This article has been
cited by other articles in ScienceCentral.
Abstract
Sciatic nerve (SN) is the thickest and longest nerve of the body. Deviations from the normal anatomical origin and level of bifurcation of SN have been frequently reported. In the present case, we are presenting a unique scenario of origin of terminal branches of the SN-tibial nerve (TN) and common peroneal nerve (CPN) in the pelvic region itself from divisions arising directly from the lumbosacral plexus. This variation was associated with origin of posterior femoral cutaneous nerve from the superior division of CPN with anomalous communicating branches between pudendal nerve and TN. The unique characteristics of the present case are the presence of ‘pseudoganglion’ found on the inferior division of TN. The present case stands out as the first of its kind to mention such pseudoganglion. Knowledge of some unusual findings like presence of pseudoganglion and intercommunications between nerves have clinical implications in anesthesiology, neurology, sports medicine, and surgery.
Go to :

Keywords: Sciatic nerve, Tibial nerve, Pseudoganglion, Pudendal nerve, Posterior femoral cutaneous nerve
Introduction
Sciatic nerve (SN) is the largest and thickest nerve of the human body that arises from L4 to S3 vertebral segments [
1]. It leaves the pelvis and enters the gluteal region through the greater sciatic foramen inferior to piriformis muscle (PM). It enters the posterior compartment of thigh at the lower margin of quadratus femoris where it innervates all the muscles that flex the knee joint including ischial part of adductor magnus [
1]. It then divides into tibial (anterior divisions of L4 to S3) and common peroneal nerve (CPN, posterior divisions of L4 to S2) at any level between the pelvis or below the popliteal fossa but mostly at the level of apex of popliteal fossa [
2-
4]. SN, via these divisions, provides innervation to all muscles in the leg, foot and sensory innervation to skin on lateral side of leg, sole and dorsum of the foot [
1].
The posterior femoral cutaneous nerve (PFCN) arises from the sacral plexus from the ventral ramii of S1, S2 and S3 segments of the spinal cord. The pudendal nerve (PN) originates from the sacral plexus from the ventral ramii of S2, S3 and S4 segments of spinal cord. Both PFCN and PN enter the gluteal region through the greater sciatic foramen inferior to PM medial to SN.
The PN, after entering the gluteal region, gives no branches in the gluteal region [
1]. The course of the PN in the gluteal region is often short and often hidden by overlying upper margin of sacrotuberous ligament. It passes over the sacrospinous ligament and immediately after that through the lesser sciatic foramen to enter the perineum where it is the major somatic nerve [
1].
Variations in the origin, course of SN and the presence of anomalous communicating branches (CBs) between PN, SN and PFCN are commonly seen [
2-
4]. Present case is a unique case of high division of SN into tibial and CPN with anomalous CBs between tibial nerve (TN) and PN with origin of PFCN from CPN.
We also observed a ‘pseudoganglion’ associated with the nerve division contributing to the formation of TN which was confirmed with histopathological examination. Occurrence of pseudoganglion near peripheral nerves of the lower limb is very rare. To the best of our knowledge, a pseudoganglion associated with branches of a SN dividing in the pelvis itself has not been found on literature search.
The purpose of this case report is to highlight neuromuscular variations which will be of great help to surgeons during sciatic neurolysis, piriformis tendon release, gluteoplasty and treatment of unusual muscle pain [
5,
6].
Go to :

Case Report
During routine cadaveric dissection of lower limb in 52 years old female cadaver, we observed a unique variation of SN. After removal of skin, superficial fascia, massive gluteal fat pad and reflecting the gluteus maximus muscle from its origin towards its insertion, it came to our notice that there was pelvic division of SN. After further dissection, 2 terminal branches (TBs) namely tibial and CPN found passing through the PM. The PN and the PFCN appeared to be normal. On tracing origin, it was found that both tibial and CPN were arising from 2 divisions—superior and inferior (as shown in
Fig. 1). The inferior division (ID) of tibial and superior division (SD) of CPN was arising directly from the lumbosacral plexus while the SD of tibial and ID of common peroneal shared a common trunk (CT) arising from lumbosacral plexus. The CPN was formed by joining of 2 divisions. Two branches (AB1 and AB2) from the ID of CPN innervated the gluteus medius muscle.
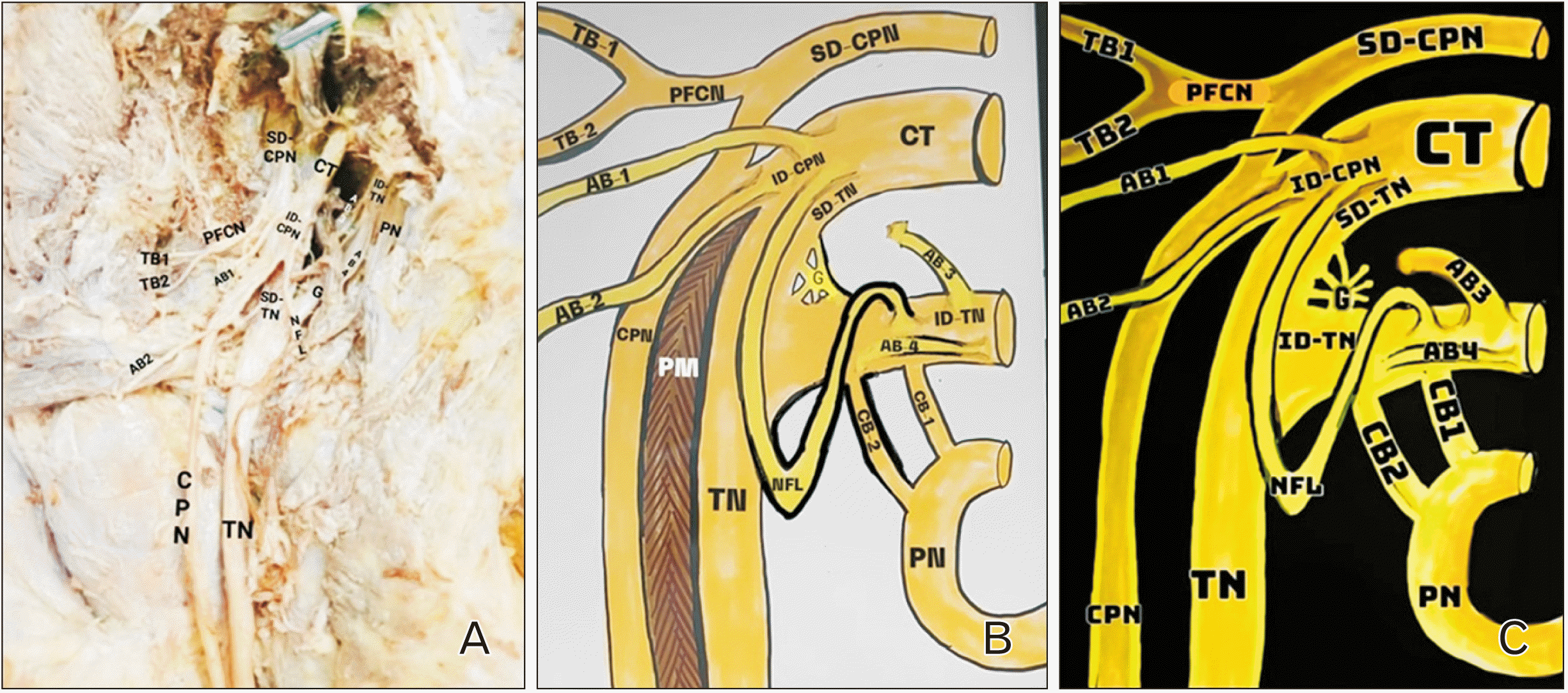 | Fig. 1Showing (A) dissection image of origin of tibial and CPN directly from lumbar plexus. (B) Schematic representation of same dissection image. (C) Schematic representation of only nerves depicted in dissection image. TN, tibial nerve; CPN, common peroneal nerve; SD, superior division; ID, inferior division; NFL, nerve fibre loop; CT, common trunk; AB, anomalous branch; TB, terminal branch; PFCN, posterior femoral cutaneous nerve; G, ganglion; PM, piriformis muscle; PN, pudendal nerve. 
|
The PFCN originated directly from the SD of CPN (SD-CPN). The PFCN then further divided 2 TBs. Two anomalous branches (AB3 and AB4) arose from the ID of TN (ID-TN). These 2 ABs innervated the gluteus medius muscle. Another AB arose from the SD of TN, curved downwards and joined ID-TN nerve fibre loop or pulley-like structure. There were 2 unique CBs (CB1 and CB2) between the PN and the ID-TN, thereby forming a “H”-shaped communicating plexus. No reunification of tibial and CPN was observed.
When traced to the origin, we noticed that SD-CPN was arising from L4 whereas the CT was having root value as L5, S1, S2. The ID-TN was arising from S3 while PN was taking contribution from S4.
A ganglion was found near ID-TN giving ramii to the 2 divisions of TN (as shown in
Fig. 2). We have done histopathological examination of the ganglion and confirmed it as ‘pseudoganglion’ showing fat and connective tissue without nerve cell bodies like genuine ganglion (as shown in
Fig. 3).
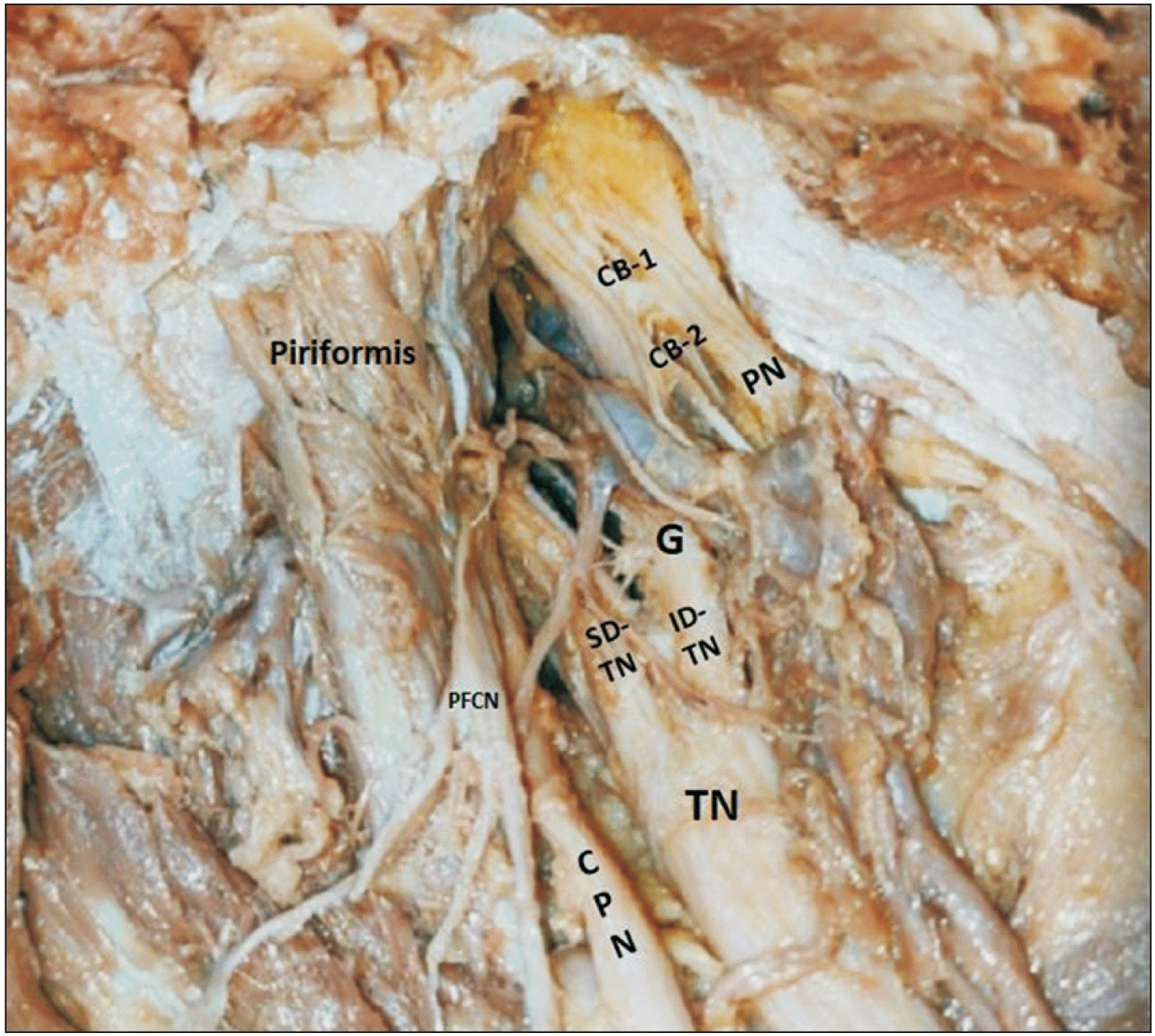 | Fig. 2Dissection image highlighting pseudoganglion associated with TN. TN, tibial nerve; PFCN, posterior femoral cutaneous nerve; SD, superior division; ID, inferior division; G, ganglion; CB, communicating branch; PN, pudendal nerve; CPN, common peroneal nerve. 
|
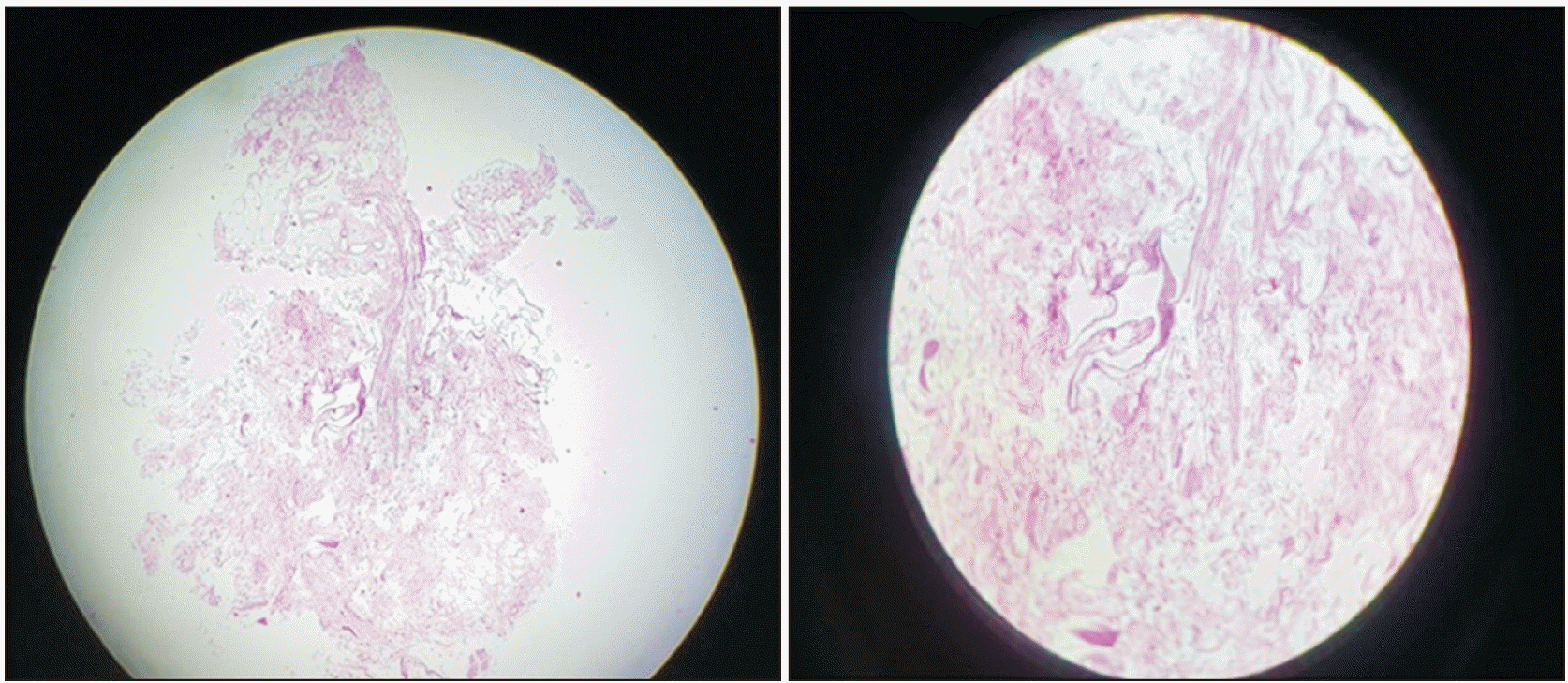 | Fig. 3Showing microanatomy of pseudoganglion after tissue processing in pathology (H&E staining, magnification: ×10). 
|
Go to :

Discussion
The long course of SN makes it vulnerable to nerve injury [
7]. Variations in the origin and course of SN in relation to PM have been commonly observed [
8]. These variations were categorized into 7 categories by Beaton and Anson [
9] in 1936 via the dissection of 6,000 cadavers which are as follows:
· Type A: SN exits the pelvis undivided below the PM.
· Type B: SN divides in the pelvis, CPN pierces the PM, and TN lies below the PM
· Type C: SN divides in the pelvis, CPN courses over the PM, and TN lies below the PM.
· Type D: SN exits the pelvis undivided piercing the PM.
· Type E: SN divides in the pelvis, CPN courses over the PM, and TN pierces the PM.
· Type F: SN exits the pelvis undivided coursing over the PM.
· Type G: SN divides in the pelvis, both CPN and TN coursing separately below the PM.
Our case does not fit into any of these categories because after their formation, both tibial and CPNs enter the gluteal region through greater sciatic foramen by piercing the PM. This makes our case even more unique and novel. However, higher division of SN has been observed earlier [
10].
Trifurcation, reunification and quadrification of SN have also been seen [
11-
14]. A complete SN block or popliteal fossa block may not be possible in our case, since there is higher division of SN into tibial and CPNs [
11]. Possibility of higher division of SN should be kept in mind by surgeons and anaesthesiologists during hip arthroplasty, hemiarthroplasty, piriformis syndrome, sciatica, coccydynia, and muscle atrophy [
11]. In our case, there are 2 CBs between TN and PN. On literature search, CBs between PN and SN have been observed by Ranjan et al. [
3] and Agarwal et al. [
15]. However, communication between PN and branches of SN undergoing higher division along with the origin of PFCN from one of the branches of SN was not encountered during our review of literature.
The anomalous CBs between SN and PN is of great clinical significance because the neurotization of PN by transplanting motor fascicles from SN is important to treat bladder incontinence [
15].
The development of anomalous communicating plexus between TN, CPN, the PFCN and the PN could be due to abnormal migration and joining of proprioceptive or sensory fibres during intrauterine life that later resulted in the formation of this anomalous communicating plexus [
5]. The responsible transcription factors may include transforming growth factor-β, Sonic Hedgehog, bone morphogenetic proteins 4 and 7, PAX3, PAX6, NKX2.2, and NKX6.1 [
15].
Ranjan et al. [
3] categorized the variations in the course of PN on the basis of number of trunks contributing to the formation of PN. Our case can be included in type I wherein a single PN was identified. This classification does not involve the CBs as seen in our case. This creates need for revised classification.
In our case, PFCN was taking origin from the SD-CPN. The anomalous CBs of PFCN with tibial and CPN should be considered while using the free inferior gluteal flap as a major secondary choice of autologous tissue for breast reconstruction if the transverse rectus abdominis musculocutaneous flap is not an option [
5].
In the present case, a ‘pseudoganglion’ was found near ID-TN giving ramii to the 2 divisions of TN. Such a pseudoganglion associated with TN had never been reported to the best of our knowledge on review of literature. The histopathological examination showed no nerve cell bodies like genuine ganglion. Instead it showed only fat and connective tissue confirming it as ‘pseudoganglion.’
In conclusion, knowledge of the level of bifurcation and distribution of the SN and its location is important. The anatomical variants associated with a high division of the SN, must always be born in mind, as they are relatively prevalent, and have important clinical implications, namely in anesthesiology, neurology, sports medicine and surgery. The uncommon anatomical findings described here are relevant to surgeons to enable them to perform efficient surgical procedures and avoid errors.
Go to :






 PDF
PDF Citation
Citation Print
Print





 XML Download
XML Download