordinating meeting on use of irradiation to control infectivity of food-borne parasites; 1993. Mexico City; Joint FAO/IAEA Division of Neclear Techniques in Food and Agriculture; 1993. p 43-8.
.
218. Seo M, Kho BM, Guk SM, Lee SH, Chai JY. Radioresistance of Anisakis simplex third-stage larvae and the possible role of superoxide dismutase. J Parasitol 2006;92:416-8.
.
219. Jeon JH and Jee CH. Larval migration inhibition activity of ivermectin, doramectin and ethanol against Anisakis simplex in vitro. Korean J Vet Res 2007;47:197-202.
.
220. Lee SH and Jee CH. Effect of ivermectin and doramectin for larval (Anisakis sp.) migratory inhibition in rats. Korean J Vet Serv 31:93-100.
.
221. Kwon HN and Jee CH. Inhibitory effects of herbal extracts (Meliae ezadarach, Dryopteris crassirhizoma, Quisqualis indica var villosa) on larval migration of Anisakis spp. in vitro. Korean J Vet Res 2008;48:473-80.
.
222. Oh SR, Zhang CY, Kim TI, Hong SJ, Ju IS, Lee SH, et al. Inactivation of Anisakis larvae in salt-fermented squid and pollock tripe by freezing, salting, and combined treatment with chlorine and ultrasound. Food Contr 2014;40:46-9.
.
223. Lee KH, Park SY, Ha SD. Inactivation of Anisakis simplex L3 in the flesh of white spotted conger (Conger myriaster) by high hydrostatic pressure and its effect on quality. Food Addit Contaminants (Part A) 2016;33:1010-5.
.
224. Nam UH, Lee SY, Lee JH, Kim JH. Survival of Anisakis species larvae of chub mackerel (Scomber japonicus) in different kinds of condiments. J Fish Pathol 2021;34:249-53.
.
Go to : 
Table 5
Marine fish, cephalopods, or other marine food dishes consumed by anisakidosis patients in Korea (1971-2022)
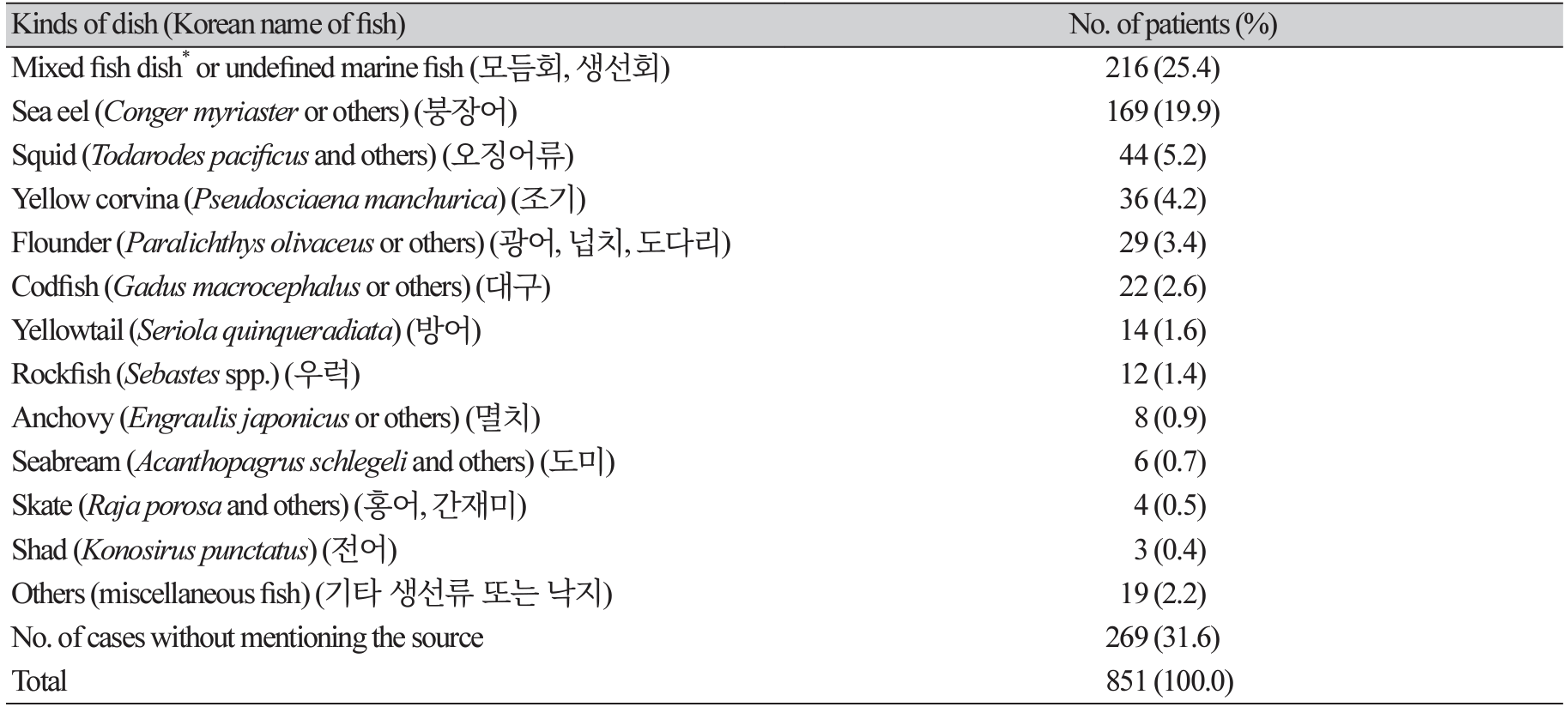
Table 7
pecies of marine fish examined for anisakid larvae* in Korea (1968-2012)†(continued)
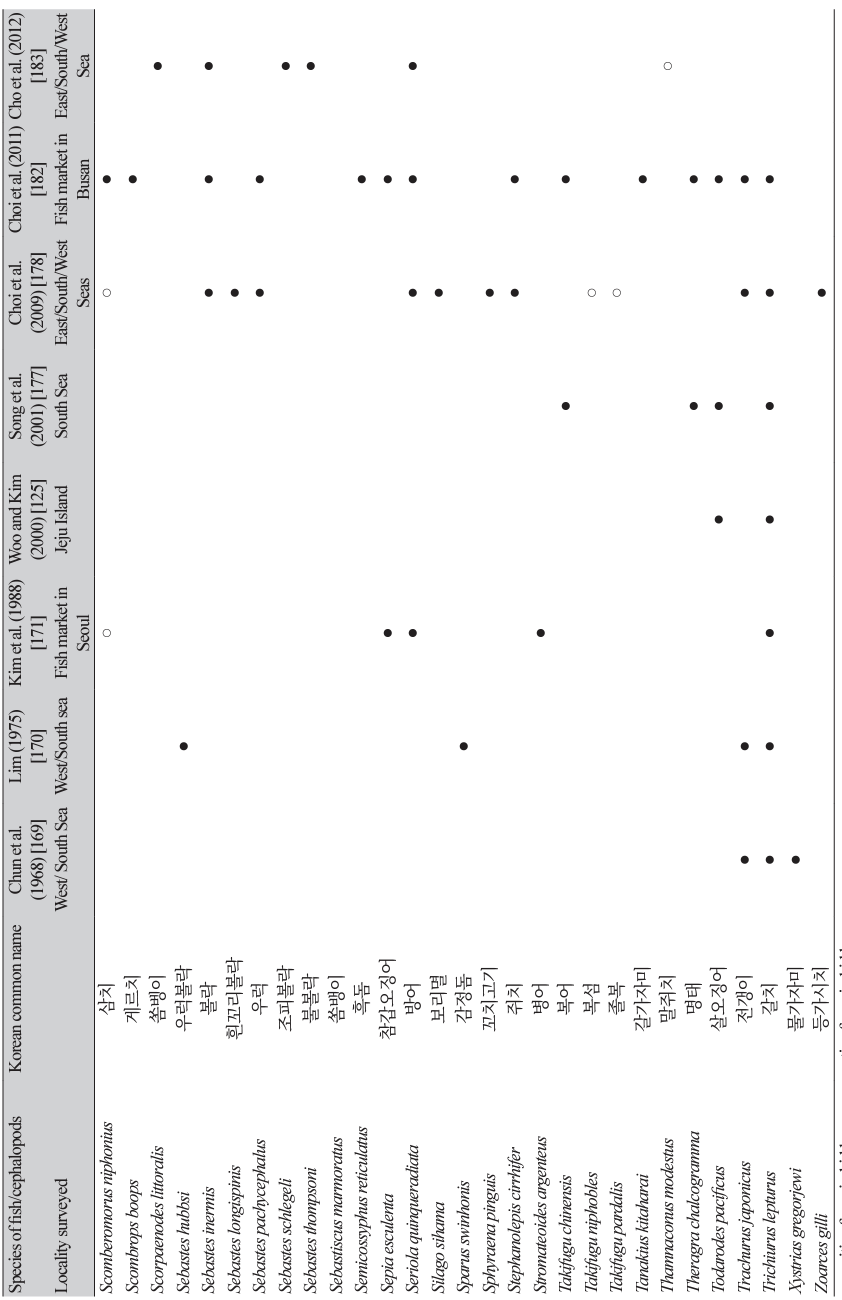
● positive for anisakid larvae; ○ negative for anisakid larvae. *Anisakid larvae recovered were mostly Anisakis type I (A. pegreffii or A. simplex) followed by Contracaecum type A, B, C, C′, D, D′ (probably Contracaecum osculatum for all these types), Contracaecum type V or others, Terranova type A (P. decipiens) or type B (P. decipiens), Raphidascaris sp., and very rarely Anisakis type II (A. simplex). †Only studies which examined more than 7 species of fish were included in this table.
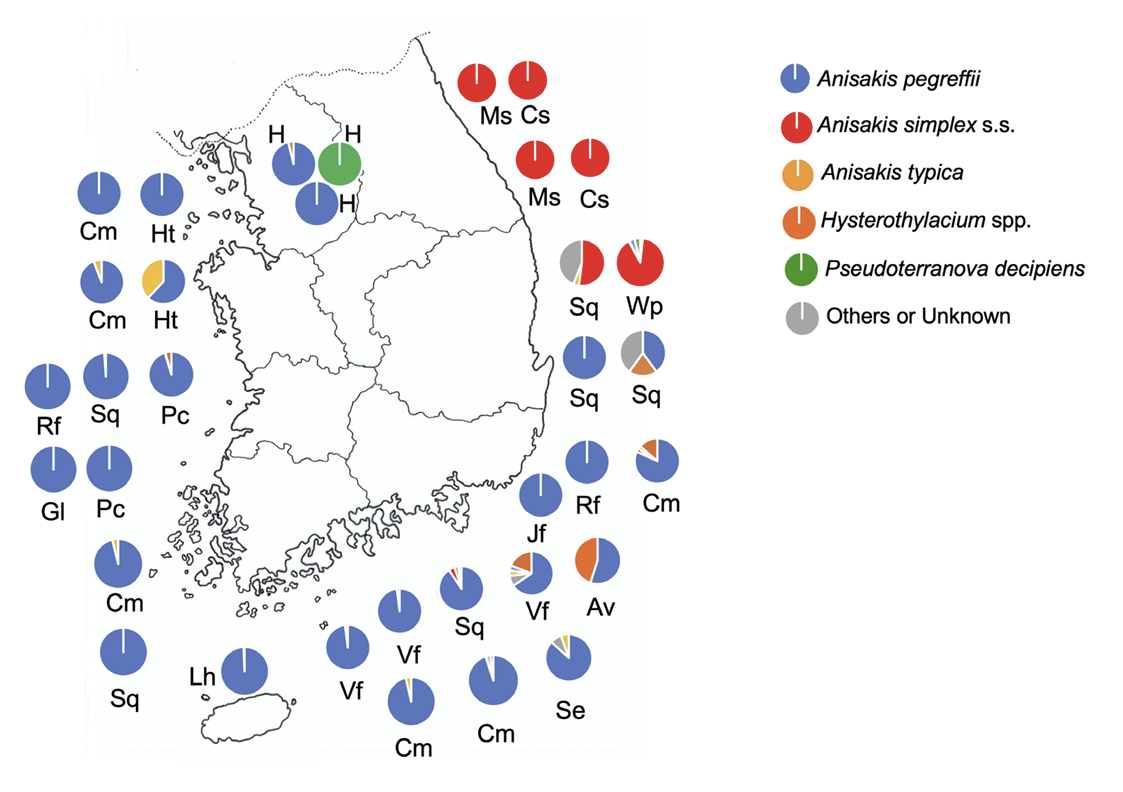 | Fig. 4.Species of anisakid larvae detected in humans (nationwide, mostly from Seoul) and fish/ squids from the eastern, southern, and western seas of Korea based on molecular analyses [3,107,113,178,180,181,184-189,205-213]. Av, anchovy; Cm, chub mackerel; Cs, chub salmon; Jf, Japanese flounder; Gl, greenling; H, human; Ht, hairtail; Lh, large-head hairtail; Ms, masou salmon; Pc, Pacific cod; Rf, rockfish; Se, sea eel; Sq, squid; Vf, various fish; Wp, walleye pollock. |




 PDF
PDF Citation
Citation Print
Print


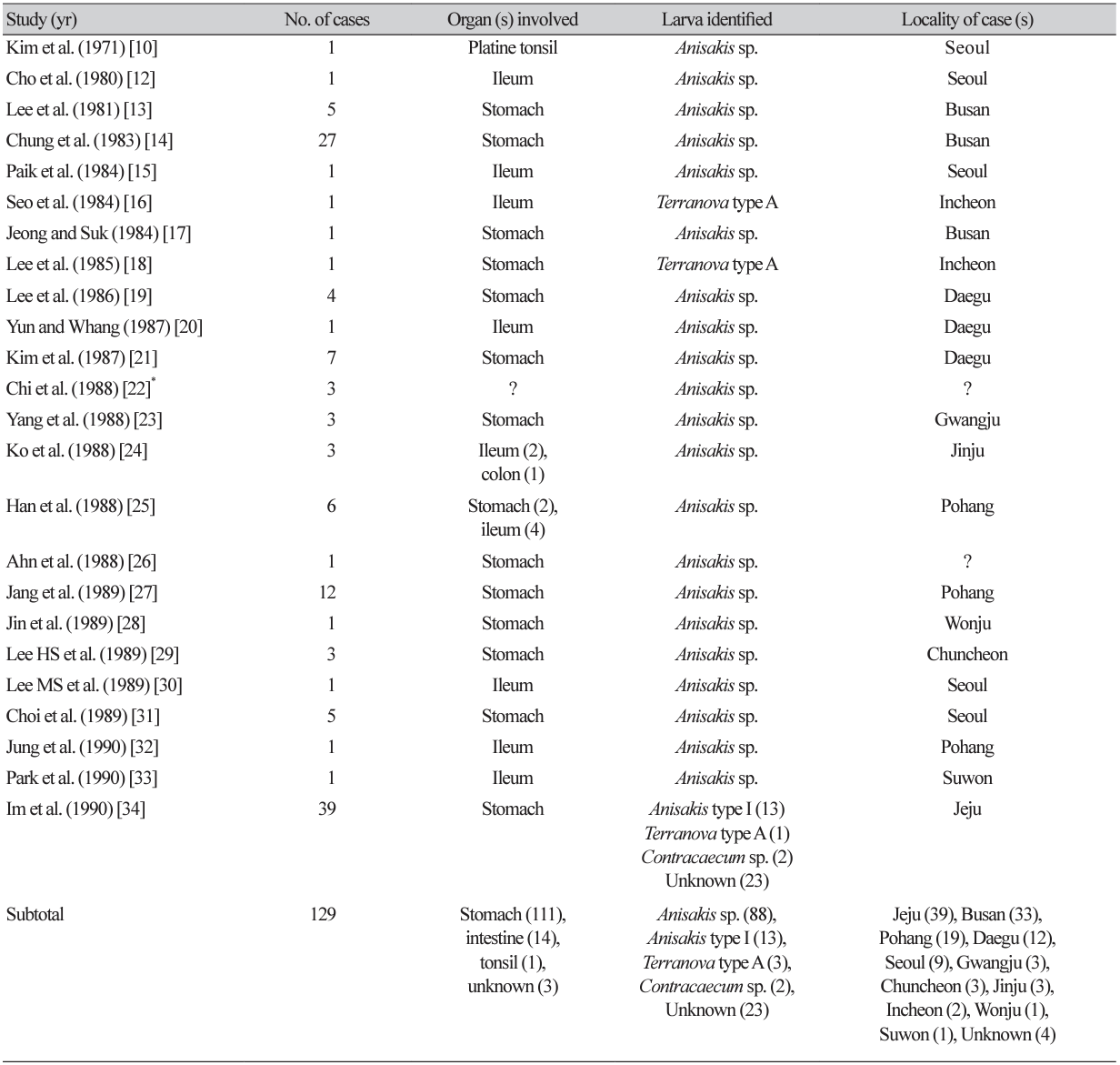
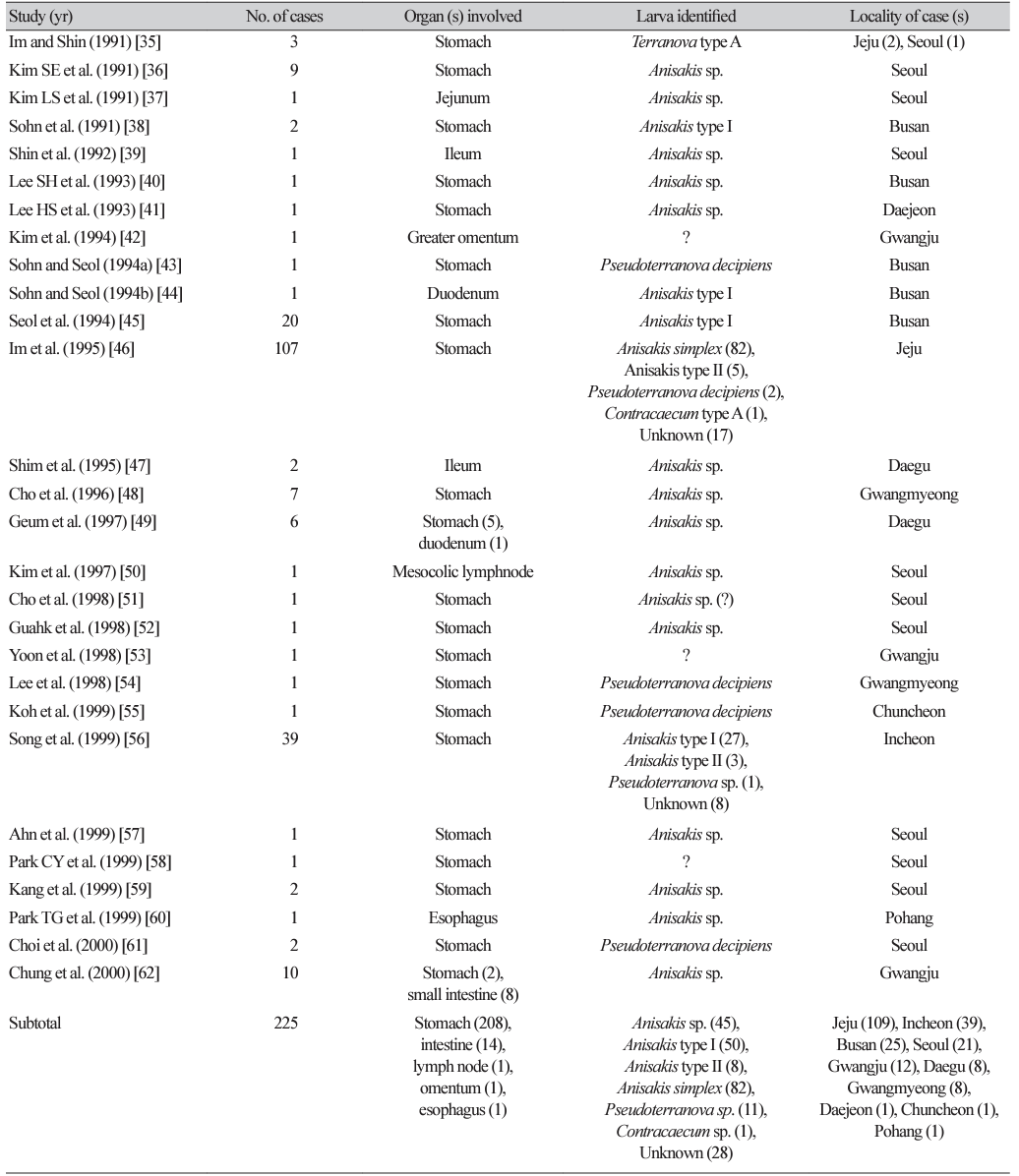
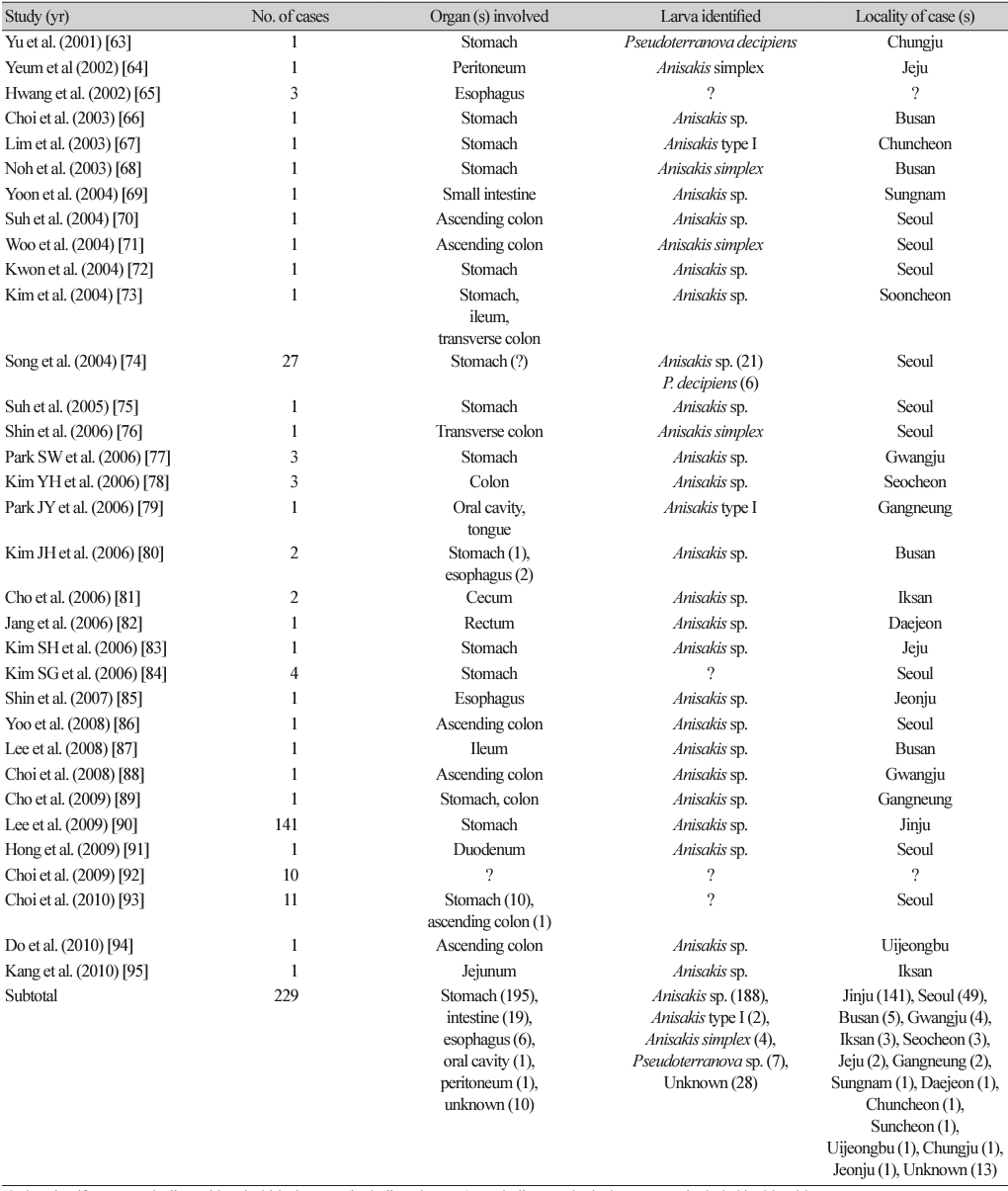
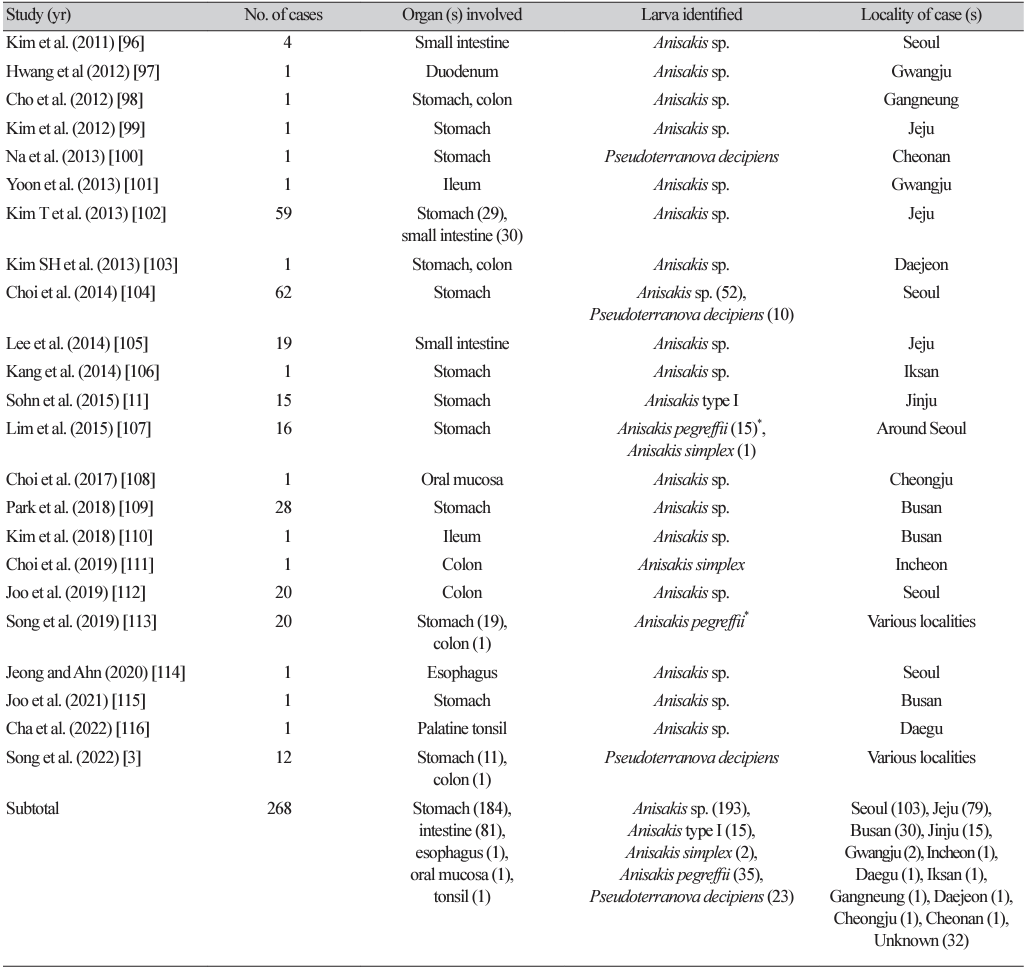
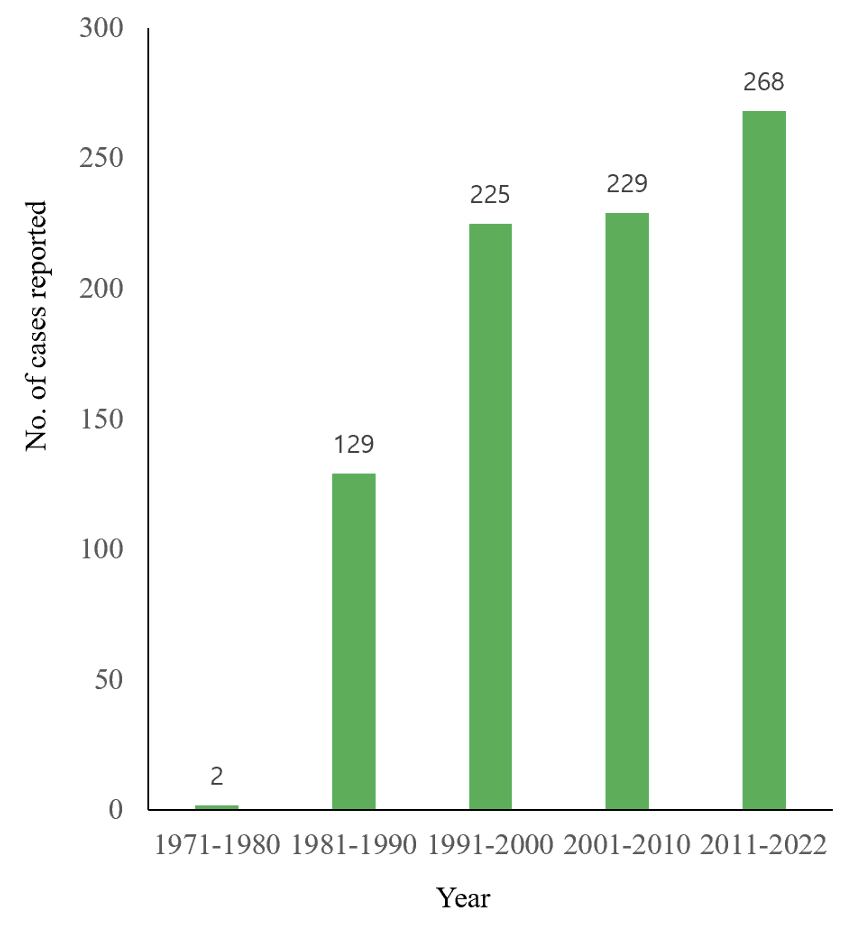
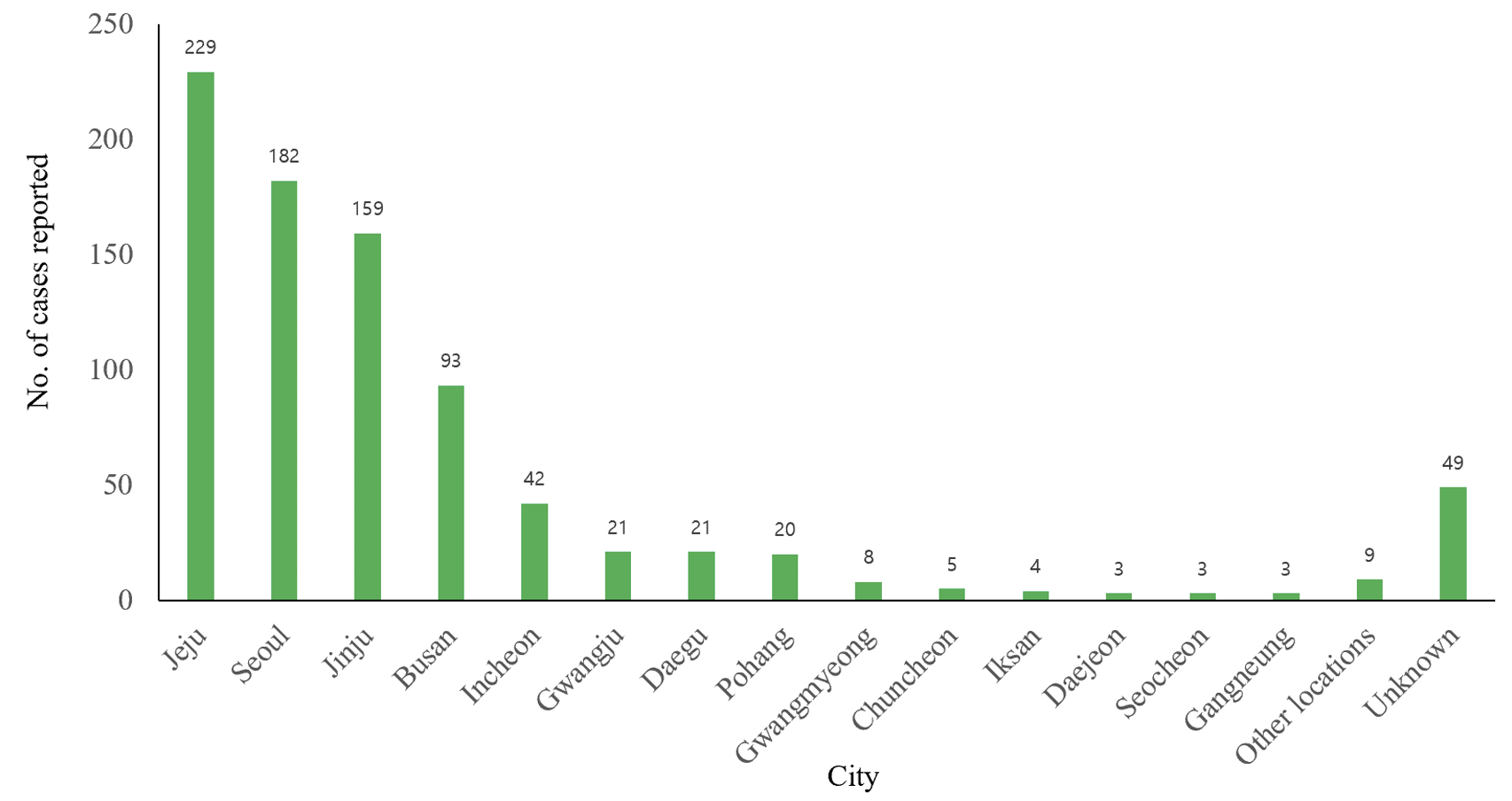
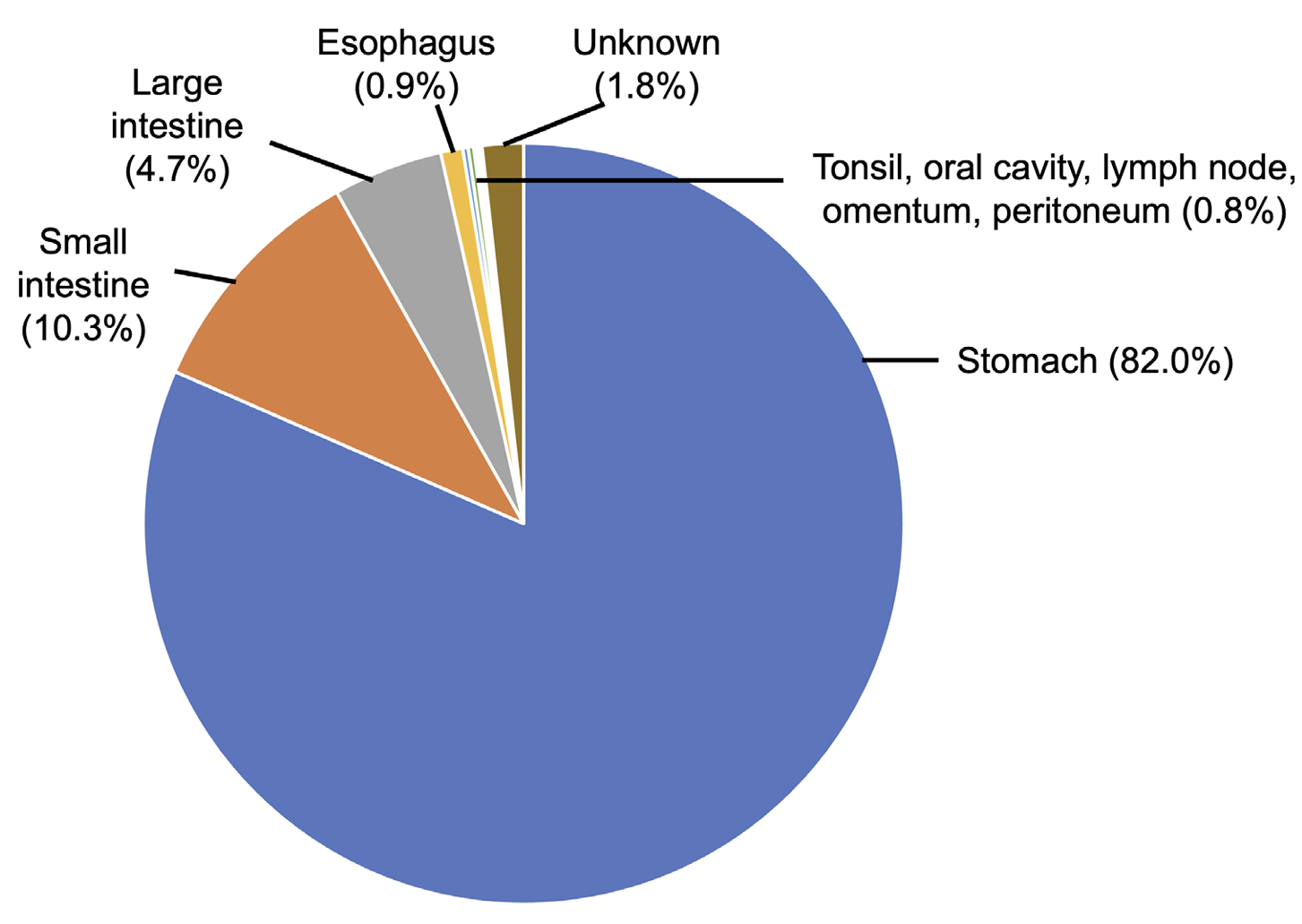

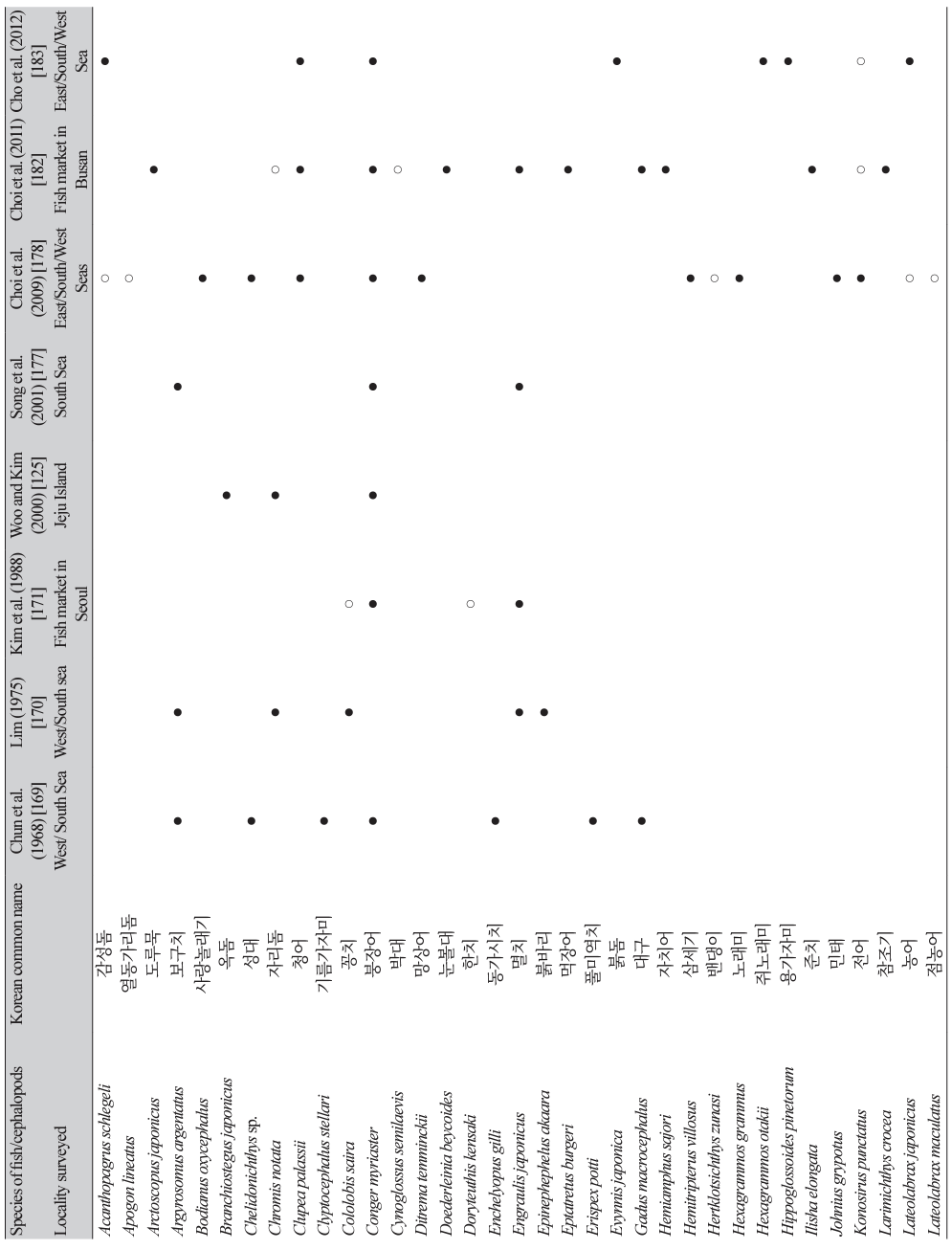
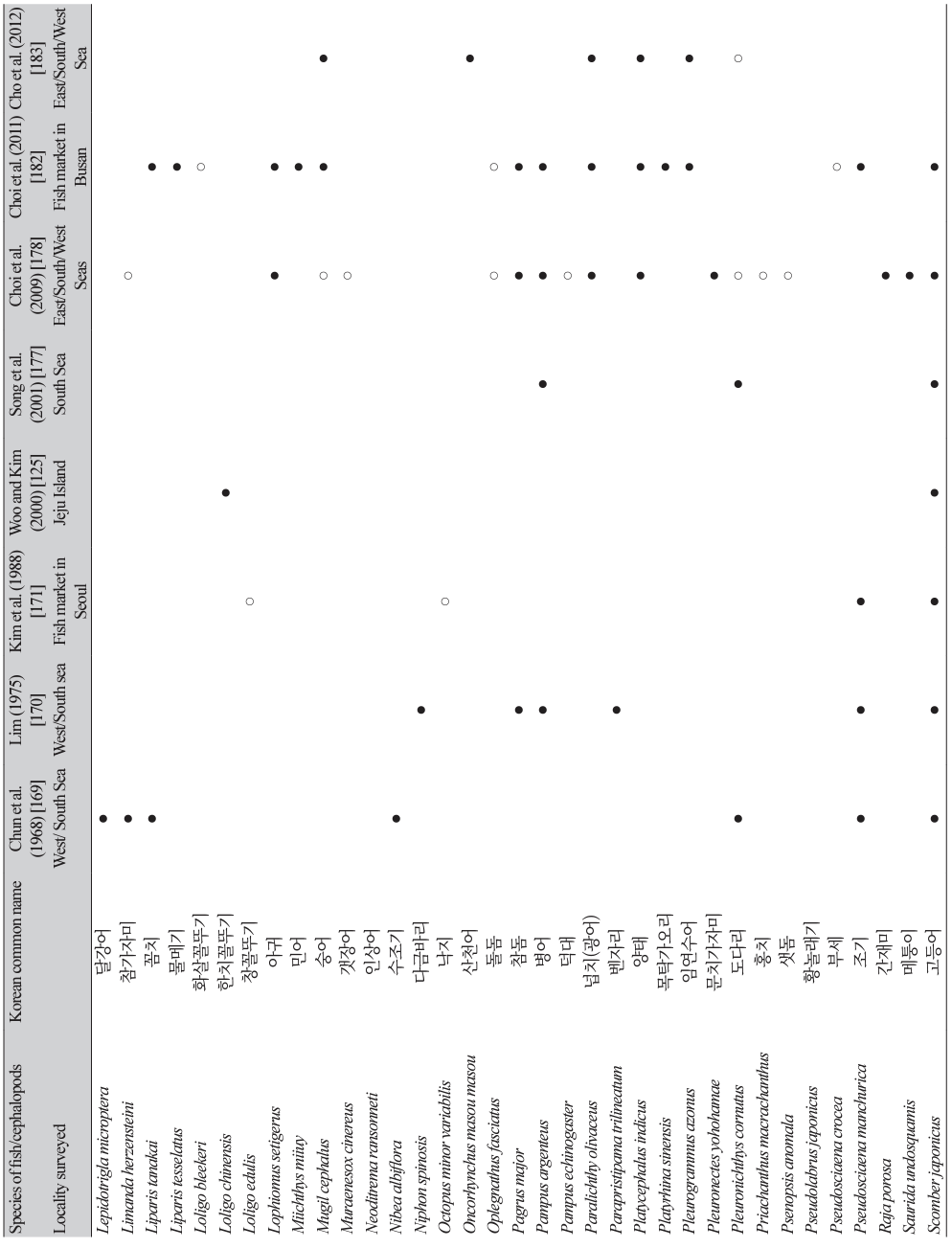
 XML Download
XML Download