Abstract
Striated muscle insertions into the skin and mucosa are present in the head, neck, and pelvic floor. We reexamined the histology of these tissues to elucidate their role in transmission of the force. We examined histological sections of 25 human fetuses (gestational ages of ~11–19 weeks and ~26–40 weeks) and 6 cadavers of elderly individuals. Facial muscle insertion or terminal almost always formed as an interdigitation with another muscle or as a circular arrangement in which muscle fiber insertions were sandwiched and mechanically supported by other muscle fibers (like an in-series muscle). Our examination of the face revealed some limited exceptions in which muscle fibers that approached the dermis were always in the nasalis and mentalis muscles, and often in the levator labii superioris alaeque nasi muscle. The buccinator muscle was consistently inserted into the basement membrane of the oral mucosa. Parts of the uvulae muscle in the soft palate and of the intrinsic vertical muscle of the tongue were likely to direct toward the mucosa. In contrast, the pelvic floor did not contain striated muscle fibers that were directed toward the skin or mucosa. Although ‘cutaneous muscle’ is a common term, the actual insertion of a muscle into the skin or mucosa seemed to be very rare. Instead, superficial muscle insertion often consisted of interdigitated muscle bundles that had different functional vectors. In this case, the terminal of one muscle bundle was sandwiched and fixed mechanically by other bundles.
Many striated muscles in the head, neck, and pelvic floor are attached to or even insert into the skin and mucosa. All facial muscles, including the platysma muscle, are referred to as ‘cutaneous muscles’. The pharyngeal constrictor muscles surround the pharyngeal mucosa, but have an origin in the hard tissue and an insertion in the pharyngeal raphe. The levator veli palatini and stylopharyngeus muscles penetrate a ‘muscle tube’ of the constrictors and approach the mucosa [1-3]. Likewise, the levator veli palatini and uvulae muscles may approach the oral mucosa, because they are interdigitated in the soft palate [4-9]. The pelvic floor also contains two striated muscle sphincters—the external urethral and anal sphincters—and parts of them attach to subcutaneous genital tissue [10-12].
Do some striated muscle fibers of cutaneous and submucosal muscles insert into the dermis or the mucosal epithelium? No previous studies have thoroughly examined the functional connections of striated muscles in subcutaneous and submucosal tissues. When a striated muscle makes a fold or pouch of the skin or mucosa, a specific fibrous tissue is likely to be necessary at the interface to transmit force from the muscle to the skin or mucosa.
The aim of this study was to re-examine the histology of the insertions of striated muscles into subcutaneous and submucosal tissues in different parts of the body. We therefore examined mid-term and near-term human fetuses, and adult specimens were also necessary in two regions: the pelvic floor, because subcutaneous sphincters seem to be underdeveloped in fetuses [13, 14]; and the tongue and facial region, because these mucosae are very thin in fetuses [15].
The study was performed in accordance with the provisions of the 1995 Declaration of Helsinki, as revised in 2013. Histological sections were from 15 midterm fetuses, 10 near-term human fetuses, and 6 adult cadavers. All histology photographs were taken with a Nikon Eclipse 80 microscope (Nikon).
The paraffin-embedded sections of 15 midterm fetuses (gestational age [GA]: ~11–19 weeks, crown-rump length [CRL]: 62–170 mm) and 10 near-term fetuses (GA: ~26–40 weeks, CRL: 215–334 mm) were part of the large collection at the Department of Anatomy of the Universidad Complutense, Madrid. All fetuses were from miscarriages or ectopic pregnancies at the University Department of Obstetrics. There was no information on the genetic backgrounds of the fetuses or the reasons for the abortions. The sectional plane was sagittal (8 fetuses), frontal (8 fetuses), or transversal (9 fetuses). These sections were stained with hematoxylin and eosin (H&E), Azan, Masson’s trichrome, or Verde Luz-orange G-acid fuchsin. This study was approved by the Ethics Committees of Complutense University (B08/374) and Tokyo Dental College (No. 932-2).
The histological sections of 6 adult cadavers (thickness: 7–10 µm at 1-mm intervals) were previously prepared by our group for studies of the tongue [15] and pelvic floor [12, 16, 17] and were stained with H&E or Azan. These specimens were obtained by dissection of 6 cadavers (4 males and 2 females, age range: 69–78 years), and were donated to the Tokyo Dental College for research and education on human anatomy. All cadavers were fixed by arterial injection of a 10% neutral formalin solution in water and stored in 50% ethanol for more than three months. The Tokyo Dental College committee approved the use of these cadavers for this research (No. 922-2).
Muscle fibers of the orbicularis oculi ran parallel to the conjunctiva and skin (Fig. 1A–D), and were in the deep side of the hair follicle and papilla and the abundant glands of conjunctiva (Fig. 1D). There were no fibrous connections between the striated muscle fibers and the skin or conjunctiva. Depending on the sectional plane, the orbicularis oculi muscle consisted of a cluster of fiber bundles that made a core of the eyelid (Figs. 1F, 2A). Thus, the muscle fibers did not approach the dermis or conjunctival epithelium. Likewise, the muscle fibers of the levator palpebrae superioris were present in multiple bundles, and did not approach the dermis or conjunctival epithelium (Fig. 1E).
Bundles of the orbicularis oculi muscle surrounded the lacrimal canaliculi, and these bundles were thinner than those in the eyelid (Fig. 2B). A bundle around the canaliculi consisted of 5 to 200 fibers, and a bundle in the eyelid consisted of 10 to 500 fibers. Immediately below the lower eyelid, fibers of the levator labii superioris alaeque nasi muscle ran inferiorly, and some of these fibers approached the dermis of the upper part of the nose (Fig. 2C). This was one of the few cases in which striated muscle fibers were directed to the skin.
The nasalis muscle was characterized by nearly straight fibers that ran superficially and orthogonal to the skin (Fig. 3A, C). Some of them reached the dermis at the nasal orifice, which contained abundant glands and hair follicles, but most of these fibers ended in the subcutaneous tissue (Fig. 3B, D, E). The gland appeared to disturb the course of the muscle fiber, making this course circuitous (Fig. 3B, E). Overall, the nasalis muscle was unique, in that striated muscle fibers were likely to insert into the dermis.
At the insertion of the levator anguli oris and risorius muscles, the muscle bundles were ‘sandwiched’ or interdigitated with other muscle bundles of the depressor anguli oris and orbicularis oris (Fig. 4A–C). The depressor anguli oris muscle bundle consisted of 3 to 20 fibers, and the orbicularis oris muscles consisted of 50 to 200 fibers. Thus, the bundle was much thinner in the levator and risorius muscles than in the depressor and orbicularis muscles. Likewise, muscle bundles of the zygomaticus major and levator labii superioris were interdigitated with those of the orbicularis oris at the insertion. Because the muscle bundles of the depressor labii inferioris were a bit deeper than those other muscles, they were interdigitated with the orbicularis muscle and the buccinator muscle. Therefore, the orbicularis oris muscle bundles provided mechanical support to the terminal regions of the superficial muscles, and provided them with insertions. There was no fibrous connection between the perimysium of the orbicularis oris muscle bundle and the oral mucosa.
Near the angle of mouth, the mentalis muscle fibers passed between muscle bundles of the orbicularis oris and extended toward the skin (Fig. 3F). However, the muscle fibers did not reach the dermis in midterm fetuses (Fig. 3F). The mentalis muscle was an exception, in that these striated muscle fibers were directed toward the skin. In addition, an inferior part of the platysma muscle had a free end without a specific fibrous structure in the anterior thoracic subcutaneous tissue (not shown).
Muscle bundles of the levator veli palatini varied considerably in thickness, and the number of fibers ranged from 5 to 250. These bundles were roughly divided by fibrous septa that extended to the oral side (Fig. 4D, E), and provided an insertion for the levator muscle. In the lateral part of the soft palate, the septa extended to or contained the uvulae muscle bundle, which had 5 to 20 fibers. At the oral side, most of the uvulae muscle fibers ended at or continued to the septa, but some fibers passed between glands and inserted deeply toward the lamina propria of the oral mucosa (Fig. 4F, G). Therefore, the uvulae muscle was atypical, in that its striated muscle fibers were directed toward the mucosa.
Muscle fibers of the pharyngeal constrictors ran parallel to the pharyngeal mucosa (not shown): this was similar to the topographical relation between the orbicularis oculi muscle fiber and palpebral conjunctiva (see above the first subsection). The stylopharyngeus muscle passed inferiorly through a ‘window’ of the constrictors, and did not reach the mucosa, but interdigitated with the mucosal-sided fibers of the constrictors.
The buccinator muscle fibers were not bundled by the perimysium, but appeared as solitary fibers with a dense arrangement (Fig. 5), a morphology quite different from the adjacent orbicularis oris (Fig. 4B, C). The buccinator fibers were separated from the mucosal epithelium by submucosal tissue in the smaller fetuses (Fig. 5A, B). Later in development, the muscle terminal provided a fibrous lamina near the epithelium (Fig. 5C, F), and the muscle fibers ultimately developed inserts into the basement membrane of the epithelium (Fig. 5D, E). Therefore, the buccinator muscle was unique, in that its striated muscle fibers were likely to reach the mucosa.
The superior longitudinal muscle and vertical muscle were near the upper mucosal surface of the tongue (Fig. 6). The longitudinal muscle had clusters of muscle bundles that ran parallel to the mucosa; in contrast, the vertical muscle had thin muscle bundles consisting of 2 to 10 muscle fibers when the muscle approached the mucosa. In late-stage fetuses, some of the thin muscle bundles reached the dense fibrous lamina of the submucosal tissue (Fig. 6A, B). However, in adult specimens, the perimysium of the vertical muscle bundle appeared to disperse in the submucosal tissue (Fig. 6C, D). Therefore, none of the striated muscle fibers reached the oral mucosa in the tongue.
The subcutaneous part of the external anal sphincter was divided into large masses by fibrous septa that appeared to correspond to the endomysium. The septa extended superficially, and dispersed the subcutaneous tissue (Fig. 7A). In each of the striated muscle masses, muscle fibers were bundled by the perimysium (Fig. 7B) and this basic structure was underdeveloped in fetuses (Fig. 7C). The external urethral sphincter surrounded the urethra, and the bilateral sphincters were ‘sandwiched’ by the medial edge of the levator ani muscle (Fig. 7D). The endomysium of each muscle fiber in the external urethral sphincter extended to collagenous fibers that covered the urethral wall, which consisted of an outer layer of smooth muscle and an inner layer of elastic fibers (Fig. 7E). Therefore, none of the striated muscle fibers directed toward or approached the skin in the pelvic floor sphincters.
Although there are many facial muscles in subcutaneous tissues, our results showed the insertion and terminal regions almost always had one of two types of configuration (Fig. 8, Table 1): 1) an insertion formed by interdigitation with another muscle (e.g., the levator anguli oris and orbicularis oris) or 2) a circular arrangement in which muscle fiber insertion was ‘sandwiched’ and mechanically supported by other muscle fibers (e.g., the orbicularis oculi). It was extremely rare for muscle fibers to be directed toward the skin or mucosa. Our analysis of the first configuration showed no specific fibrous structure for facial muscle insertions, such as the modiolus, but this might develop postnatally. Our analysis of the second configuration showed that this morphology seemed to correspond to an in-series architecture, in which a muscle fiber passes contraction force to another fiber via a common endomysium.
The insertion of a striated muscle into the skin or mucosa was the most striking feature in the present observations. In the face, these morphologies were always in the nasalis and mentalis muscles, and often in the levator labii superioris alaeque nasi muscle. The buccinator muscle was consistently inserted into the basement membrane of the oral mucosa. It is likely that the uvulae muscle of the soft palate and the intrinsic vertical muscle of the tongue were directed toward the mucosa. However, the pelvic floor did not contain striated muscle fibers that were directed toward the skin or mucosa. To our regret, we have no information about the extracellular matrix at the exceptional muscle insertion into the skin or mucosa. Indeed, as seen in the external urethral sphincter, elastic fibers have a significant role in establishing the interface between the mucosa and striated muscle. However, a mesh of type I collagen fibrils also seemed to function by buffering the peak contraction load.
Our observations of the orbicularis oculi, orbicularis oris, and pharyngeal constrictor muscles indicated the circularly-arrayed striated muscle (i.e., in-series architecture, as described in the Introduction) did not have a specific interface tissue or an insertion into the skin or mucosa. Instead, the perimysium or endomysium seemed to establish a sliding surface in the subcutaneous or submucosal tissue. Likewise, our analysis of the external anal sphincter and the levator veli palatini muscle showed that the perimysium seemed to establish a sliding surface with the thick septa, because many muscle fibers were bundled by the perimysium and faced the septa. Notably, the septa pulled up the skin or mucosa (i.e., an upward load), whereas the composite muscle fibers ran along the medio-lateral or antero-posterior axis (i.e., orthogonal to the septa). There may be a specific extracellular matrix that reduces frictional stress at the sliding surface.
As seen in the angle of the mouth, muscle insertions often consisted of interdigitated muscle bundles with different functional vectors. Therein, one muscle bundle terminal (the lateral tractor bundle) was ‘sandwiched’ and fixed mechanically by the other bundles (depressor bundles). Thus, their functional vectors were almost orthogonal. The morphology of the different load vectors seemed likely because transmission of the force of muscle contraction occurs via the fascia or epimysium, even for synergic and antagonistic muscle groups [18]. To minimize mechanical stress and improve function, when the depressor contracts the lateral tractor should also contract to achieve fixation; if the depressor fully relaxes, then it loses the insertion. Therefore, the nervous system must meticulously control of timing, duration, and strength of muscle contraction. Recent studies of surface electromyography identified which muscles are activated for specific facial expressions [19, 20]. These studies of various types of facial expression showed that the orbicularis oris, levator anguli oris, and depressor anguli oris muscles altogether had relatively high activity. Some muscle fibers should make the specific facial expression, while the other fibers may play our-hypothesized role of the fixation of muscle terminals.
The different types of striated muscle fibers transmit a contraction force to the target, such as a bone, via associated fibrous tissues, including the tendon, myotendinous junction, epimysium, perimysium, and endomysium [21, 22]. These mechanical forces are coordinated and pass between adjacent muscle cells via cell-matrix interactions and the endomysial connective tissue that links these cells together [23-25]. Striated muscle fibers can have a non-spanning architecture or a spanning architecture [21, 22]. The non-spanning architecture is characterized by a series of muscle fibers that use the same endomysium for the transmission of force to connect the origin and insertion (in-series muscle fibers). The spanning architecture is characterized by single muscle fibers that extend from the origin to the insertion, even when the muscle is very long, such as the sartorius. Using transmission electron microscopy, further studies seemed to be necessary to identify connecting fibrils and/or substances between the striated muscle fiber and the skin or mucosa.
We did not examine facial muscles in adult specimens. However, we believe that late-term fetuses and adults have a similar morphology. However, at the peripheral and lateral regions, it is likely that parts of the facial striated muscle fibers inserted into a tight subcutaneous tissue, the ‘superficial musculoaponeurotic system’ [26, 27], a structure that is underdeveloped in fetuses. Likewise, the platysma muscle of fetuses had a free end without a specific fibrous structure in the anterior thoracic subcutaneous tissue.
Notes
References
1. Ohtsuka K, Tomita H, Murakami G. 2002; Anatomy of the tonsillar bed: topographical relationship between the palatine tonsil and the lingual branch of the glossopharyngeal nerve. Acta Otolaryngol Suppl. 546:99–109. DOI: 10.1080/00016480260046472. PMID: 12132628.

2. Meng H, Murakami G, Suzuki D, Miyamoto S. 2008; Anatomical variations in stylopharyngeus muscle insertions suggest interindividual and left/right differences in pharyngeal clearance function of elderly patients: a cadaveric study. Dysphagia. 23:251–7. DOI: 10.1007/s00455-007-9131-2. PMID: 18427898.

3. Abe S, Fukuda M, Yamane S, Saka H, Katori Y, Rodríguez-Vázquez JF, Murakami G. 2013; Fetal anatomy of the upper pharyngeal muscles with special reference to the nerve supply: is it an enteric plexus or simply an intramuscular nerve? Anat Cell Biol. 46:141–8. DOI: 10.5115/acb.2013.46.2.141. PMID: 23869261. PMCID: PMC3713278.

4. Rohan RF, Turner L. 1956; The levator palati muscle. J Anat. 90:153–4. DOI: 10.1097/00006534-196811000-00036. PMID: 13295160. PMCID: PMC1244830.
5. Doménech-Ratto G. 1977; Development and peripheral innervation of the palatal muscles. Acta Anat (Basel). 97:4–14. DOI: 10.1159/000144712. PMID: 66841.

6. Stål PS, Lindman R. 2000; Characterisation of human soft palate muscles with respect to fibre types, myosins and capillary supply. J Anat. 197(Pt 2):275–90. DOI: 10.1017/S0021878299006627. PMID: 11005719. PMCID: PMC1468126.

7. Langdon HL, Klueber K. 1978; The longitudinal fibromuscular component of the soft palate in the fifteen-week human fetus: musculus uvulae and palatine raphe. Cleft Palate J. 15:337–48. PMID: 281277.
8. Klueber K, Langdon HL. 1979; Anatomy of musculus levator veli palatini in the 15-week human fetus. Acta Anat (Basel). 105:94–105. DOI: 10.1159/000145113. PMID: 525252.

9. Shimokawa T, Yi SQ, Izumi A, Ru F, Akita K, Sato T, Tanaka S. 2004; An anatomical study of the levator veli palatini and superior constrictor with special reference to their nerve supply. Surg Radiol Anat. 26:100–5. DOI: 10.1007/s00276-003-0183-1. PMID: 14586563.

10. Hinata N, Murakami G. 2014; The urethral rhabdosphincter, levator ani muscle, and perineal membrane: a review. Biomed Res Int. 2014:906921. DOI: 10.1155/2014/906921. PMID: 24877147. PMCID: PMC4022307.

11. Sasaki H, Hinata N, Kurokawa T, Murakami G. 2014; Supportive tissues of the vagina with special reference to a fibrous skeleton in the perineum: a review. Open J Obstet Gynecol. 4:144–57. DOI: 10.4236/ojog.2014.43025.

12. Kim JH, Kinugasa Y, Yu HC, Murakami G, Abe S, Cho BH. 2015; Lack of striated muscle fibers in the longitudinal anal muscle of elderly Japanese: a histological study using cadaveric specimens. Int J Colorectal Dis. 30:43–9. DOI: 10.1007/s00384-014-2038-0. PMID: 25331031.

13. Arakawa T, Hayashi S, Kinugasa Y, Murakami G, Fujimiya M. 2010; Development of the external anal sphincter with special reference to intergender difference: observations of mid-term fetuses (15-30 weeks of gestation). Okajimas Folia Anat Jpn. 87:49–58. DOI: 10.2535/ofaj.87.49. PMID: 20882767.

14. Arakawa T, Hwang SE, Kim JH, Wilting J, Rodríguez-Vázquez JF, Murakami G, Hwang HP, Cho BH. 2016; Fetal growth of the anal sinus and sphincters, especially in relation to anal anomalies. Int J Colorectal Dis. 31:493–502. DOI: 10.1007/s00384-015-2455-8. PMID: 26615552.

15. Yamamoto M, Hirota Y, Watanabe G, Taniguchi S, Murakami G, Rodríguez-Vázquez JF, Abe SI. 2024; Development and growth of median structures in the human tongue: a histological study using human fetuses and adult cadavers. Anat Rec (Hoboken). 307:426–41. DOI: 10.1002/ar.25198. PMID: 36939757.

16. Ogawa Y, Hinata N, Murakami G, Bando Y, Kitamura K, Hussein AA, Guru K, Abe SI, Fujisawa M. 2018; Aspects of lymphatic vessel configuration of the human male urinary bladder and adjacent organs: a histological basis for understanding the spread of cancer metastases. Transl Res Anat. 11:10–7. DOI: 10.1016/j.tria.2018.05.001.

17. Hinata N, Hussein AA, Bando Y, Terakawa T, Murakami G, Yamamoto M, Abe SI, Guru K, Fujisawa M. 2021; Histologic investigation of the female vesicourethral junction and adjacent tissues for nerve-sparing radical cystectomy. Urology. 149:161–7. DOI: 10.1016/j.urology.2020.12.001. PMID: 33309709.

18. Huijing PA, van de Langenberg RW, Meesters JJ, Baan GC. 2007; Extramuscular myofascial force transmission also occurs between synergistic muscles and antagonistic muscles. J Electromyogr Kinesiol. 17:680–9. DOI: 10.1016/j.jelekin.2007.02.005. PMID: 17383898.

19. Schumann NP, Bongers K, Scholle HC, Guntinas-Lichius O. 2021; Atlas of voluntary facial muscle activation: visualization of surface electromyographic activities of facial muscles during mimic exercises. PLoS One. 16:e0254932. DOI: 10.1371/journal.pone.0254932. PMID: 34280246. PMCID: PMC8289121.

20. Mueller N, Trentzsch V, Grassme R, Guntinas-Lichius O, Volk GF, Anders C. 2022; High-resolution surface electromyographic activities of facial muscles during mimic movements in healthy adults: a prospective observational study. Front Hum Neurosci. 16:1029415. DOI: 10.3389/fnhum.2022.1029415. PMID: 36579128. PMCID: PMC9790991.

21. Trotter JA. 1993; Functional morphology of force transmission in skeletal muscle. A brief review. Acta Anat (Basel). 146:205–22. DOI: 10.1159/000147459. PMID: 8317197.
22. Huijing PA. 1999; Muscle as a collagen fiber reinforced composite: a review of force transmission in muscle and whole limb. J Biomech. 32:329–45. DOI: 10.1016/S0021-9290(98)00186-9. PMID: 10213024.

23. Light N, Champion AE. 1984; Characterization of muscle epimysium, perimysium and endomysium collagens. Biochem J. 219:1017–26. DOI: 10.1042/bj2191017. PMID: 6743238. PMCID: PMC1153576.

24. Purslow PP. 2002; The structure and functional significance of variations in the connective tissue within muscle. Comp Biochem Physiol A Mol Integr Physiol. 133:947–66. DOI: 10.1016/S1095-6433(02)00141-1. PMID: 12485685.

25. Sleboda DA, Stover KK, Roberts TJ. 2020; Diversity of extracellular matrix morphology in vertebrate skeletal muscle. J Morphol. 281:160–9. DOI: 10.1002/jmor.21088. PMID: 31840868. PMCID: PMC7137673.

26. Mitz V, Peyronie M. 1976; The superficial musculo-aponeurotic system (SMAS) in the parotid and cheek area. Plast Reconstr Surg. 58:80–8. DOI: 10.1097/00006534-197607000-00013. PMID: 935283.

27. Watanabe K, Han A, Inoue E, Iwanaga J, Tabira Y, Yamashita A, Kikuchi K, Haikata Y, Nooma K, Saga T. 2023; The key structure of the facial soft tissue: the superficial musculoaponeurotic system. Kurume Med J. 68:53–61. DOI: 10.2739/kurumemedj.MS682008. PMID: 37062726.

Fig. 1
Striated muscles in the eyelid skin and mucosa of fetuses. Two horizontal sections of a fetus with a crown-rump length of 262 mm. Panel (A) is 2.6 mm superior to panel (C); panels (B, E) are magnified views of rectangles in panel (A); and panels (D, F) are magnified views of rectangles in panel (C). In the upper eyelid, muscle fibers of the orbicularis oculi muscle (OOM) ran parallel to the skin and conjunctiva (B, D), but the levator palpebrae superioris muscle (LPSM) consisted of muscle bundles that cut transversely (E). (F) Shows the orbicularis oculi muscle bundle cut transversely in the lower eyelid. Insert at the bottom of the figure shows topographical relation of the orbicularis oculi and levator palpebrae superioris muscles with the skin (S) or mucosa (M). (A–D, F) Scale bars=1 mm, (E) scale bars=0.1 mm; H&E staining: all panels. FB, frontal bone.
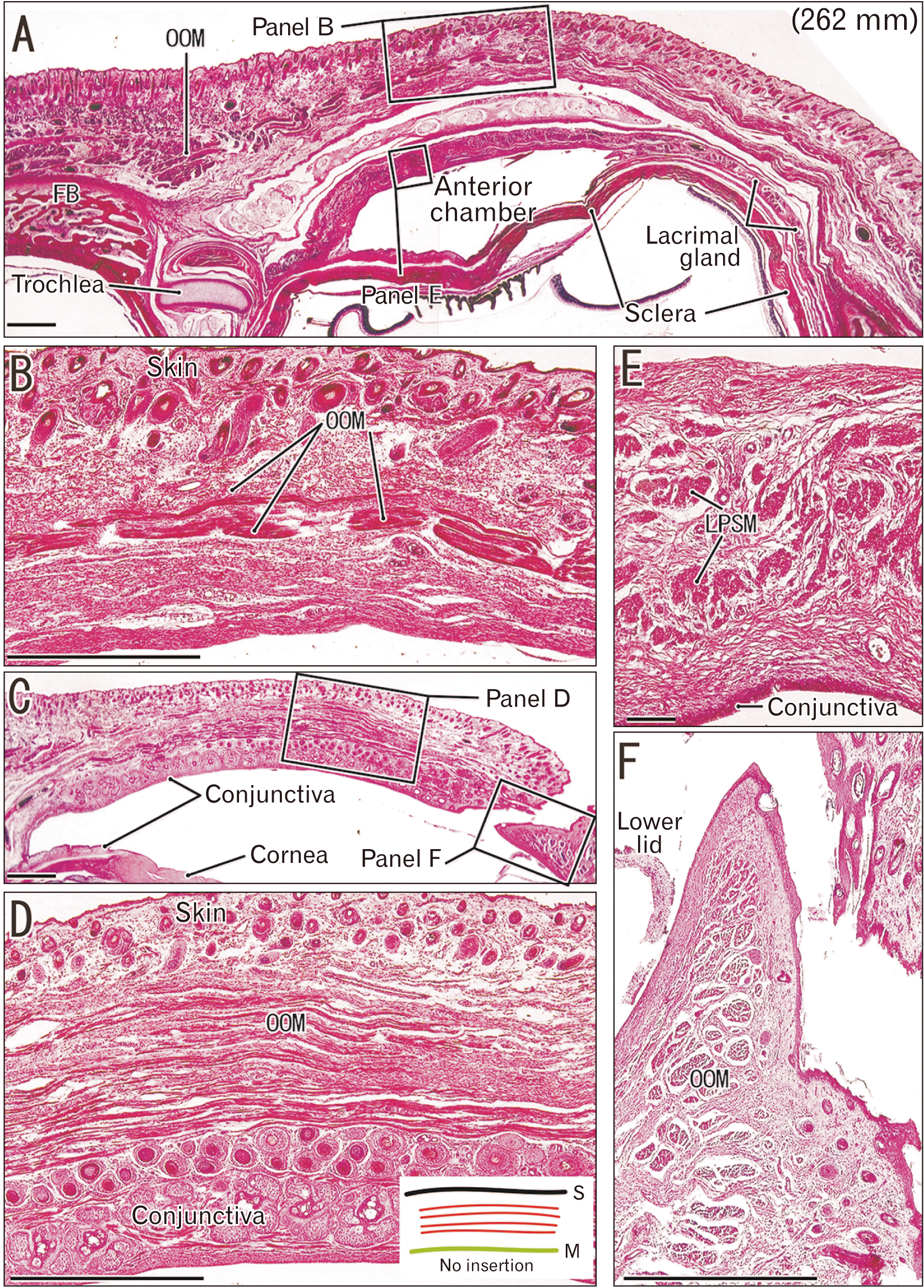
Fig. 2
Circularly-arrayed muscle in the eyelid skin and mucosa and another muscle near the eye of fetuses. Sagittal section of a fetus with a crown-rump length of 328 mm. Panels (B, C) are magnified views of the rectangles in panel (A). Eyelids contain many muscle bundles of the orbicularis oculi muscle (OOM in A). Small muscle bundles surround the lacrimal canaliculus (stars in B). Some fibers (arrows in C) of the levator labii superioris alaeque nasi muscle (LLSANM) are superficial to (but do not reach) the dermis. Insert at the bottom of the figure shows topographical relation of the levator labii superioris alaeque nasi muscle with the skin (S). Scale bars=1 mm. H&E staining: all panels. LB, lacrimal bone; LLSM, levator labii superioris muscle; MX, maxilla; NM, nasalis muscle.
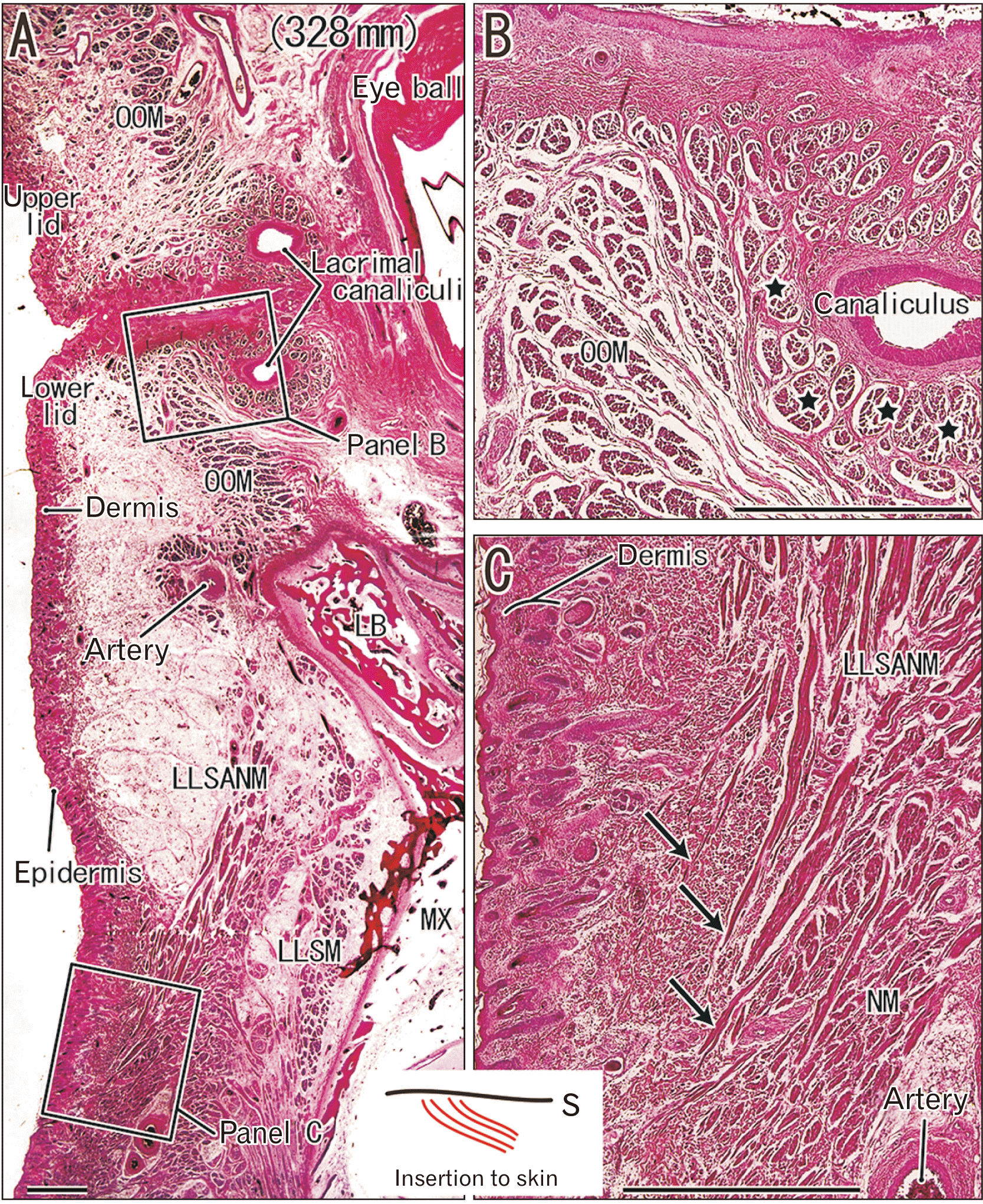
Fig. 3
Nasalis muscle in the nose skin and the mentalis muscle in the lip skin in near-term fetus with 215 mm crown-rump length (CRL). Frontal sections of a fetus with a CRL of 215 mm (A, B, E, F) and a fetus with a CRL of 170 mm (C, D); panels (B, D, E) are magnified views of the rectangles in panels (A, C). Some fibers of the nasalis muscle (NM) ran toward the skin and inserted into the dermis (arrows in D), but did not reach the basement membrane (stars in B, D, E). Fibers of the mentalis muscle (MM) were orthogonal to the skin and they approach the hair follicle (hair, F). Insert in panel (E) shows the topographical relation of the mentalis and nasalis muscles with the skin (S). (A, C, D, F) Scale bars=1 mm, (B, E) scale bars=0.1 mm. (A, B, E, F) H&E staining, (C, D) Azan staining. MA, mandible; NS, nasal septum cartilage; OORM, orbicularis oris muscle.
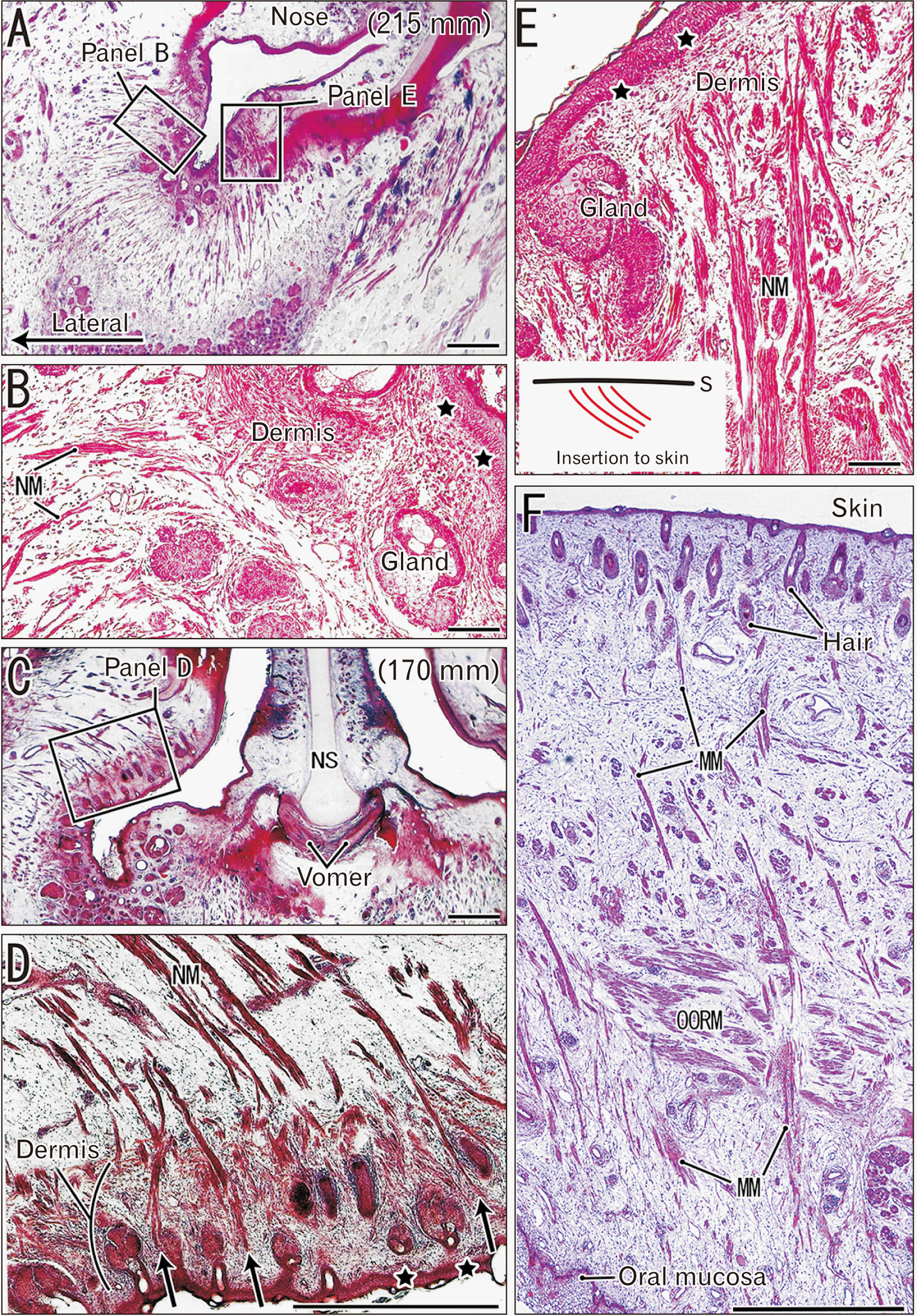
Fig. 4
Muscle fiber configuration in the skin at the angle of mouth and in the mucosa of the soft palate of fetuses. Frontal sections of a fetus with a crown-rump length (CRL) of 334 mm (A, C) showing muscle convergence at the angle of the mouth; sagittal sections of a fetus with a CRL of 328 mm (D, G) showing interdigitation of the levator veli palatini (LVPM) and uvulae muscles (UM); panels (B, C) are magnified views of the rectangles in panel (A); panels (E, F) are magnified views of the rectangles in panel (D). In the subcutaneous tissue at the angle of mouth, the risorius muscle (RM) interdigitated with the depressor anguli oris (DAOM) and orbicularis oris muscles (OORM) (B). Likewise, the levator anguli oris muscles (LAOM) interdigitated with the orbicularis oris muscles (C). In the soft palate, the levator veli palatini muscle was divided into multiple bundles by the uvulae muscle (E). A solitary fiber or bundle of the uvulae muscle approached the oral mucosa (F), and ended in the dense submucosal tissue (G). Insert in panel (C) shows a facial muscle interdigitation providing the insertions at the angle of mouth. Another insert in panel (F) shows the topographical relation of the uvulae muscle with the mucosa (M). (A, D, E, F) Scale bars=1 mm, (B, C, G) scale bars=0.1 mm. H&E staining: all panels.

Fig. 5
Insertion of the buccinator muscle into the oral mucosa of fetuses. Sagittal sections of fetuses with a crown-rump length (CRL) of 62 mm CRL (A, B), a CRL of 150 mm (C, F), and a CRL of 215 mm (D, E); panels (B, E, F) are magnified views of the rectangles in panels (A, D, C). Muscle fibers of the buccinator muscle (BM) ran along the oral mucosa (B, E, F), and some of them (E) appeared to reach the basement membrane (stars). The muscle fibers ended at a dense fibrous layer (arrowheads in F) that was distant from the basement membrane. Insert at the lower right-hand angle of the figure shows the topographical relation of the buccinator muscle with the mucosa (M). (A–E) Scale bars=1 mm, (F) scale bars=0.1 mm. (A, B) Azan staining, (C–F) H&E staining. MA, mandible; MX, maxilla; PM, platysma muscle.
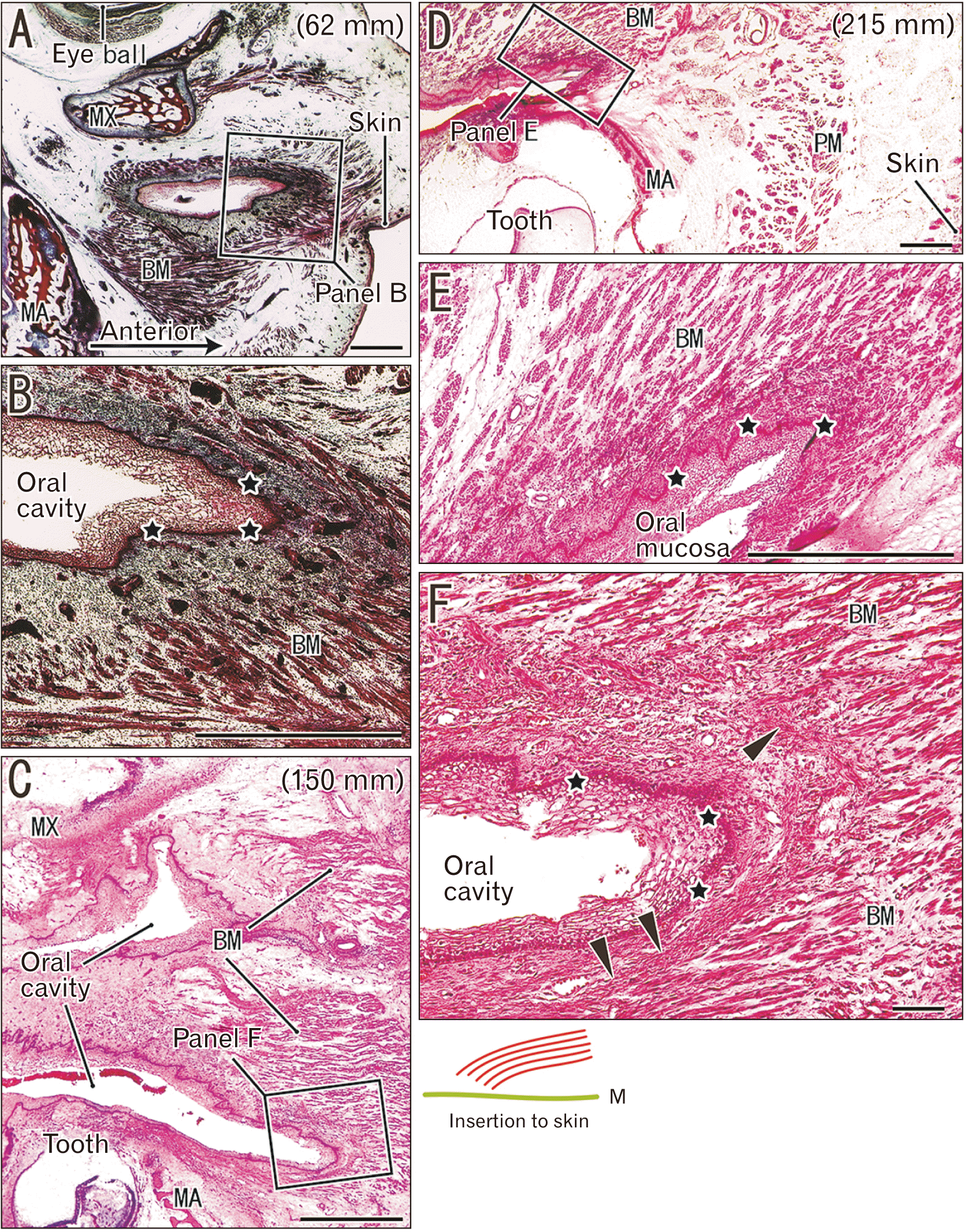
Fig. 6
Intrinsic vertical muscle approaching the lingual mucosa of fetuses and adults. Frontal sections of fetuses with a crown-rump length (CRL) of 70 mm (A) and a CRL of 258 mm (B), and of a 72-year-old female (C, D); panel (D) is a magnified view of the rectangle in panel (C). Intrinsic vertical muscle fibers of the tongue (VM) approached the lingual mucosa, but did not reach the basement membrane of the squamous epithelium (stars in A, B, C). A dense fibrous lamina was in the submucosal tissue of fetuses (arrowheads in A, B). The epimysium appeared to continue to a fibrous band in adults (triangles in D) and disperse into the submucosal tissue. Insert in panel (A) shows the topographical relation of the intrinsic tongue muscles with the mucosa (M). (A–C) Scale bars=1 mm, (D) scale bars=0.1 mm. (A) Verde Luz-orange G-acid fuchsin staining, (B) Masson’s trichome staining, (C, D) Azan staining. SLM, superior longitudinal muscle; TM, transverse muscle.
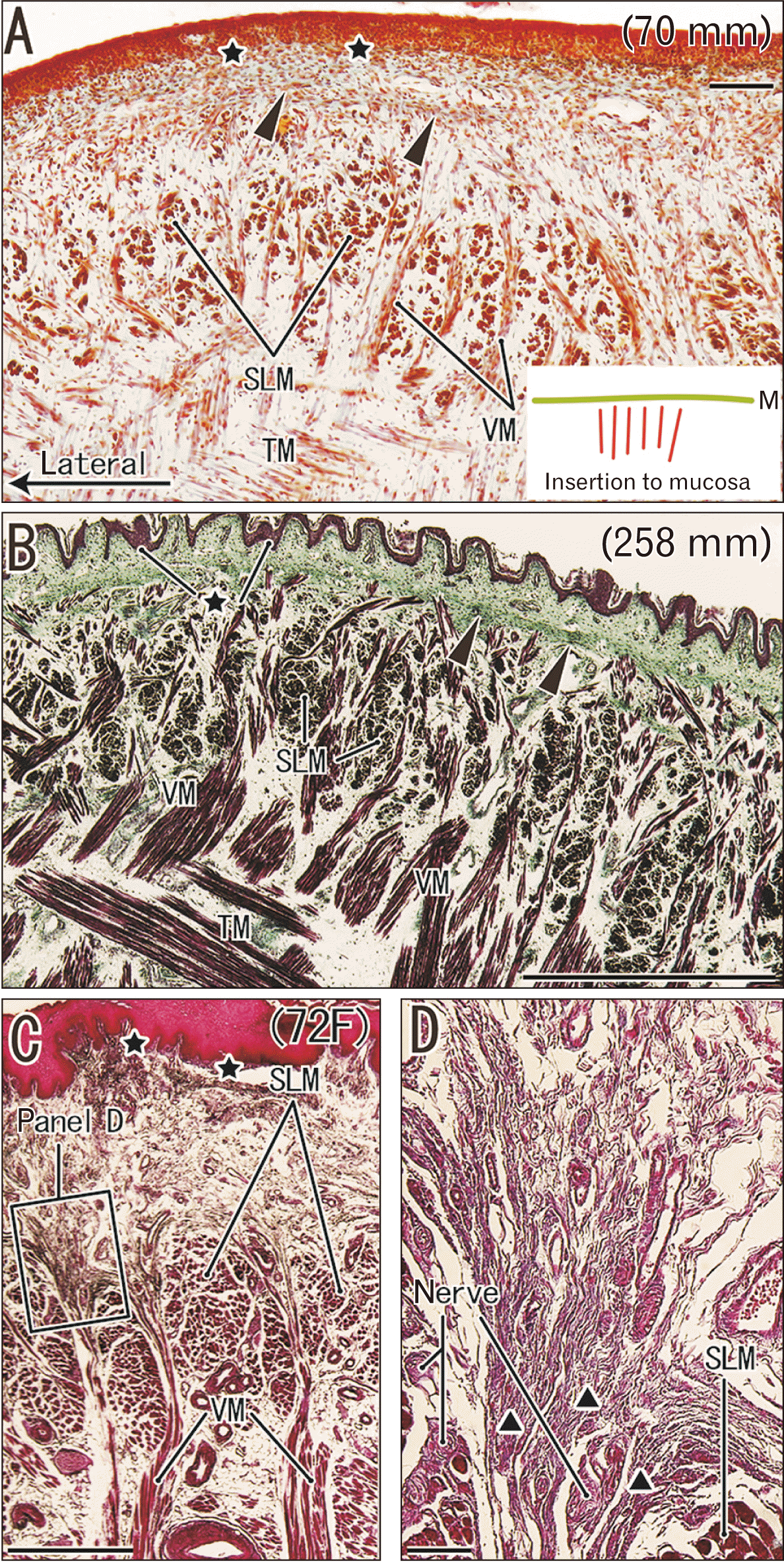
Fig. 7
Striated muscle sphincters beneath the anal and urethral mucosae of fetuses and adults. Frontal sections of the subcutaneous part of the external anal sphincter (EAS) in a 75-year-old male (A, B); anal sphincters of a fetus with a crown-rump length of 100 mm (C); and external urethral sphincter (URS) in a 69 year-old male (D, E); panel (E) is a magnified view of the rectangle in panel (D). The adult lateral anal wall (A) had subcutaneous striated muscle fibers (B) of the sphincter that were bundled by fibrous tissue septa (arrows in A), and the septa were dispersed into subcutaneous tissue. The fetal external sphincter became thick before the smooth muscle-made internal sphincter (IAS in C). The external urethral sphincter (D) and the external anal sphincter were ‘sandwiched’ or suspended by the bilateral levator ani muscles (LAM; C, D). Adult muscle fibers of these sphincters did not reach or connect to the dermis or mucosa (B, E). Adult septa (stars in E) divided the external urethral sphincter into multiple bundles or sheets, and they joined to insert into the urethral wall. Insert in panel (A) shows the topographical relation of the external sphincters of the anus and urethra with the mucosa (M). (D) Scale bars=10 mm, (A, C, E) scale bars=1 mm, (B) scale bars=0.1 mm. (A, B) H&E staining, (C) Azan staining, (D, E) Elastic-Masson’s staining.

Fig. 8
Schematic representation of muscle insertions beneath the skin. The perimysium (green), endomysium (orange), and basement membrane (black dots and lines). (A) Left shows striated muscle fibers that approached the skin with rare morphologies, in which muscles were almost orthogonal to the dermis (a) or were oblique to the dermis (b). (A) Right shows the more usual morphologies, in which muscle fibers were parallel to the skin and were solitary fibers enclosed by the endomysium (c) or were in a bundle surrounded by the perimysium (d). (B) Shows the usual insertion of cutaneous and submucosal striated muscles, in which the two muscles with different functional vectors (levator and lateral tractor muscles) were interdigitated and mechanically fixed due to bundle-to-bundle crossing with perimysial contact.
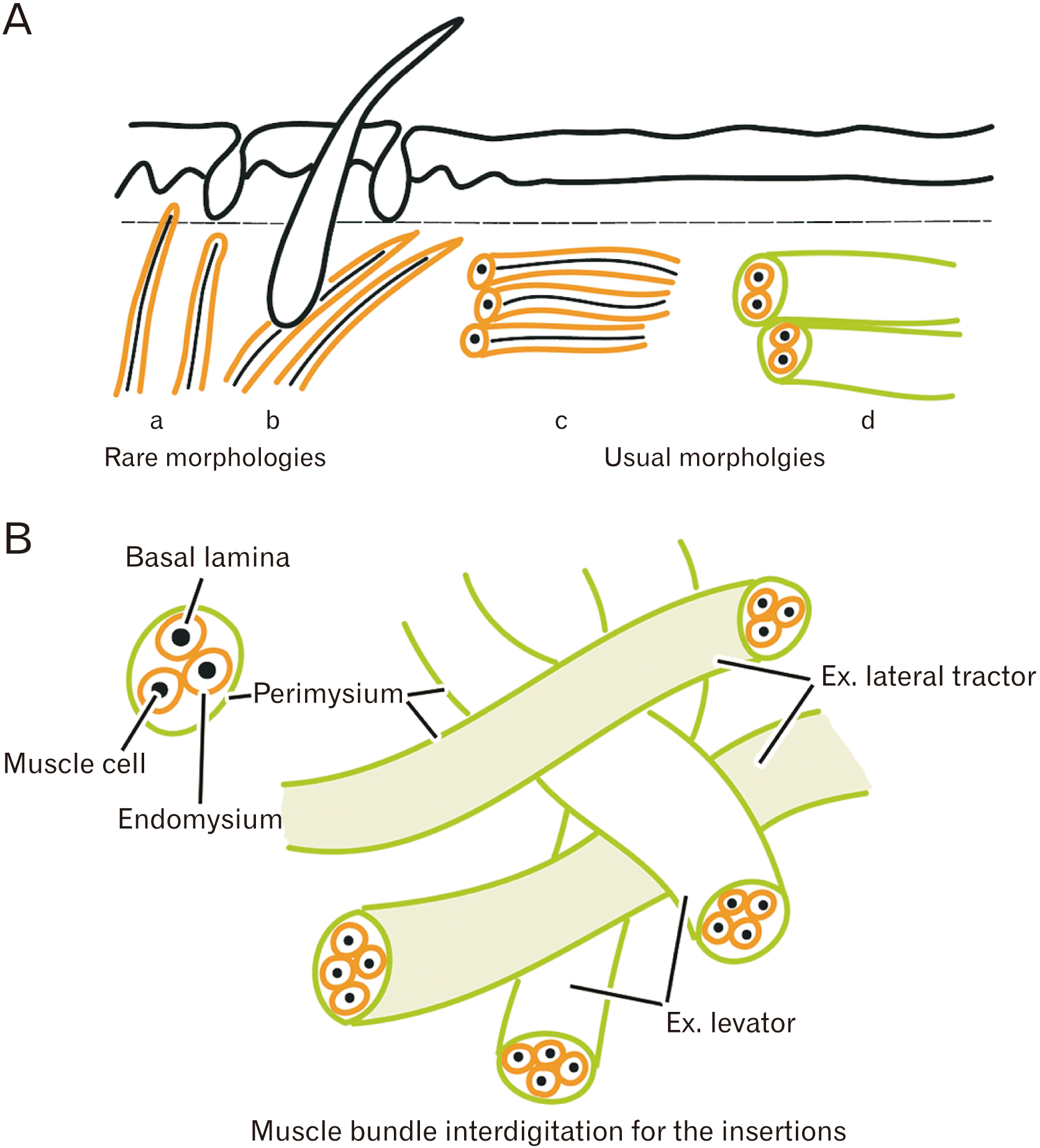
Table 1
Summary of the present observations
| Muscle name or group | Insertion to the skin or mucosa |
|---|---|
| Levator palpebrae superioris (Fig. 1) | No insertion but bundled in the subcutaneous tissue |
| Orbicularis oculi (Figs. 1, 2) | Same as above |
| Levator labii superioris alaeque nasi (Fig. 2) | To the subcutaneous tissue and sometimes* to the dermis |
| Nasalis (Fig. 3) | To the dermis at the external nasal orifice |
| Mentalis (Fig. 3) | To the dermis |
| Buccinator (Fig. 5) | To the lamina propria and sometimes* to the basement membrane |
| Facial muscles at the angle of mouth (Fig. 4) | Interdigitation each other instead of the insertion |
| Uvulae (Fig. 4) | To the submucosa and sometimes* to the lamina propria |
| Levator veli palatini (Fig. 4) | No insertion but bundled by septa in the submucosa |
| Intrinsic tongue muscles (Fig. 6) | To the submucosa |
| External sphincter of the anus and urethra (Fig. 7) | No insertion but bundled by septa in the submucosa |




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download