Abstract
The carotid duct (CD) is a transient embryological structure connecting the 3rd and 4th aortic arches. We found a persisting CD in an adult female case, by studying the computed tomography angiogram. On the left side, the proximal external carotid artery (ECA) agenesis was noted. The CD was inserted into the left subclavian artery and continued upwards to reach the level of the atlas, and then it descended to connect to a normally configured segment of that ECA. It could be speculated that the CD-to-ECA connection was possible via unregressed 1st and/or 2nd aortic arches. The segmental ECA agenesis is extremely rare, while its supply via a persisting patent CD was not reported previously to the authors’ knowledge. The variants are extremely important during neck surgery because damaging the CD could determine hemorrhage, as well as ischemia in the ECA territory.
In stage 13 in human embryos, blood exits the heart via the outflow tract and enters the extrapericardial aortic sac that further connects with the bilateral dorsal aortae via transient aortic arches (AAs) [1-3]. Normally, the 1st and 2nd AAs disappear [3]. With development, the 3rd AA forms the common carotid artery (CCA) and the first segment of the internal carotid artery (ICA); the remainder of the ICA is formed by the cranial portion of the dorsal aorta [3]. The external carotid artery (ECA) is regarded as a sprout of the 3rd AA [3]. As the ECA emerges from the base of the 3rd AA, the stem of the ventral pharyngeal artery is incorporated within it, and the maxillary branch of the ventral pharyngeal artery communicates with the common trunk of origin of maxillary and mandibular branches of the stapedial artery and annex their branches [4]. The 4th AA evolves in an asymmetrical fashion: on the left, it forms part of the arch of the aorta, between the left common carotid and the left subclavian arteries (SAs), and on the right, it forms the most proximal segment of the right SA [3]. The dorsal aorta between the entrance of the 3rd and 4th AAs is known as the carotid duct (CD, ductus caroticus), and it normally regresses [3, 5-8].
To the authors’ knowledge, an isolated unilateral persistence of the CD was not reported previously in adults, nor was it encountered associated with the segmental agenesis of the proximal ECA.
During a retrospective study on computed tomography angiograms (CTAs) a peculiar anatomical variant was found in a 71 year-old female case. The research was conducted ethically in accordance with The Code of Ethics of the World Medical Association (Declaration of Helsinki). The Ethics Committee of the University Emergency Hospital Bucharest approved the study (approval no. 10540/16.02.2022). The CTAs were performed with a 32-slice scanner (Siemens Multislice Perspective Scanner), with a 0.6 mm collimation and a reconstruction of 0.75 mm thickness, with 50% overlap for a multiplanar maximum intensity projection and three-dimensional (3D) volume rendering technique. The cases were documented using Horos for iOS (Horos Project). Findings were checked on 2D planar reconstructions and were documented with 3D volume renderings.
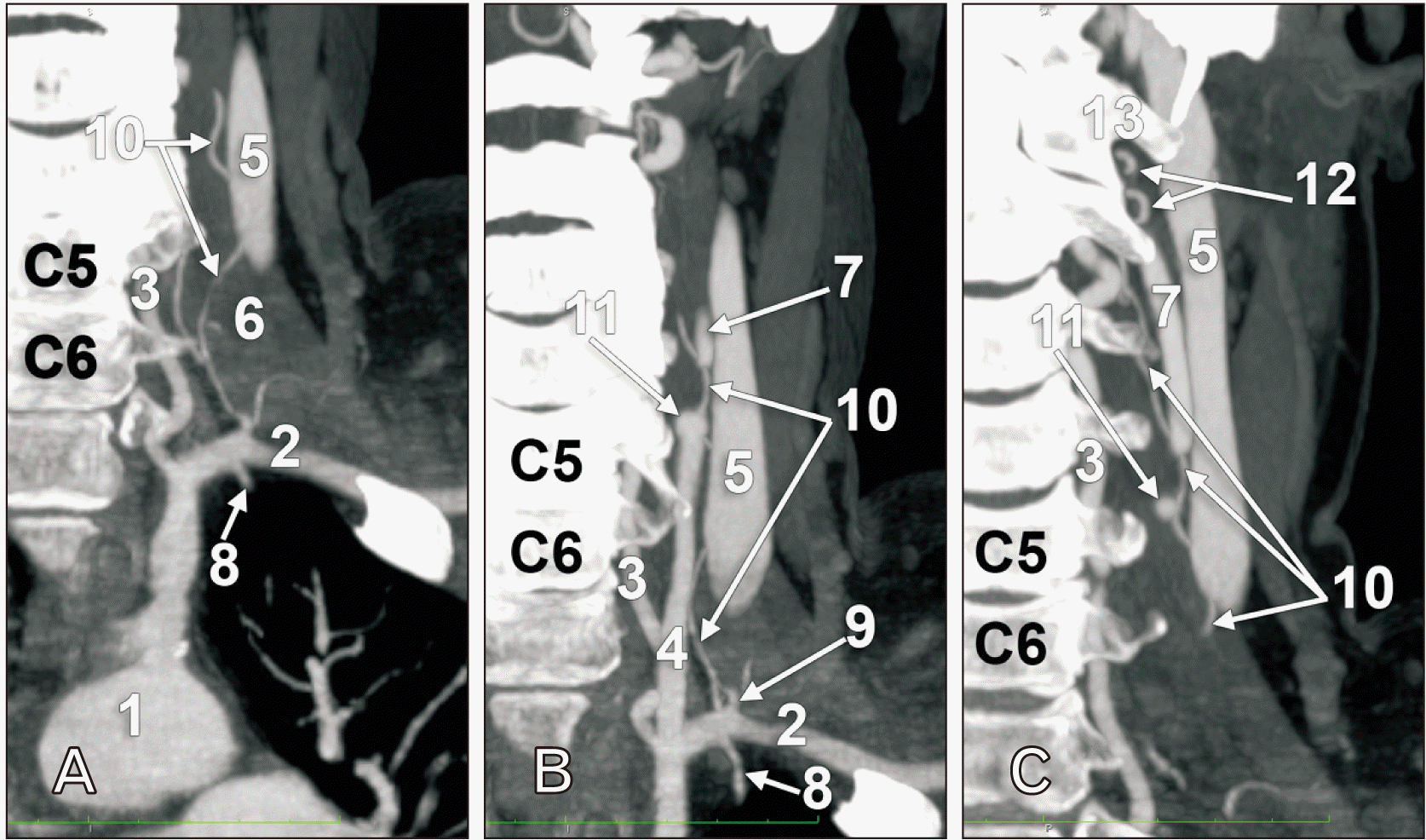
It was identified the common origin from the AA of the brachiocephalic trunk (innominate artery) and left CCA (Fig. 1). Then a CD was found leaving the left SA at 7.58 mm postero-lateral to the left CCA, at the medial border of the left anterior scalene muscle, and posterior to the left internal jugular vein (IJV) (Fig. 2A). At the same level of the left SA left its descending branch, the internal thoracic artery and immediately lateral to the SA origin of the CD was found the origin of the costocervical trunk. The origin of the CD was at 1.43 cm lateral to the origin of the left vertebral artery. The latter was initially kinked anterior to the transverse process of the 7th cervical vertebra and continued through the transverse foramen of the 6th cervical vertebra. The CD kept a relatively constant calibre of 0.82 mm. It initially ascended on the anterior scalene muscle, on the posterior side of the IJV up to the level of the upper 3rd of the 5th cervical vertebra. At that level the left ICA continued from the left CCA, while the normal ECA origin was replaced by a short blunt trunk of 2 mm (Fig. 2B). The retrojugular segment of the CD continued superiorly to cross from lateral to medial the posterior side of the left ICA at the level of the 4th cervical vertebra (Fig. 2C). It further ascended on the medial side of the left ICA, had to successive kinks inferior to the transverse process of atlas and reached anterior to that process, where it turned downwards (Fig. 1). The last, descending segment of the CD was anterior to the ascending one, antero-medially to the left ICA. At the level of the 3rd cervical vertebra the CD ended into a suprahyoid segment of the left ECA (Fig. 1). The trunk of ECA inferior to the CD insertion gave off the superior thyroid and lingual arteries while above the CD insertion were found the ECA origins of the occipital and facial arteries. The further morphology of the left ECA, as well as that of the right ECA, were normal.
Very few direct data are available on the persisting CD [7]. Our results allow us to reasonably speculate the following mechanism: (a) the segmental agenesis of the proximal segment of the left ECA indicates that the ventral end of the 3rd AA did not sprout the ECA during morphogenesis; (b) a persisting left CD should connect the derivatives of the left 3rd and 4th AAs, the left SA and the CCA-ICA axis; (c) the CD in the present case continued up to the level of atlas to descend and further insert into an anatomically normal segment of ECA above the hyoid—this could’ve been accomplished during morphogenesis via persisting 1st and/or 2nd AAs; (d) as the left ICA formed normally, the primitive left dorsal aorta could not be involved in this mechanism. The cardiac neural crest is essential for the persistence of an arch artery, but not its formation [9]. Therefore, an abnormal deficit of the neural crest could be suspected in this case. However, it remains speculative also. Padget [10], quoted in [11], described that in human embryos, the stapedial artery contributes to the distal ECA. This could justify a normal anatomical branching of the distal ECA combined with its proximal segment agenesis. As discussed by Rana et al. [1], theoretically, the absence of CCAs should result from agenesis or involution of the third AAs, and such a configuration, in turn, would require persistence of the CD in order to maintain a cranial circulation. We found here, however, co-existing CCA and persisting CD that supplies the absence of the proximal segment of the ECA.
Suntratonpipat et al. [5] reported a pediatric case with an interrupted AA between the origins of the left CCA and SA. The ascending aorta gave off the right and left CCAs. On both sides were observed large collateral arteries consistent with persistent CDs inserted into the carotid bifurcations, further giving off the ICAs and ECAs [5]. The right one gave rise to the right SA and vertebral artery. The left one was connected to the left SA and the descending aorta [5]. No segmental agenesis of any ECA was found then. In a different case of interrupted AA, the descending aorta was supplied only by a small persistent 3rd AA artery [6].
Segmental agenesis of ECA was found previously, but it was not supplied by a persisting CD. Furtado et al. [12] reported a case in which the left CCA continued as the ICA and the ECA was not visualized on the left CCA injection. The left ECA was reformed by muscular branches of the vertebral artery in the early phase, and the distal occipital artery was reformed by muscular branches of the vertebral artery in the later phase [12]. That occipital artery reformed the ECA in its entirety [12].
Although extremely rare the variant reported here is surgically important during different procedures, such as neck dissections. Surgeons should be carefully dissect the major vessels of neck, as damaging of such persisting CD could interrupt the only feeder of an ECA with proximal agenesis. A preoperative imaging evaluation of the carotid system could be extremely useful for identifying such a persisting CD and would prevent haemorrhagic, or ischemic events.
Notes
References
1. Rana MS, Sizarov A, Christoffels VM, Moorman AF. 2014; Development of the human aortic arch system captured in an interactive three-dimensional reference model. Am J Med Genet A. 164A:1372–83. DOI: 10.1002/ajmg.a.35881. PMID: 23613216.

2. Anderson RH, Bamforth SD. 2022; Morphogenesis of the mammalian aortic arch arteries. Front Cell Dev Biol. 10:892900. DOI: 10.3389/fcell.2022.892900. PMID: 35620058. PMCID: PMC9127140.

3. Sadler TW, Langman J. Langman's medical embryology. 12th ed. Wolters Kluwer Health/Lippincott Williams & Wilkins;2012. p. 384.
4. Devadas D, Pillay M, Sukumaran TT. 2016; Variations in the origin of superior laryngeal artery. Anat Cell Biol. 49:254–8. DOI: 10.5115/acb.2016.49.4.254. PMID: 28127500. PMCID: PMC5266101.

5. Suntratonpipat S, Bamforth SD, Johnson AL, Noga M, Anderson RH, Smallhorn J, Tham E. 2015; Childhood presentation of interrupted aortic arch with persistent carotid ducts. World J Pediatr Congenit Heart Surg. 6:335–8. DOI: 10.1177/2150135114560830. PMID: 25870362.

6. Ekteish FM, Hajar R, Folger GM Jr. 1986; Persistence of third aortic arch with fourth aortic arch agenesis. Br Heart J. 55:607–9. DOI: 10.1136/hrt.55.6.607. PMID: 3718803. PMCID: PMC1236772.

7. Elumalai G, Chodisetty S, Usen BO, Patel RD. 2016; "patent ductus caroticus" embryological basis and its clinical significance. Elixir Physio Anat. 98:42439–42.
8. Maybody M, Uszynski M, Morton E, Vitek JJ. 2003; Absence of the common carotid artery: a rare vascular anomaly. AJNR Am J Neuroradiol. 24:711–3. PMID: 12695210. PMCID: PMC8148690.
9. Waldo KL, Kumiski D, Kirby ML. 1996; Cardiac neural crest is essential for the persistence rather than the formation of an arch artery. Dev Dyn. 205:281–92. DOI: 10.1002/(SICI)1097-0177(199603)205:3<281::AID-AJA8>3.0.CO;2-E. PMID: 8850564.

10. Padget DH. 1948; The development of the cranial arteries in the human embryo. Contrib embryol. 32:205–61.
11. Hiruma T, Nakajima Y, Nakamura H. 2002; Development of pharyngeal arch arteries in early mouse embryo. J Anat. 201:15–29. DOI: 10.1046/j.1469-7580.2002.00071.x. PMID: 12171473. PMCID: PMC1570898.

12. Furtado SV, Quryshi SA, Jagannatha AT, Hegde AS. 2021; Segmental agenesis of external carotid artery from common carotid artery with anomalous reformation through occipital artery anastomoses. Neurol India. 69:1824–7. DOI: 10.4103/0028-3886.333480. PMID: 34979699.

Fig. 1
Three-dimensional volume rendering, left side, medial view of carotid system. 1. Aortic arch; 2. common trunk of origin of the brachiocephalic trunk and left common carotid artery; 3. brachiocephalic trunk; 4. left common carotid artery; 5. left subclavian artery; 6. vertebral artery; 7. carotid duct (arrowheads); 8. blunt trunk at the origin of the left external carotid artery; 9. thyroid cartilage; 10. greater horn of hyoid bone; 11. superior thyroid artery; 12. lingual artery; 13. facial artery; 14. occipital artery; 15. maxillary artery; 16. internal jugular vein; 17. retromandibular vein; 18. external carotid artery; 19. internal carotid artery; 20. styloid process; 21. tip of the transverse process of atlas.
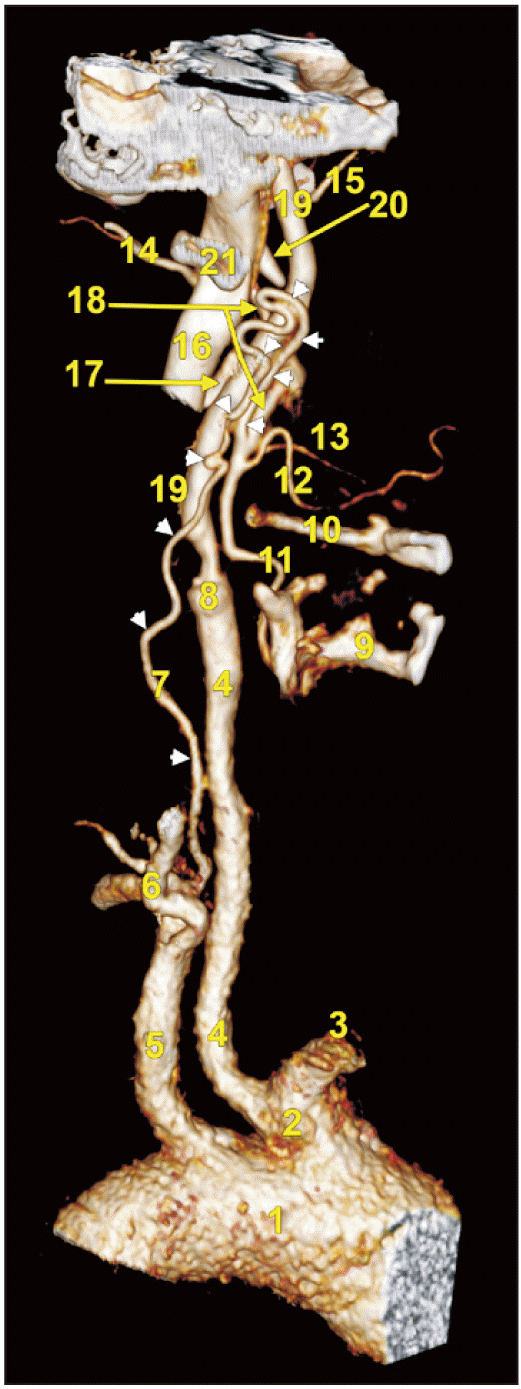
Fig. 2
Coronal slices through the left subclavian (A), left common carotid (B) and left internal carotid (C) arteries. Anterior views. The 5th and 6th cervical vertebrae are indicated. 1. Aortic arch; 2. subclavian artery; 3. vertebral artery; 4. common carotid artery; 5. internal jugular vein; 6. anterior scalene muscle; 7. internal carotid artery; 8. internal thoracic artery; 9. costocervical trunk; 10. carotid duct; 11. blunt origin of the external carotid artery; 12. superior kinks of carotid duct; 13. transverse process of atlas.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download