Abstract
Acknowledgements
Notes
References
Fig. 1
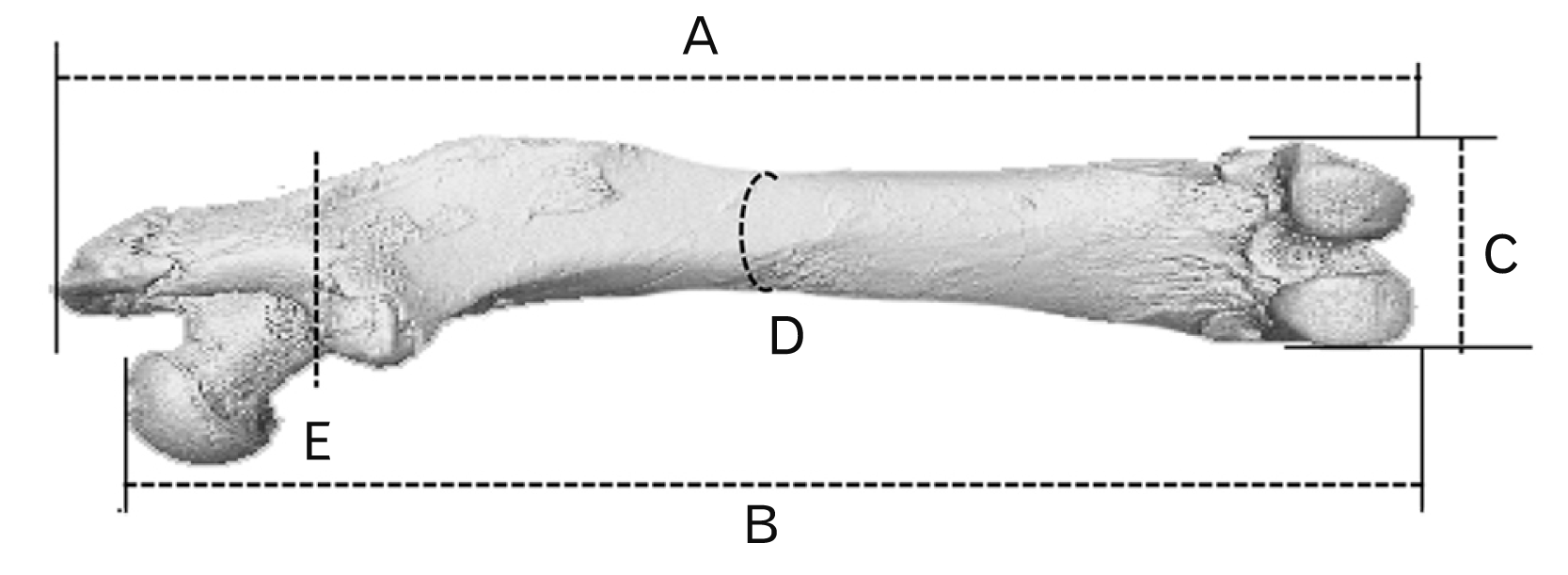
Fig. 2
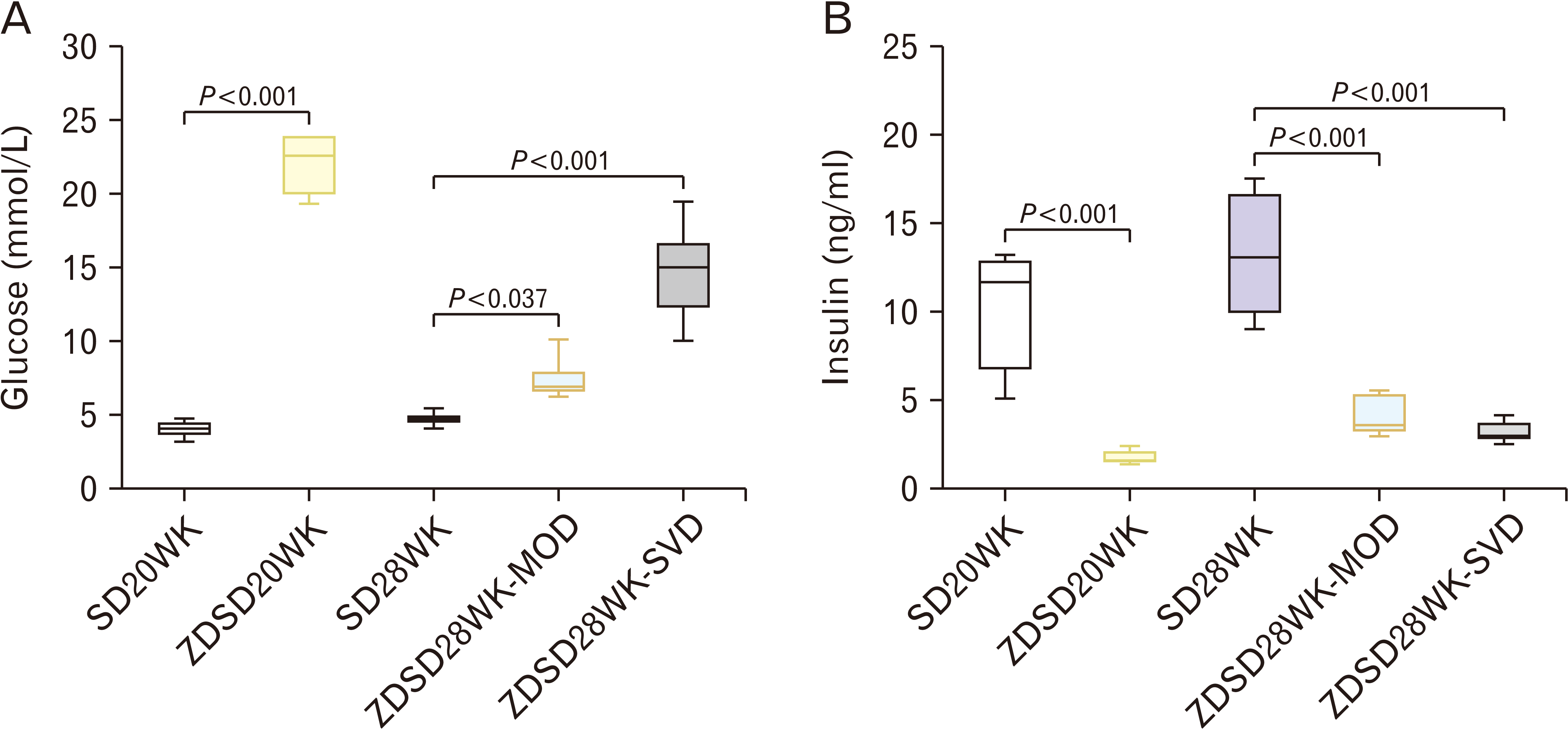
Fig. 3
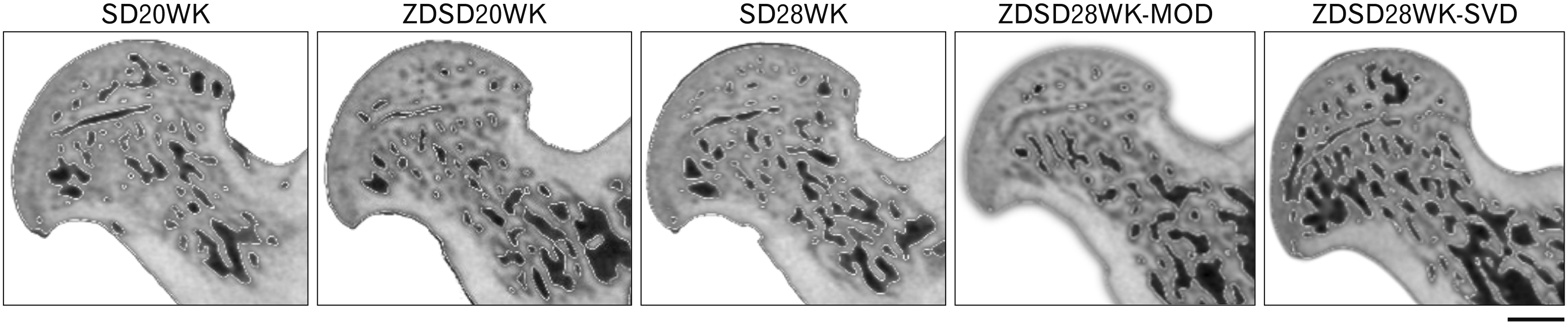
Fig. 4
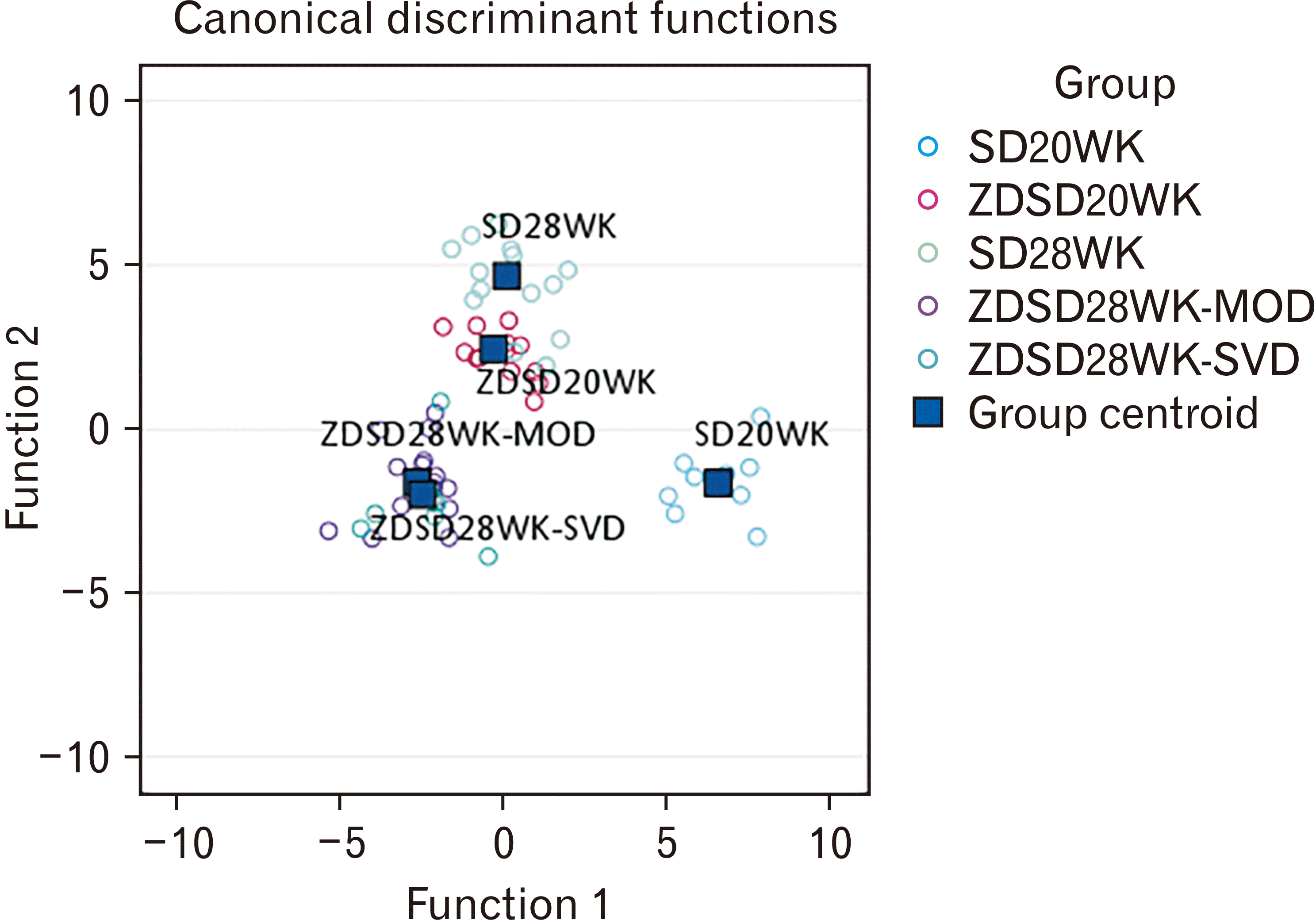
Table 1
Table 2
Table 3
| Property | Week-20 | P-value | Week-28 | P-value | |||
|---|---|---|---|---|---|---|---|
| SD (n=12) | ZDSD (n=12) | SD (n=12) | ZDSD-MOD (n=12) | ZDSD-SVD (n=12) | |||
| Bone mass (g) | 1.35±0.09 | 1.19±0.04 | <0.001 | 1.26±04 | 1.25±0.06 | 1.16±0.11 |
0.005, <0.001a) <0.001b) 0.020c) 0.014 |
| Full length (mm) | 39.71±0.83 | 39.57±0.37 | 0.545 | 40.45±0.31 | 39.37±0.28 | 39.13±0.85 |
<0.001a) 0.001b) 0.354c) 0.059 |
| Biomechanical length (mm) | 38.59±0.53 | 38.43±0.41 | 0.584 | 39.03± 0.53 | 37.85±0.68 | 37.18±0.47 |
<0.001, 0.003a) 0.105b) 0.024c) <0.001 |
| Midshaft mediolateral diameter (mm) | 5.13±0.17 | 4.75±0.17 | <0.001 | 5.05±0.26 | 3.61±0.35 | 3.83±0.44 |
<0.001a) 0.188b) <0.001c) |
| Bicondylar width (mm) | 7.78±0.26 | 6.91±0.19 | <0.001 | 7.14±0.21 | 7.11±0.07 | 6.85±0.25 |
0.003, <0.001a) <0.001b) 0.200c) 0.051 |
| Robusticity index (%) | 22.49±1.17 | 21.13±0.44 | 0.002 | 21.21±0.85 | 16.65±1.39 | 16.71±1.04 |
<0.001a) 0.003b) <0.001c) |
Values are presented as mean±standard deviation. SD, Sprague–Dawley; ZDSD, Zucker Diabetic Sprague–Dawley; MOD, moderately diabetic; SVD, severely diabetic. a)Respective comparison of SD28WK with ZDSD28WK-MOD and ZDSD28WK-SVD. b)P-value comparing SD20WK against SD28WK, c)P-value comparing ZDSD20WK against ZDSD28WK-MOD and ZDSD28WK-SVD, respectively.
Table 4
| Property | Week-20 | P-value | Week-28 | P-value | |||
|---|---|---|---|---|---|---|---|
| SD (n=12) | ZDSD (n=12) | SD (n=12) | ZDSD-MOD (n=12) | ZDSD-SVD (n=12) | |||
| BV (mm3) | 30.90±8.45 | 26.69±3.55 | 0.039 | 34.08±2.96 | 27.93±3.01 | 27.73±2.87 |
0.002a) 0.116b) 0.505, 0.604c) |
| BV/TV (%) | 26±2 | 18±5 | <0.001 | 19±9 | 19±2 | 18±2 |
0.944, 0.955a) <0.001b) 0.828, 0.936c) |
| TbTh (mm) | 0.33±0.14 | 0.31±0.04 | 0.692 | 0.46±0.10 | 0.35±0.08 | 0.32±0.07 |
0.002, 0.001a) 0.001b) 0.403, 0.842c) |
| TbN | 2.40±1.25 | 0.57±0.18 | <0.001 | 0.39±0.20 | 0.43±0.21 | 0.67±0.98 |
0.884, 0.368a) <0.001b) 0.639, 0.740c) |
| TbSp (mm) | 0.39±0.62 | 1.59±.0.61 | 0.050 | 2.50±1.66 | 2.50±1.66 | 2.61±1.36 |
0.851, 0.858a) 0.001b) 0.115, 0.143c) |
| Cortical area (50th percentile, mm2) | 19.97±2.58 | 18.26±0.71 | 0.056 | 20.41±2.09 | 19.26±2.37 | 20.83±1.56 |
0.164, 0.635a) 0.619b) 0.226, 0.005c) |
| Medullary area (50th percentile, mm2) | 9.39±0.67 | 12.48±2.66 | <0.001 | 9.39±0.67 | 10.34±1.02 | 11.43±1.34 |
0.146, 0.011a) 0.161b) 0.733, 0.202c) |
Values are presented as mean±standard deviation. BV/TV, bone volume to bone tissue volume ratio; TbTh, trabecular thickness; TbN, trabecular number; TbSp, trabecular spacing; SD, Sprague–Dawley; ZDSD, Zucker Diabetic Sprague–Dawley; MOD, moderately diabetic; SVD, severely diabetic. a)Respective comparison of SD28WK with ZDSD28WK-MOD and ZDSD28WK-SVD. b)P-value comparing SD20WK against SD28WK, c)P-value comparing ZDSD20WK against ZDSD28WK-MOD and ZDSD28WK-SVD respectively.
Table 5
| Structure matrix | |||
|---|---|---|---|
| Parameter | Function | ||
| 1 | 2 | 3 | |
| Robusticity index | 0.57a) | 0.45a) | –0.35a) |
| Midshaft mediolateral diameter | 0.51a) | 0.46a) | –0.08 |
| Bicondylar width (mm) | 0.36a) | –0.39a) | 0.07 |
| TbN | 0.29 | –0.15 | 0.00 |
| BV/TV | 0.28 | –0.13 | 0.06 |
| Midshaft medullary area (mm2) | 0.17 | 0.01 | 0.31a) |
| Biomechanical length (mm) | 0.15 | 0.26 | 0.04 |
| Bone mass (g) | 0.14 | –0.31a) | –0.04 |
| Full length (mm) | 0.08 | 0.22 | 0.16 |
| Midshaft cortical area (mm2) | 0.01 | –0.03 | 0.32a) |
| TbTh | –0.01 | 0.15 | 0.34a) |
| TbSp | –0.16 | 0.04 | 0.18 |
| Bone volume | –0.18 | 0.01 | 0.24 |
| Percentage of variance (%) | 58.7 | 33.4 | 6.1 |




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download