Abstract
The antidepressant drug trazodone (TRZ) is commonly used for treating depression, anxiety, and insomnia, however, it causes cardiotoxicity, which is one of its limitations. The objective of this work was to investigate the impact of sage (Salvia officinalis) in rats against cardiotoxicity induced by TRZ and to investigate the mechanisms involved in its cardio-protective properties through autophagy and oxidative stress. Fifty male albino rats were split randomly into five experimental groups: control group, sage oil group (100 mg/kg), TRZ group (20 mg/kg), protective group, and curative group. Cardiac function biomarkers (aspartate aminotransferase [AST], creatine kinase-MB [CK-MB], and cardiac troponin T [cTnI]) were assessed in serum. Oxidative stress and inflammatory biomarkers in cardiac tissue (total antioxidant capacity, malondialdehyde, and tumor necrosis factor-α) were evaluated. Heart tissues were subjected to histological, immunohistochemical, and ultrastructural evaluations. DNA damage also evaluated. Significant rise in the levels of AST, CK-MB, and cTnI were observed with enhanced autophagy along with marked histopathological changes in the form of interrupted muscle fibers with wide interstitial spaces with areas of hemorrhage and extravasated blood and interstitial mononuclear cellular infiltration in TRZ group. DNA damage was also significantly increased in TRZ group. However, administration of sage in both protective and curative groups show marked improvement of the cardiac alterations. In conclusion, sage ameliorated the alterations in the heart induced by trazadone through modulation of autophagy and oxidative stress.
The drug trazodone (TRZ) was first clinically approved in 2001 and acts as an antagonist of serotonin receptor type 2 and reuptake inhibitor. Despite being approved for treating insomnia, several adverse effects of TRZ including hypotension, syncope, headaches, dizziness, and confusion have been reported [1]. TRZ has been associated with a number of cardiotoxicity reports over the past few years, which suggests TRZ may have cardiovascular adverse effects [2]. Postural hypotension, electrocardiogram (ECG) abnormalities, and cardiac arrhythmias have been observed after TRZ therapy at both therapeutic and subtherapeutic dosages [3].
It has been shown that free radicals and reactive oxygen species (ROS) are among the most common mechanisms of cardiotoxicity induced by TRZ. Tumor necrosis factor (TNF) serves as a crucial inflammatory biomarker of cardiotoxicity, which is regulated by ROS [4, 5]. In this regard, the inhibition of TNF-α using cardioprotective agents could be therapeutically useful.
Autophagy performs a crucial role both physiologically and pathologically. Upon stress stimuli, autophagy maintains cellular homeostasis by removing damaged organelles, misfolded/aggregated proteins, and intracellular pathogens [6]. Moreover, autophagy can also assist in mobilizing energy stores [7]. There are four processes involved in autophagy, the most important of which is autophagosome formation [8]. Microtubule-associated protein 1A/1B-light chain 3 (LC3) are inextricably linked to autophagosome formation. LC3 protein is a marker for autophagosome formation by converting LC3-I to LC3-II [9].
An essential role for autophagy in cardiac homeostasis has been identified. In cardiomyopathic mice, inhibition of autophagy gene 5 (ATG5) induced myocardial dysfunction, and disruption of ATG6 (known as Beclin1) caused heart failure [4, 10]. Furthermore, myocyte death by autophagy was observed in heart failure patients. Apoptosis, necrosis, or autophagy of cardiomyocytes cause heart dysfunction and exacerbate heart failure by destroying their proliferative potential [11].
P62, also called sequestosome 1 (SQSTM1), has a significant impact on the regulation of selective autophagy [12]. Physiologically, P62 levels are maintained at relatively low levels by basal autophagy. Nevertheless, various diseases can result in elevated P62 levels because of increased transcription or decreased autophagy [13]. It has been demonstrated that P62 plays various roles in inhibiting or inducing the death of cancer cells [14].
The acridine orange/ethidium bromide (AO/EB) double fluorescent staining method is an indirect technique used to assess genotoxicity and screen for potential harmful effects of a substance. It detects an increase in dead or damaged cells, indicating possible genotoxic effects. The comet assay is a widely used, cost-effective, reliable, and easy-to-use method for evaluating genotoxicity by detecting DNA damage [15]. Several studies [1, 16, 17] have investigated the genotoxic effects of TRZ on male rats. They discovered that TRZ caused dose-dependent cardiotoxicity and reproductive toxicity in male rats. Higher doses of TRZ were associated with increased DNA damage.
The perennial round shrub Salvia officinalis L. (known as sage) is a member of Labiatae/Lamiaceae family. It contains several active compounds, including tune, cineol, borneol, pensions, phalanges, saponins, glycosides, resins, vitamins E and C, tannins, and gummy substances [18]. The Middle East and Mediterranean are its native regions, though it is widely distributed. Currently, S. officinalis is used as an antibacterial, antifungal, antiviral, antioxidant, anti-inflammatory, diuretic, and anti-stress [19]. Through potentiation of the antioxidant defense system, S. officinalis ethanolic extracts used for four weeks inhibited alterations brought on by chlorpyrifos and methomyl in rats’ hearts and testes [20]. In rats subjected to 5-fluorouracil-induced cardiotoxicity, S. officinalis oil reduced biochemical cardiotoxicity, oxidative stress and antioxidant parameters [21].
Consequently, the current study assessed sage oil’s protective and curative effects on trazadone-induced cardiotoxicity in rats with investigations of possible involved mechanisms.
Using distilled water, TRZ hydrochloride Trittico® (EIPICO) was prepared. A bottle of pure sage oil (30 ml, 255 mg/ml; Hauppauge) was obtained from Nature’s Answer Company Co. and suspended in water with 0.1 ml of Tween 80 (Merck).
The study was carried out at the Department of Human Anatomy and Embryology, Faculty of Medicine, Menoufia University. Fifty male rats of the Sprague–Dawley strain, between 150 to 180 g, aged 10 to 12 weeks, took part in this study. Animals were obtained from the National Research Center animal house, in Cairo, Egypt. Before the experiment, a stainless steel cage was used to acclimate rats for two weeks. They were fed standard laboratory pellets ad libitum in addition to tap water. Optimal humidity and temperature were maintained throughout the room with light and darkness last for 12 hours each.
All animal experiments were conducted according to the National Institutes of Health guide for the care and use of laboratory animals (NIH Publications No. 8023, revised 1978). The ethical approval number in the Research Ethics Committee, Faculty of Medicine, Menoufia University is (12/2022 ANAT 7-1).
Two weeks after acclimatizing, animals were divided into five groups randomly (group size: 10):
Control group: received 0.1 ml tween 80 in distilled water/orally by gavage.
Sage oil group: received daily sage oil by oral gavage 100 mg/kg/orally for 3 weeks [22].
TRZ group: received an oral dose of TRZ (20 mg/kg/day) for 28 days [23].
Protective group: received sage oil of 100 mg/kg/day/orally for 3 weeks followed by TRZ (20 mg/kg/day/orally) for 28 days.
Curative group: received an oral dose of TRZ (20 mg/kg/day) for 28 days followed by daily sage oil 100 mg/kg/orally for 3 weeks.
Upon completion of the experiment, rats were fasted throughout the night and weighed. Under ether anesthesia then cervical dislocation was done. Samples of blood were gathered and then separated at ×708 g, using a cooling centrifuge (Laborzentrifugen 3-3OK; Sigma). For biochemical analysis, serum samples were stored at –80°C. Dissection of rats’ hearts was performed after scarification, rinsing with normal saline, drying, and weighing. Small pieces of the heart were cut out. One portion for histopathological examination was fixed in 10% formalin, a second portion for biochemical analysis was reserved at −80°C, whereas other portions were processed for transmission electron microscope (TEM). For the DNA fragmentation, viability assay, and comet assay, blood was collected, aliquoted in EDTA tubes, mixed thoroughly, and held on ice until required. These tests were assessed in leukocytes.
The calculation of heart weight to body weight ratio was done as follows: heart weight (g)/final body weight (g)×100 [24].
Aspartate aminotransferase (AST) and creatine kinase-MB (CK-MB) levels were determined spectrophotometrically with Biotecnica Instruments SpA kits. The manufacturer’s instructions were followed during all procedures [25]. CK-MB was measured as U/L while AST as IU/L. For CK-MB, the limit of detection was 2–2,000 U/L, and for AST, it was 4–700 IU/L. As per the manufacturer’s instructions, cardiac troponin T (cTnI) levels were measured using the technique of sandwich enzyme-linked immunosorbent assay (ELISA) and a rat cTnI ELISA kit was used (Sun-Red Biotechnology Company®), the cTnI detection limit was 4–1,000 ng/L.
Malondialdehyde (MDA), the biomarker of lipid peroxidation, was measured using a Spectrum Diagnostics® assay kit. The assay relies on the interaction between MDA and thiobarbituric acid (TBA) leading to the formation of an MDATBA2 adduct with a strong absorption at 532 nm. In this study, MDA was determined (nmol/g tissue) using a molar extinction coefficient of 1.56×105 M−1 cm−1 [26]. To determine cardiac total antioxidant capacity (TAC), an ELISA kit was used [27].
Heart tissue sections from each group were mounted on slides and stained with hematoxylin and eosin (H&E). By using slide microtomes, paraffin tissue blocks were sectioned at 4 microns and then deparaffinized. Afterward, routine histological evaluation was conducted with H&E-stained sections. To demonstrate collagen fibers, Masson’s trichrome staining was performed [29].
Four μm-thick paraffin sections were deparaffinated and rehydrated by xylene and graded alcohols. After being washed in tap water, incubation of tissue sections in methanol with 0.3% H2O2 was carried out at room temperature for 20 minutes. Phosphate-buffered saline (PBS) was used to wash the sections then blocked them with goat serum in PBS. Afterward, incubated with rabbit anti-LC3 polyclonal antibody (Abcam), mouse anti-Beclin1 monoclonal antibody (Abcam), mouse anti-P62 monoclonal antibody (Santa Cruz Biotechnology). Then, the sections were incubated for 30 minutes with biotinylated anti-mouse IgG (Vectastain Elite Avidin-Biotin Complex kit; Vector Labs). After incubation with avidin and biotinylated horseradish peroxidase macromolecular complex (Vector Laboratory), sections were stained with 3-amino-9-ethylcarbazole (Vector Laboratory) and hematoxylin. The primary antibody was omitted from the negative control. Under a light microscope, the samples were examined [30].
For each specimen, ten non-overlapping fields (×400) were examined at the Anatomy Department, Faculty of Medicine, Menoufia University. To avoid inter-observer errors, all measurements were conducted by the same investigator. Images J 1.47 v software was used for morphometric measurements of the area percentage of immunoexpression of LC3, Beclin1, and P62 and the area percentage of collagen fibers.
Left ventricle pieces (12 mm) were fixed in glutaraldehyde (2.5%) in 0.1 M cacodylate buffer (PH, 7.2) for one hour at 4°C. and then washed in cacodylate buffer. Afterward, these were postfixed in 1% osmium tetraoxide in cacodylate buffer, dehydrated in acetone, and embedded in epoxy araldite. An ultramicrotome (Leica EMUC6) and copper grids were used to obtain ultrathin sections and stain them with uranyl acetate and lead citrate [31]. A detailed examination was carried out at the Faculty of Science, Alexandria University, Egypt, with a JSM-1400 PLUS electron microscope (JEOL) at 80 KV.
According to El-Garawani [32], approximately 2 ml of peripheral venous blood was mixed with an erythrocyte-lysing buffer containing 0.015 M NH4CL, 1 mM NaHCO3, and 0.1 mM EDTA at a five-volume ratio. The mixture was then centrifuged at 1,000 rpm for 5 minutes, using a cooling centrifuge (Sigma 3K 30; Sigma-Aldrich). Repeating the centrifugation step was done multiple times until a white pellet formed, indicating the successful removal of erythrocytes.
DNA extraction and fragmentation detection were performed following the “salting out extraction method” described by Aljanabi and Martinez [33], with slight modifications made by Hassab El-Nabi and Elhassaneen [34]. The cells were lysed overnight at 37°C using a lysing buffer comprising 10 mM Tris base, 10 mM NaCl, 10 mM Na2 EDTA, 0.5% SDS, and adjusted to pH 8.3. Following lysis, 4M NaCl was added to the lysed cell samples. The mixture was then centrifuged at 10,000 rpm for 10 minutes, and carefully transferring the resulting supernatant to a new tube. Precipitation of DNA from the supernatant was done by adding 1 ml of cold isopropanol, followed by centrifugation at 12,000 rpm for 5 minutes. The resulting DNA pellets were washed with cold 70% ethanol and then resuspended in 10% glycerol in TE buffer (10 mM Tris, 1 mM EDTA, pH 8).
To prepare the samples for analysis, a 10% loading mixture (1X) was added to the extracted DNA samples, and the samples were stored at –20°C until further use. The samples were then incubated at 37°C for 30–60 minutes with 0.1% RNase and loading buffer. Following the incubation, the samples were loaded onto an agarose gel for further analysis.
A fluorescent labeling method called AO/EB was employed to evaluate the viability of leukocytes. In summary, cell suspensions (5 μl) from both the control and the exposed groups were stained with a combination of AO/EB (10 μg/ml) on a clean glass slide. The stained cells were promptly examined using a fluorescent microscope (Olympus BX 41) at 400× magnification. Identification of two distinct cell types was done based on their fluorescence emission: viable cells exhibited a consistent bright green color with an intact structure, whereas dead cells appeared yellowish to red, showing chromatin condensation or fragmentation. Approximately 1,000 cells were examined and assessed [35].
Blood samples were used following the three-layer procedure described by Singh et al. [36] with slight modifications. The cells were immobilized within a gel matrix composed of 0.7% low melting agarose sandwiched between two layers of 0.5% ultrapure agarose on a clean microscopic slide. Lysis of the cells was achieved by treating them with detergents and a high salt solution at a highly alkaline pH of 13. Subsequently, the denatured cells were subjected to electrophoresis in a running buffer, during which the damaged DNA migrated toward the anode. All chemical reagents used in the experiment were sourced from Sigma-Aldrich in Germany. For analysis, a total of 100 cells were randomly selected and observed using a fluorescence microscope. The cells were classified into two categories, based on the length of the DNA tail: damaged and strongly damaged nuclei. The extent of DNA damage was evaluated by visually estimating the migrated DNA percentage and the length of migration.
The data were analyzed using the Statistical Package for Social Sciences version 25 (IBM Co.). To compare the study groups, analysis of variance (ANOVA) was used, followed by Tukey’s post-hoc test. The results are displayed as the mean±SD. A significance level of P<0.05 was considered.
Regarding all the examined parameters, there was no statistically significant difference between the control group and the sage oil group.
There was no significant difference in body weight between the studied groups. But heart weight and heart weight/final body weight ratio were significantly higher in the TRZ group. Moreover, sage oil treatment either with or after TRZ treatment decreased these 2 parameters (Table 1).
TRZ significantly raised cardiac biomarkers levels in rat serum compared to control (AST, 300.5±13.19 vs. 111.0±5.727, P<0.001; CK-MB, 381.5±15.15 vs. 178.3±12.68 P<0.001; cTnI, 1.942±0.144 vs. 0.1417±0.0387, P<0.001; Fig. 1). Pretreatment with sage oil significantly prevents the induction of those cardiac biomarkers compared to the TRZ group (AST, 132±7.537 vs. 300.5±13.19, P<0.001; CK-MB, 225.8±12.12 vs. 381.5±15.15, P<0.001; cTnI, 0.3983±0.1457 vs. 1.942±0.144, P<0.001; Fig. 1). Similarly, administration of sage oil after treatment with TRZ significantly attenuated the production of those cytokines in the curative group compared to the TRZ group (AST, 160±15.9 vs. 300.5±13.19, P<0.001; CK-MB, 264.2±14.22 vs. 381.5±15.15, P<0.001; cTnI, 0.9567±0.1681 vs. 1.942±0.144, P<0.001; Fig. 1). However, there was a significant decrease in levels of those cardiac biomarkers in the protective group in comparison to the curative group (P<0.001, Fig. 1).
MDA and TNF-α levels significantly increased (68.33±4.502 and 142.1±13.35 respectively, P<0.001) with a decrease in TAC level (0.3567±0.03559, P<0.001) in comparison to the control group. Nevertheless, pretreatment with sage oil in the protective group exerted antioxidant and anti-inflammatory effects and showed significantly low levels of MDA and TNF-α (41.17±1.472 and 89.30±7.134 respectively, P<0.001) with a high level of TAC (0.07333±0.0242, P<0.001) in comparison to the TRZ group. Moreover, the curative group showed a significant reduction of MDA and TNF-α levels (48.17±2.639 and 104.5±6.822 respectively, P<0.001) with an increase in TAC level (0.1867±0.0509, P<0.001) compared to the TRZ group. But sage oil was effective as a protective more than a curative agent (P<0.001, Fig. 2).
Stained sections with H&E: group I (control) and group II (sage oil) cardiac sections revealed normal architecture with striated branching and anastomosing cylindrical cardiac muscle fibers cardiomyocytes showed acidophilic cytoplasm and central oval vesicular nuclei. Intercalated discs can also be detected (Fig. 3A, B). Cardiac sections of group III (TRZ) revealed interrupted muscle fibers with wide interstitial spaces containing mononuclear cellular infiltration (Fig. 3C). The sarcoplasm shows vacuolization and cardiomyocytes with pyknotic nuclei and others surrounded by a perinuclear halo. Both protective (Fig. 3E) and curative (Fig. 3F) groups show marked improvement in muscle fiber striation, which almost looks like control. However, wide interstitial spaces, myocytes with dark pyknotic nuclei, and interstitial mononuclear cellular infiltration were still evident in the treated group (Fig. 3F).
Masson’s trichrome stained cardiac tissue sections of the control (Fig. 4A) and sage oil (Fig. 4B) groups showed cardiomyocyte fibers normally separated by collagen fibers. Cardiomyocytes and blood capillaries deposited significantly more collagen fibers (63.33±6.887 vs. 3.383±0.9908) in the TRZ group (Fig. 4C) compared to the control group. Comparatively, sections of protective (Fig. 4D) and curative (Fig. 4E) groups revealed fewer collagen deposits between cardiomyocytes and around blood capillaries than in the TRZ group (11.27±2.38 and 25.02±3.386 respectively vs. 63.33±6.887).
Immunohistochemically, a significant upregulation (P<0.001) in the expression of Beclin1 in the TRZ group in comparison to the control group (45.12±5.077 vs. 3.733±1.125). Sage oil treatment either as a protective or a curative agent significantly attenuated (P<0.001) the expression of Beclin1 (9.380±1.585 and 18.07±4.504 respectively vs. 45.12±5.077) compared to the TRZ group (Fig. 5A–E).
Regarding P62 expression, a significant higher expression (P<0.001) was revealed in the TRZ group (37.90±6.605 vs. 2.733±0.9933) in comparison to the control group. Furthermore, a significant reduction (P<0.001) in its expression was detected in both the protective and the curative groups (16.48±2.169 and 20.30±2.06 vs. 37.90±6.605) compared to the TRZ group (Fig. 5F–J).
TRZ administration dramatically upregulated (P<0.001) the expression of LC3 (60.30±4.179 vs. 2.983±0.6047) compared to the control group. As a protective or curative agent, sage oil significantly (P<0.001) attenuated LC3 expression (19.30±4.169 and 23.75±3.454 vs. 60.30±4.179) compared to the TRZ group (Fig. 5K–O).
Ultrathin sections of cardiomyocytes from rats in the control and sage oil groups showed parallel myofibrils and numerous mitochondria interspersed between them. Sarcomeres made up the myofibrils, which consisted of dark and light bands that alternated between Z lines. Across the light band, there is a Z line. Euchromatic nuclei were observed. Intercalated discs connected adjacent cardiomyocytes (Fig. 6A, B). Disorganized fragmented myofibrils were seen in cardiomyocytes in sections of the TRZ group. There was a malformation and disarray of mitochondria, and the intercalated discs were disfigured and interrupted. Phagocytic vacuoles and bizarre nuclei with indentation appeared (Fig. 6C, D). The protective group showed well-defined Z-Bands and myofibrils but swollen mitochondria and irregular nuclei were still detected (Fig. 6E). The protective group cardiomyocyte with the euchromatic nucleus and well-defined Z lines are noticed. However, less regular arrays of myofibrils and irregular mitochondria were detected (Fig. 6F).
In order to evaluate the protective and curative effects of sage oil on the TRZ-exposed group, a double-strand genomic DNA fragmentation assay was conducted. The results, as shown in Fig. 7, indicated a significant (P<0.001) increase in the intensity of fragmented DNA in the TRZ-exposed group compared to the control group. However, the protective group and curative group displayed a significant (P<0.001) decrease in DNA fragmentation intensity by approximately 81.35% and 67.62%, respectively, in comparison with the TRZ-exposed group. Additionally a significant (P<0.01) difference was observed between the protective and curative groups, with the curative group exhibiting a 76.98% increase in intensity compared to the protective group. These findings recommend that sage oil has a protective effect against TRZ-induced DNA fragmentation, and when used as a curative treatment, it can significantly reduce the extent of DNA fragmentation.
To assess cell viability in both the control and exposed groups, a fluorescent staining technique using AO and EB double staining was used. Under this staining, viable cells exhibited a uniform fluorescent green color, while dead cells were marked by a yellow-green color, as shown in Fig. 8.
The results revealed a significant increase (P<0.01) in the percentage of dead cells in the TRZ-exposed group, with a rise of approximately 144.11% compared to the control group. Moreover, the other treatment groups, including protective group, and curative group, displayed significant decreases in the percentage of dead cells in comparison to the TRZ-exposed group. The recorded percentages decrease for these groups were 36.59% and 32.69%, respectively, in comparison to the TRZ-exposed group.
Based on these results, TRZ treatment led to a significant increase in cell death, while sage oil and other treatment groups indicated no significant differences in cell viability in comparison to the control group.
DNA damage in blood cells of both control and exposed groups was evaluated using comet assay, Fig. 9. The results demonstrated the protective effect of sage oil against the damaging effects of TRZ and its curative effect. In comparison to the control group, the TRZ-exposed group exhibited a significant and substantial increase in the frequency of DNA damage in the blood, reaching thousands of times higher levels.
Furthermore, the protective group demonstrated a significant (P<0.001) reduction in DNA damage by approximately 69.78%, while the curative group exhibited a decrease of around 90.79%, compared with TRZ-exposed group. It is indicating the effectiveness of sage oil in reducing DNA damage caused by TRZ exposure.
Acute or chronic pharmacological treatment can lead to cardiotoxicity by affecting the mechanical function and structure of the myocardium, such as morphological damage and cellular/subcellular loss [37]. In the present study, TRZ cardiovascular adverse effects were compared to S. officinalis combination with TRZ.
Trazadone is a triazolopyridine antidepressant of second generation. It has sedative and hypnotic effects [38]. Treatment with TRZ has been associated with electrophysiological abnormalities in patients with and without premorbid cardiac co-morbidities, according to a number of case reports and case series. A wide variety of abnormalities were observed, including prolonged PR intervals, heart block, QT prolongation, bradycardia, supraventricular tachycardia, and torsades de pointes [39]. Hypertrophy and other structural abnormalities such as cardiomyopathy have also been linked with TRZ prolonged PR intervals [1].
In the current study, an increase in serum biomarkers (CK-MB, AST, and cTnI) after TRZ administration was found which indicated cardiotoxicity. These biomarkers are released by myocytes after structural damage occurs [40]. Although AST levels are not completely specific to the myocardium, high levels in blood reflect myocardial damage. Due to the short half-life of CK-MB, that indicates myocardial injury, it is crucial to determining acute myocardial damage [41, 42]. In clinical and animal model studies, troponins are considered the most accurate biomarkers of myocardial ischemic damage due to their cardiac specificity [43, 44]. As a result of the increased levels of CK-MB, AST, and troponin-T following TRZ administration, we concluded that TRZ causes myocardial injury in rats. Other studies have also reported ischemic findings with TRZ treatment [39, 45, 46].
The current study demonstrated significant reductions in serum levels of CK-MB, AST, and cTnI in the protective group in comparison to the curative group after receiving 100 mg/kg sage oil for 3 weeks. Previous studies have shown that S. officinalis oil ameliorated the elevated levels of cTnI, CK-MB, NO, and MDA induced by 5-fluorouracil [21]. Furthermore, Yousry et al. [22] noted an improvement in chronic heart failure treated with sage oil as a curative rather than prophylactic measure. ROS are generated in the heart by normal mitochondrial functions as well as by enzymatic reactions [47].
In the present study, following TRZ treatment, an increased MDA level was reported, in addition to a decrease in TAC level, in comparison to the control, sage oil, protective and curative groups. Accordingly, the observed cardiac toxicity may have been accompanied by an oxidative stress in cardiac tissue that was lessened by sage oil administration. Moreover, TRZ and its reactive metabolite were found to cause oxidative stress as they increased MDA levels and depleted glutathione levels in vitro [48]. However, sage oil reduced ROS and increased endogenous antioxidants, so it restored oxidative stress.
Moreover, TNF-α has been reported to be a marker for cardiac remodelling and the progression of congestive heart failure, which may help to explain why TRZ induced cardiotoxicity linked with elevated TNF-α [49]. Additionally, in peripheral muscles and cardiac myocytes, TNF-α triggers the production of ROS [50]. Cardiomyopathy and ventricular remodeling have been linked to TNF-α in infarcted hearts [51, 52].
Sage oil’s potent antioxidant properties can be linked to its ingredients, such as rosmarinic acid, the major phenolic compound found in sage [53]. The two ortho-dihydroxy groups (catechol structure), present in it, are thought to be responsible for its antioxidant effect [54, 55]. Additionally, sage contains phenolic diterpenes, which are antioxidants, including carnosic acid, carnosol, caffeine, and luteolin-7-glucoside [56]. Also, the main monoterpene ingredient in sage essential oil, 1,8-cineole, is also anti-inflammatory, antioxidant, and anti-nociceptive [57]. Salvia officinalis essential oil’s antioxidant benefits, according to Amensour et al. [58], are principally brought on by its redox characteristics, which adsorb and neutralize free radicals, quench singlet oxygen, and break down peroxide.
Upon further examination of cardiac tissues, light microscopic examination confirmed the above-mentioned biochemical changes, which resulted in changes in the shape of cardiac cells, myocardial necrosis and degeneration. Along with irregular myofibrils, hypertrophy, and fibrotic heart muscle, vacuoles were seen in the sarcoplasm. In cardiac tissue, a number of degenerative alterations were discovered, including fibrosis, asymmetrical cardiac myocyte hypertrophy, irregular myofibrils, myofibrillar loss, edema, and inflammation. Additionally, dilated blood vessels and inflammatory cell infiltration were also present. Furthermore, rats treated with TRZ developed large abnormal mitochondria with few cristae and severe heterogeneous subcellular changes in cardiomyocytes. A clear matrix was also revealed. Moreover, some mitochondria appeared markedly swollen. Also, the marked increase in the number of collagen fibers stained with Masson revealed the existence of cardiac fibrosis. Cardiomyopathy, cardiac hypertrophy, interstitial cardiac fibrosis, endothelial dysfunction, and contractile protein malfunction may all be caused by increased oxidative stress in cardiac tissue [59]. It has also been suggested that fibrosis serves as a barrier between cardiomyocytes that impairs electrical coupling between them and malfunction of the cardiac systole [60].
During times of cellular stress, a cysteine protease cleaves ATG5, an essential protein for the production of autophagy precursors. ATG12 plays a crucial role in the formation of autophagosomes. ATG12 is subjected to ATP-dependent conjugation to ATG5, which is carried out through the mediation of ATG7 and ATG10. The ATG12-ATG5 complex then engages in an interaction with ATG16L1, resulting in the formation of an ATG12-ATG5-ATG16L1 conjugate which is required for the successful conjugation of Atg8/LC3 to phagophore membranes [61]. Nevertheless, when autophagy is induced, beclin-1, which typically binds to Bcl-2 and inhibits autophagy, is competitively displaced, boosting autophagy process [4]. Furthermore, P62 promotes the development of aggresomes, stimulates autophagy, and shields cardiomyocytes from proteotoxic stress [62].
ATG7, ATG3, and the ATG12-ATG5-ATG16L1 complex strongly conjugate MAP1LC3-I with phosphatidylethanolamine following the induction of autophagy to produce MAP1LC3-II, which is subsequently attracted to the inner and outer surfaces of the autophagosome. Autophagosomes are easily identified by immunohistochemistry due to the formation of MAP1LC3-positive puncta [63]. In the present study, TRZ-treated group showed high Beclin-1, LC3II, and P62 immuno-expression. Sage administration dramatically lessened TRZ’s effects and the expression of Beclin-1, LC3, and P62 levels as compared to the TRZ-treated group. These effects could be attributed to TRZ’s elevated oxidative stress, which sage’s antioxidant and scavenging activities reduced.
Currently, research strongly suggests that autophagy serves a protective role, defending cells against apoptosis during DNA damage. Recent studies highlight autophagy not only as a fundamental response to genotoxic agents but also as a crucial contributor to the cellular response under genotoxic stress [64]. Comet assay is commonly utilized to assess the mutagenic and genotoxic effects caused by xenobiotics [16, 65]. While there is limited research investigating the genotoxic effects of TRZ, studies have demonstrated that TRZ can induce cardiotoxicity in rats following repeated doses and in vitro, in human lymphocytes [1, 16]. Our study yielded significant findings, indicating a notable increase in DNA damage in rats treated with TRZ while significant decrease in DNA damage was noticed in both protective and curative groups when compared to TRZ group. Furthermore, other investigations have suggested that TRZ could induce oxidative stress, with ROS potentially playing a crucial role in TRZ-induced genotoxicity in vitro [1, 16, 23]. The role of autophagy in enhancing cell survival following DNA damage has opened avenues for the development of new protective agents or intervention strategies against the genotoxic impact of xenobiotics on DNA [64].
Moreover, histopathological analysis of myocardial sections from both the protective and curative groups revealed some signs of reduced autophagy, a partial reduction in myocardial fibrosis, and a partial improvement in myocardial affection, with more improvement in protective group compared to the curative one. This was also demonstrated morphometrically by the notable reductions in collagen fibre area percentage, area percentage of immunoexpression of LC3, Beclin1 and P62 of both protective and curative groups when compared to TRZ group. Our study’s results agree with those of earlier research that showed that S. officinalis extract exhibited nearly normal structure in hearts subjected to chlorpyrifos or methomyl [20]. According to Yousry et al. [22], S. officinalis essential oil improved myocardial affection, decreased apoptosis, and partially attenuated myocardial fibrosis in rats treated with isoprenaline.
In the current study, elevated cardiac enzyme levels and histological abnormalities, such as persistent inflammatory cells with localized fragmentation of myocardial fibers and nuclei loss, showed that TRZ therapy caused cardiotoxicity. Oxidative stress, inflammation, and autophagy were responsible for TRZ’s cardiotoxicity. Sage, on the other hand, has antioxidant and scavenging properties that ameliorate these effects.
Notes
Author Contributions
Conceptualization: MASAG. Data acquisition and Experiment Design: MASAG, ASE. Performing the experiment: MASAG, HMRH. Data analysis: MASAG, HMRH, ASE. Writing of the manuscript: MASAG, ASE, HMRH, MMS. Revision of the manuscript: MASAG, ASE. Approval of the final version of the manuscript: all authors.
References
1. Atli O, Kilic V, Baysal M, Kilic G, Gormus G, Ucarcan S, Korkut B, Ilgin S. 2019; Assessment of trazodone-induced cardiotoxicity after repeated doses in rats. Hum Exp Toxicol. 38:45–55. DOI: 10.1177/0960327118769717. PMID: 29774748.

2. Miura N, Saito T, Taira T, Umebachi R, Inokuchi S. 2015; Risk factors for QT prolongation associated with acute psychotropic drug overdose. Am J Emerg Med. 33:142–9. DOI: 10.1016/j.ajem.2014.09.048. PMID: 25445869.

3. Jaffer KY, Chang T, Vanle B, Dang J, Steiner AJ, Loera N, Abdelmesseh M, Danovitch I, Ishak WW. 2017; Trazodone for insomnia: a systematic review. Innov Clin Neurosci. 14:24–34. PMID: 29552421. PMCID: PMC5842888.
4. Xiao B, Hong L, Cai X, Mei S, Zhang P, Shao L. 2019; The true colors of autophagy in doxorubicin-induced cardiotoxicity. Oncol Lett. 18:2165–72. DOI: 10.3892/ol.2019.10576. PMID: 31452719. PMCID: PMC6676529.
5. Montalvo RN, Doerr V, Min K, Szeto HH, Smuder AJ. 2020; Doxorubicin-induced oxidative stress differentially regulates proteolytic signaling in cardiac and skeletal muscle. Am J Physiol Regul Integr Comp Physiol. 318:R227–33. DOI: 10.1152/ajpregu.00299.2019. PMID: 31774307.

6. Chun Y, Kim J. 2018; Autophagy: an essential degradation program for cellular homeostasis and life. Cells. 7:278. DOI: 10.3390/cells7120278. PMID: 30572663. PMCID: PMC6315530.

7. Cuervo AM, Macian F. 2012; Autophagy, nutrition and immunology. Mol Aspects Med. 33:2–13. DOI: 10.1016/j.mam.2011.09.001. PMID: 21982744. PMCID: PMC3996457.

8. Noda NN, Inagaki F. 2015; Mechanisms of autophagy. Annu Rev Biophys. 44:101–22. DOI: 10.1146/annurev-biophys-060414-034248. PMID: 25747593.

9. Lee Y, Kwon I, Jang Y, Song W, Cosio-Lima LM, Roltsch MH. 2017; Potential signaling pathways of acute endurance exercise-induced cardiac autophagy and mitophagy and its possible role in cardioprotection. J Physiol Sci. 67:639–54. Erratum in: J Physiol Sci 2017;68:205. DOI: 10.1007/s12576-017-0555-7. PMID: 28685325. PMCID: PMC5684252.

10. Wang H, Wang H, Liang EY, Zhou LX, Dong ZL, Liang P, Weng QF, Yang M. 2018; Thrombopoietin protects H9C2 cells from excessive autophagy and apoptosis in doxorubicin-induced cardiotoxicity. Oncol Lett. 15:839–48. DOI: 10.3892/ol.2017.7410. PMID: 29403560. PMCID: PMC5780751.

11. Christidi E, Brunham LR. 2021; Regulated cell death pathways in doxorubicin-induced cardiotoxicity. Cell Death Dis. 12:339. DOI: 10.1038/s41419-021-03614-x. PMID: 33795647. PMCID: PMC8017015.

12. Kirkin V, Rogov VV. 2019; A diversity of selective autophagy receptors determines the specificity of the autophagy pathway. Mol Cell. 76:268–85. DOI: 10.1016/j.molcel.2019.09.005. PMID: 31585693.

13. Ma S, Attarwala IY, Xie XQ. 2019; SQSTM1/p62: a potential target for neurodegenerative disease. ACS Chem Neurosci. 10:2094–114. DOI: 10.1021/acschemneuro.8b00516. PMID: 30657305. PMCID: PMC6712989.

14. Islam MA, Sooro MA, Zhang P. 2018; Autophagic regulation of p62 is critical for cancer therapy. Int J Mol Sci. 19:1405. DOI: 10.3390/ijms19051405. PMID: 29738493. PMCID: PMC5983640.

15. Ahmed MK, Kundu GK, Al-Mamun MH, Sarkar SK, Akter MS, Khan MS. 2013; Chromium (VI) induced acute toxicity and genotoxicity in freshwater stinging catfish, Heteropneustes fossilis. Ecotoxicol Environ Saf. 92:64–70. DOI: 10.1016/j.ecoenv.2013.02.008. PMID: 23474066.

16. Avuloglu Yilmaz E, Unal F, Yuzbasioglu D. 2017; Evaluation of cytogenetic and DNA damage induced by the antidepressant drug-active ingredients, trazodone and milnacipran, in vitro. Drug Chem Toxicol. 40:57–66. DOI: 10.1080/01480545.2016.1174870. PMID: 27147406.

17. Ilgın S, Aydoğan-Kılıç G, Baysal M, Kılıç V, Ardıç M, Uçarcan Ş, Atlı Ö. 2018; Toxic effects of trazodone on male reproductive system via disrupting hypothalamic-pituitary-testicular axis and inducing testicular oxidative stress. Oxid Med Cell Longev. 2018; 2018:7196142. Erratum in: Oxid Med Cell Longev 2018; 2018:8294061. DOI: 10.1155/2018/7196142. PMID: 30151072. PMCID: PMC6087606.

18. Lu Y, Foo LY. 2002; Polyphenolics of Salvia--a review. Phytochemistry. 59:117–40. DOI: 10.1016/S0031-9422(01)00415-0. PMID: 11809447.

19. Mossi AJ, Cansian RL, Paroul N, Toniazzo G, Oliveira JV, Pierozan MK, Pauletti G, Rota L, Santos AC, Serafini LA. 2011; Morphological characterisation and agronomical parameters of different species of Salvia sp. (Lamiaceae). Braz J Biol. 71:121–9. DOI: 10.1590/S1519-69842011000100018. PMID: 21437408.

20. Ahmed OM, Ashour MB, Abd El Mawgoud AA, Ali MA. 2017; Assessment of the preventive effects of Salvia officinalis and Ruta graveolens ethanolic extracts on chlorpyrifos- and methomyl-induced testicular and cardiac toxicities in albino rats. Am J Med Med Sci. 7:287–301.
21. Safwat GM, Mohammed ET. 2015; Salvia officinalis oil improves the atherogenic index and cardiotoxicity in albino rats treated with 5- fluorouracil. Int J Pharm Bio Sci. 6:59–66.
22. Yousry SM, Taha RAM, El-Banna HA, Emam SR. 2021; Curative and protective effect of Salvia officinalis oil on isoprenalineinduced congestive heart failure in rats. Adv Anim Vet Sci. 9:1895–907. DOI: 10.17582/journal.aavs/2021/9.11.1895.1907.
23. Khedr NF, Werida RH. 2022; l-carnitine modulates autophagy, oxidative stress and inflammation in trazodone induced testicular toxicity. Life Sci. 290:120025. DOI: 10.1016/j.lfs.2021.120025. PMID: 34637798.

24. Rawat PS, Jaiswal A, Khurana A, Bhatti JS, Navik U. 2021; Doxorubicin-induced cardiotoxicity: an update on the molecular mechanism and novel therapeutic strategies for effective management. Biomed Pharmacother. 139:111708. DOI: 10.1016/j.biopha.2021.111708. PMID: 34243633.

25. Gerhardt W, Waldenström J. 1979; Creatine kinase B-subunit activity in serum after immunoinhibition of M-subunit activity. Clin Chem. 25:1274–80. DOI: 10.1093/clinchem/25.7.1274. PMID: 455648.

26. Khedr NF. 2015; Protective effect of mirtazapine and hesperidin on cyclophosphamide-induced oxidative damage and infertility in rat ovaries. Exp Biol Med (Maywood). 240:1682–9. DOI: 10.1177/1535370215576304. PMID: 25787947. PMCID: PMC4935352.

27. Koracevic D, Koracevic G, Djordjevic V, Andrejevic S, Cosic V. 2001; Method for the measurement of antioxidant activity in human fluids. J Clin Pathol. 54:356–61. DOI: 10.1136/jcp.54.5.356. PMID: 11328833. PMCID: PMC1731414.

28. Markham R, Young L, Fraser IS. 1995; An amplified ELISA for human tumour necrosis factor alpha. Eur Cytokine Netw. 6:49–54. PMID: 7795175.
29. Suvarna SK, Layton C, Bancroft JD. Bancroft's theory and practice of histological techniques. 7th ed. Churchill Livingstone;2012. p. 187–214.
30. Chi RF, Wang JP, Wang K, Zhang XL, Zhang YA, Kang YM, Han XB, Li B, Qin FZ, Fan BA. 2017; Progressive reduction in myocyte autophagy after myocardial infarction in rabbits: association with oxidative stress and left ventricular remodeling. Cell Physiol Biochem. 44:2439–54. DOI: 10.1159/000486167. PMID: 29268264.

31. Bozzola JJ, Russell LD. Electron microscopy principles and techniques for biologists. 2nd ed. Jones and Bartlett;1999. p. 100–124. DOI: 10.1159/000486167.
32. El-Garawani IM. 2015; Ameliorative effect of Cymbopogon citratus extract on cisplatin-inducedgenotoxicity in human leukocytes. J Biosci Appl Res. 1:304–10. DOI: 10.21608/jbaar.2015.106040.
33. Aljanabi SM, Martinez I. 1997; Universal and rapid salt-extraction of high quality genomic DNA for PCR-based techniques. Nucleic Acids Res. 25:4692–3. DOI: 10.1093/nar/25.22.4692. PMID: 9358185. PMCID: PMC147078.
34. Hassab El-Nabi SE, Elhassaneen YA. 2008; Detection of DNA damage, molecular apoptosis and production of home-made ladder by using simple techniques. Biotechnology. 7:514–22. DOI: 10.3923/biotech.2008.514.522.

35. Liu K, Liu PC, Liu R, Wu X. 2015; Dual AO/EB staining to detect apoptosis in osteosarcoma cells compared with flow cytometry. Med Sci Monit Basic Res. 21:15–20. DOI: 10.12659/MSMBR.893327. PMID: 25664686. PMCID: PMC4332266.

36. Singh NP, McCoy MT, Tice RR, Schneider EL. 1988; A simple technique for quantitation of low levels of DNA damage in individual cells. Exp Cell Res. 175:184–91. DOI: 10.1016/0014-4827(88)90265-0. PMID: 3345800.

37. Aziz MM, Abd El Fattah MA, Ahmed KA, Sayed HM. 2020; Protective effects of olmesartan and l-carnitine on doxorubicin-induced cardiotoxicity in rats. Can J Physiol Pharmacol. 98:183–93. DOI: 10.1139/cjpp-2019-0299. PMID: 31665614.
38. Bossini L, Casolaro I, Koukouna D, Cecchini F, Fagiolini A. 2012; Off-label uses of trazodone: a review. Expert Opin Pharmacother. 13:1707–17. DOI: 10.1517/14656566.2012.699523. PMID: 22712761.

39. Li TC, Chiu HW, Ho KJ, Tzeng DS. 2011; Bradycardia following a single low dose of trazodone. Asian J Psychiatr. 4:77–9. DOI: 10.1016/j.ajp.2010.03.003. PMID: 23050923.

40. Chen CT, Wang ZH, Hsu CC, Lin HH, Chen JH. 2015; In vivo protective effects of diosgenin against doxorubicin-induced cardiotoxicity. Nutrients. 7:4938–54. DOI: 10.3390/nu7064938. PMID: 26091236. PMCID: PMC4488824.

41. Walker DB. 2006; Serum chemical biomarkers of cardiac injury for nonclinical safety testing. Toxicol Pathol. 34:94–104. DOI: 10.1080/01926230500519816. PMID: 16507550.

42. Tonomura Y, Mori Y, Torii M, Uehara T. 2009; Evaluation of the usefulness of biomarkers for cardiac and skeletal myotoxicity in rats. Toxicology. 266:48–54. DOI: 10.1016/j.tox.2009.10.014. PMID: 19854236.

43. O'Brien PJ. 2008; Cardiac troponin is the most effective translational safety biomarker for myocardial injury in cardiotoxicity. Toxicology. 245:206–18. DOI: 10.1016/j.tox.2007.12.006. PMID: 18249481.
44. Crivellente F. 2011. Aug. 23. Utilising biomarkers to eliminate drug candidates with cardiotoxicity in preclinical development [Internet]. Available from: https://www.ddw-online.com/utilising-biomarkers-to-eliminate-drug-candidates-with-cardiotoxicity-in-preclinical-development-1020-201108/. Drug Discovery World;cited 2023 Feb.
45. Selvaraj V, Ramaswamy S, Bhatia SC. 2011; Transient ischemic attack associated with trazodone therapy: a case report. Prim Care Companion CNS Disord. 13:PCC.10l01093. DOI: 10.4088/PCC.10l01093. PMID: 22132348. PMCID: PMC3219511.
46. Shell WE, May LA, Bullias DH, Pavlik SL, Silver DS. 2012; Sentra PM (a medical food) and trazodone in the management of sleep disorders. J Cent Nerv Syst Dis. 4:65–72. DOI: 10.4137/JCNSD.S9381. PMID: 23650468. PMCID: PMC3619436.

47. Keleş S, Caner İ, Ateş O, Çakici Ö, Saruhan F, Mumcu UY, Ünal D, Tekgündüz KŞ, Taştekin A, Hacimüftüoğlu A, Gürsans N, Alp HH. 2014; Protective effect of L-carnitine in a rat model of retinopathy of prematurity. Turk J Med Sci. 44:471–5. DOI: 10.3906/sag-1301-9. PMID: 25558651.

48. Peoples JN, Saraf A, Ghazal N, Pham TT, Kwong JQ. 2019; Mitochondrial dysfunction and oxidative stress in heart disease. Exp Mol Med. 51:1–13. DOI: 10.1038/s12276-019-0355-7. PMID: 31857574. PMCID: PMC6923355.

49. Mann DL. 2002; Tumor necrosis factor-induced signal transduction and left ventricular remodeling. J Card Fail. 8(6 Suppl):S379–86. DOI: 10.1054/jcaf.2002.129253. PMID: 12555149.

50. Nakamura K, Fushimi K, Kouchi H, Mihara K, Miyazaki M, Ohe T, Namba M. 1998; Inhibitory effects of antioxidants on neonatal rat cardiac myocyte hypertrophy induced by tumor necrosis factor-alpha and angiotensin II. Circulation. 98:794–9. DOI: 10.1161/01.CIR.98.8.794. PMID: 9727550.

51. Blanca AJ, Ruiz-Armenta MV, Zambrano S, Miguel-Carrasco JL, Arias JL, Arévalo M, Mate A, Aramburu O, Vázquez CM. 2016; Inflammatory and fibrotic processes are involved in the cardiotoxic effect of sunitinib: Protective role of L-carnitine. Toxicol Lett. 241:9–18. DOI: 10.1016/j.toxlet.2015.11.007. PMID: 26581635.

52. Sun M, Chen M, Dawood F, Zurawska U, Li JY, Parker T, Kassiri Z, Kirshenbaum LA, Arnold M, Khokha R, Liu PP. 2007; Tumor necrosis factor-alpha mediates cardiac remodeling and ventricular dysfunction after pressure overload state. Circulation. 115:1398–407. DOI: 10.1161/CIRCULATIONAHA.106.643585. PMID: 17353445.

53. Lima CF, Andrade PB, Seabra RM, Fernandes-Ferreira M, Pereira-Wilson C. 2005; The drinking of a Salvia officinalis infusion improves liver antioxidant status in mice and rats. J Ethnopharmacol. 97:383–9. DOI: 10.1016/j.jep.2004.11.029. PMID: 15707779.

54. Shan B, Cai YZ, Sun M, Corke H. 2005; Antioxidant capacity of 26 spice extracts and characterization of their phenolic constituents. J Agric Food Chem. 53:7749–59. DOI: 10.1021/jf051513y. PMID: 16190627.

55. Büyükbalci A, El SN. 2008; Determination of in vitro antidiabetic effects, antioxidant activities and phenol contents of some herbal teas. Plant Foods Hum Nutr. 63:27–33. DOI: 10.1007/s11130-007-0065-5. PMID: 18183488.

56. Walch SG, Tinzoh LN, Zimmermann BF, Stühlinger W, Lachenmeier DW. 2011; Antioxidant capacity and polyphenolic composition as quality indicators for aqueous infusions of Salvia officinalis L. (sage tea). Front Pharmacol. 2:79. DOI: 10.3389/fphar.2011.00079. PMID: 22194722. PMCID: PMC3242359.

57. El-Hosseiny LS, Alqurashy NN, Sheweita SA. 2016; Oxidative stress alleviation by sage essential oil in co-amoxiclav induced hepatotoxicity in rats. Int J Biomed Sci. 12:71–8. DOI: 10.59566/IJBS.2016.12071. PMID: 27493593. PMCID: PMC4947092.

58. Amensour M, Sendra E, Abrini J, Bouhdid S, Pérez-Alvarez JA, Fernández-López J. 2009; Total phenolic content and antioxidant activity of myrtle (Myrtus communis) extracts. Nat Prod Commun. 4:819–24. DOI: 10.1177/1934578X0900400616. PMID: 19634329.
59. Emran T, Chowdhury NI, Sarker M, Bepari AK, Hossain M, Rahman GMS, Reza HM. 2021; L-carnitine protects cardiac damage by reducing oxidative stress and inflammatory response via inhibition of tumor necrosis factor-alpha and interleukin-1beta against isoproterenol-induced myocardial infarction. Biomed Pharmacother. 143:112139. DOI: 10.1016/j.biopha.2021.112139. PMID: 34507121.

60. Takeda N, Manabe I. 2011; Cellular interplay between cardiomyocytes and nonmyocytes in cardiac remodeling. Int J Inflam. 2011:535241. DOI: 10.4061/2011/535241. PMID: 21941677. PMCID: PMC3175723.

61. Elnakish MT, Ahmed AA, Mohler PJ, Janssen PM. 2015; Role of oxidative stress in thyroid hormone-induced cardiomyocyte hypertrophy and associated cardiac dysfunction: an undisclosed story. Oxid Med Cell Longev. 2015:854265. DOI: 10.1155/2015/854265. PMID: 26146529. PMCID: PMC4471379.

62. Zheng Q, Su H, Ranek MJ, Wang X. 2011; Autophagy and p62 in cardiac proteinopathy. Circ Res. 109:296–308. DOI: 10.1161/CIRCRESAHA.111.244707. PMID: 21659648. PMCID: PMC3142307.

63. Martinet W, Roth L, De Meyer GRY. 2017; Standard immunohistochemical assays to assess autophagy in mammalian tissue. Cells. 6:17. DOI: 10.3390/cells6030017. PMID: 28665306. PMCID: PMC5617963.

64. Zhang SM, Shang ZF, Zhou PK. 2015; Autophagy as the effector and player in DNA damage response of cells to genotoxicants. Toxicol Res. 4:613–22. DOI: 10.1039/C5TX00043B.

65. Brambilla G, Mattioli F, Martelli A. 2009; Genotoxic and carcinogenic effects of antipsychotics and antidepressants. Toxicology. 261:77–88. DOI: 10.1016/j.tox.2009.04.056. PMID: 19410629.

Fig. 1
Status of (A) aspartate aminotransferase (AST), (B) creatine kinase-MB (CK-MB), and (C) cardiac troponin enzymatic activity in the hearts of the control, sage oil, trazodone (TRZ), protective, and curative groups. ∆∆∆P<0.001, compared to the control group; ᵒᵒᵒP<0.001, compared to the TRZ group; ■■■P<0.001, compared to the protective group. Data are expressed as means±SD.
Fig. 2
(A) Malonaldehyde (MDA), (B) total antioxidant capacity (TAC), and (C) tumor necrosis factor-α (TNF-α) levels in the hearts of the control, sage oil, trazodone (TRZ), protective, and curative groups. ∆∆∆P<0.001, compared to the control group; oooP<0.001, compared to the TRZ group; ■■■P<0.001, compared to the protective group. Data are presented as means±SD.
Fig. 3
Representative H&E staining of the rat hearts of different groups: the control (panel A) and sage oil (panel B) myocardium (A) displaying branched and anatomized muscle fibers with narrow interstitial spaces (S) that contains the nuclei of interstitial fibroblast (arrows). A central oval vesicular nucleus (N) and acidophilic sarcoplasm are seen in every cardiomyocyte. TRZ myocardium (panel C, D) shows interrupted muscle fibers with wide interstitial spaces (S) containing areas of hemorrhage and extravasated blood and interstitial mononuclear cellular infiltration (curved arrows). The sarcoplasm shows vacuolization (arrowheads) and cardiomyocytes with pyknotic nuclei (N) and others surrounded by perinuclear halo (white arrows). Both protective (panel E) and curative (panel F) groups show marked improvement in muscle fiber striation, which almost looks like control; an acidophilic sarcoplasm containing central oval vesicular nucleus (N) and interstitial fibroblast (arrow). However, wide interstitial spaces (S) contain extravasated blood. Also, myocytes with dark pyknotic nuclei (N) and interstitial mononuclear cellular infiltration (curved arrows) are still evident in the treated group (×400).
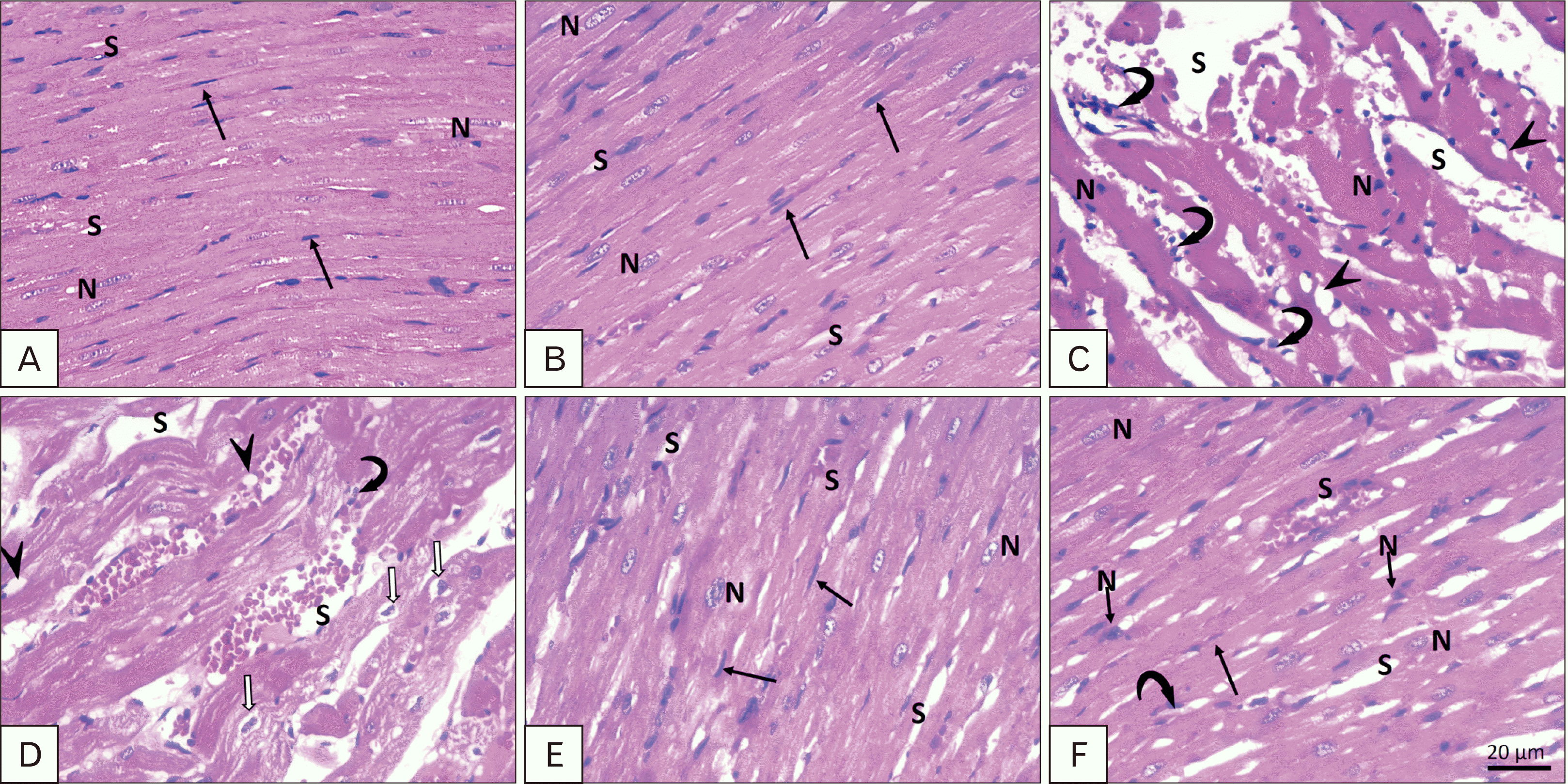
Fig. 4
Representative Mallory trichrome staining of the rat hearts of different groups: the control (A) and sage oil (B) showing scanty collagen fibers deposition. Trazodone group (C) shows higher collagen formation surrounding blood vessels and in between cardiomyocytes. Both protective (D) and curative (E) groups show a relative reduction in collagen deposition (×400).
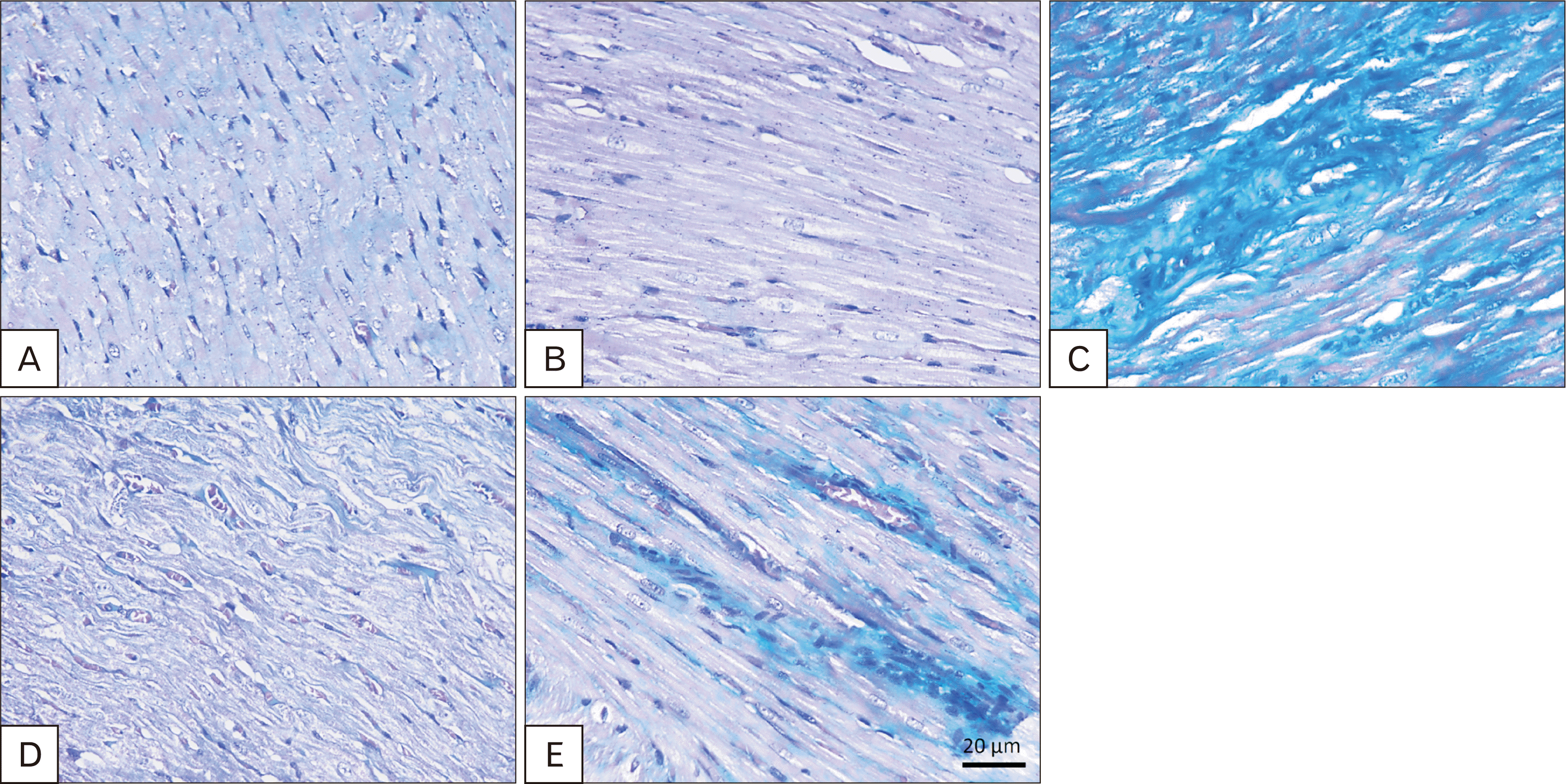
Fig. 5
Immunohistochemical expression of Beclin1, P62, and microtubule-associated protein 1A/1B-light chain 3 (LC3) in the hearts of the control, sage oil, trazodone (TRZ), protective, and curative groups (×400).
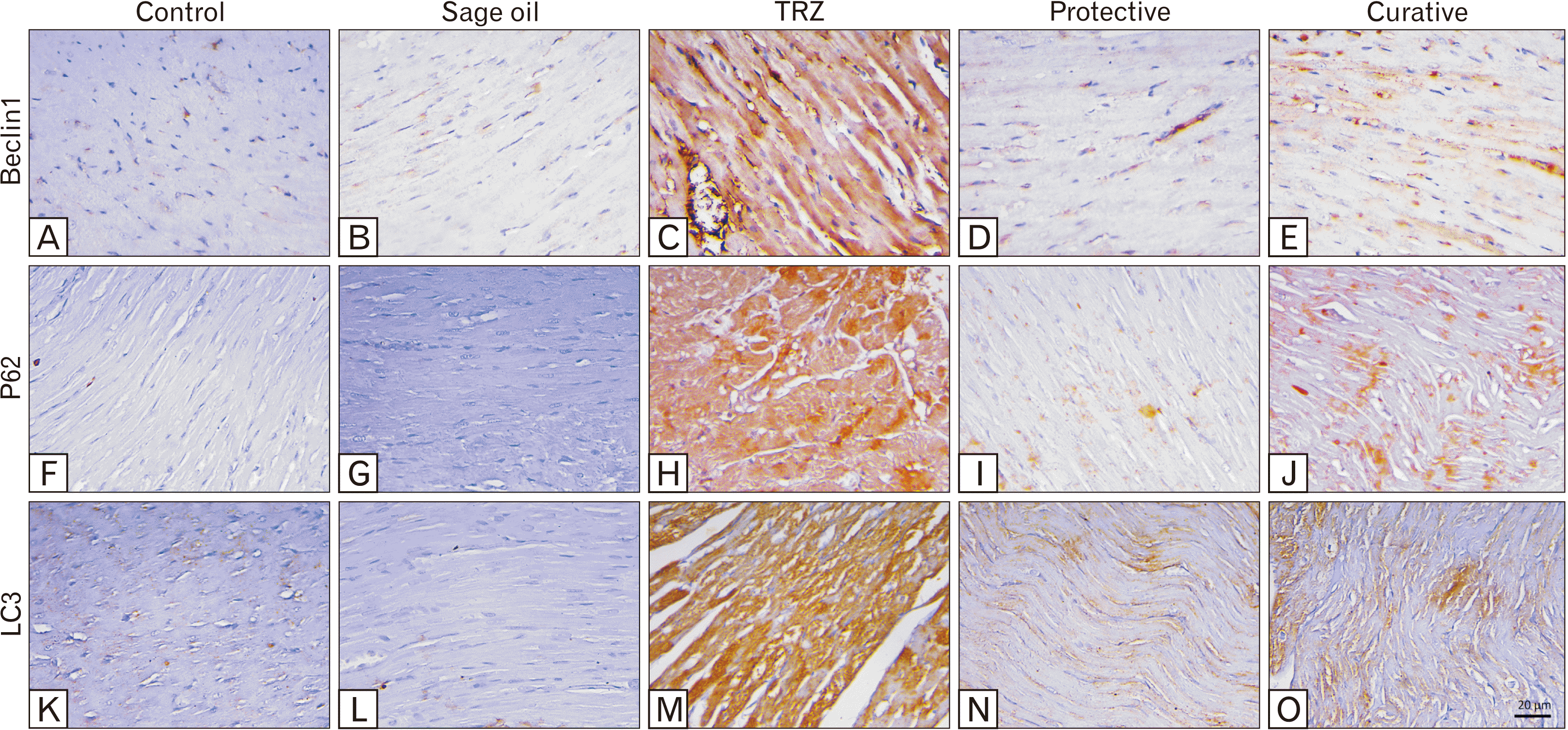
Fig. 6
Transmission electron microscope observation of the myocardium of the control (panel A) and sage oil (panel B) groups shows cardiomyocytes with an oval nucleus and extended chromatin (N) and long parallel arrays of myofibrils (F). In these myofibrils, dark and light bands alternate, as well as regular Z lines (red arrows) between the light bands. Few lysosomes (circle) and rows of mitochondria (M) are seen in the perinuclear region and between the myofibrils. Intercalated discs with a step-like pattern of desmosomes (yellow arrows) and gap junctions (J) could be seen between myocytes. (Panel C, D) in the trazodone group cardiomyocyte shows irregular arrays of myofibrils (F) and a highly irregular pattern of intercalated disc in between myocytes (yellow arrows). Between the myofibrils, irregular-shaped mitochondria (M) are detected, some of which have irregular cristae (curved arrows). Phagocytic vacuoles are indicated with (circles). An irregularly outlined nucleus (N) with condensed peripheral chromatin and prominent nucleolus (n) is revealed. (Panel E) protective group showing well-defined Z-Bands (red arrow) and myofibrils (F) but swollen mitochondria (M) and irregular nucleus (N) with prominent nucleolus (n) are still detected. (Panel F) curative group cardiomyocyte with euchromatic nucleus (N) and well-defined Z lines are noticed. However, less regular arrays of myofibrils (F) and irregular mitochondria M and phagocytic vacuole (circle) are still detected (uranyl acetate-lead citrate stain, ×5,000).
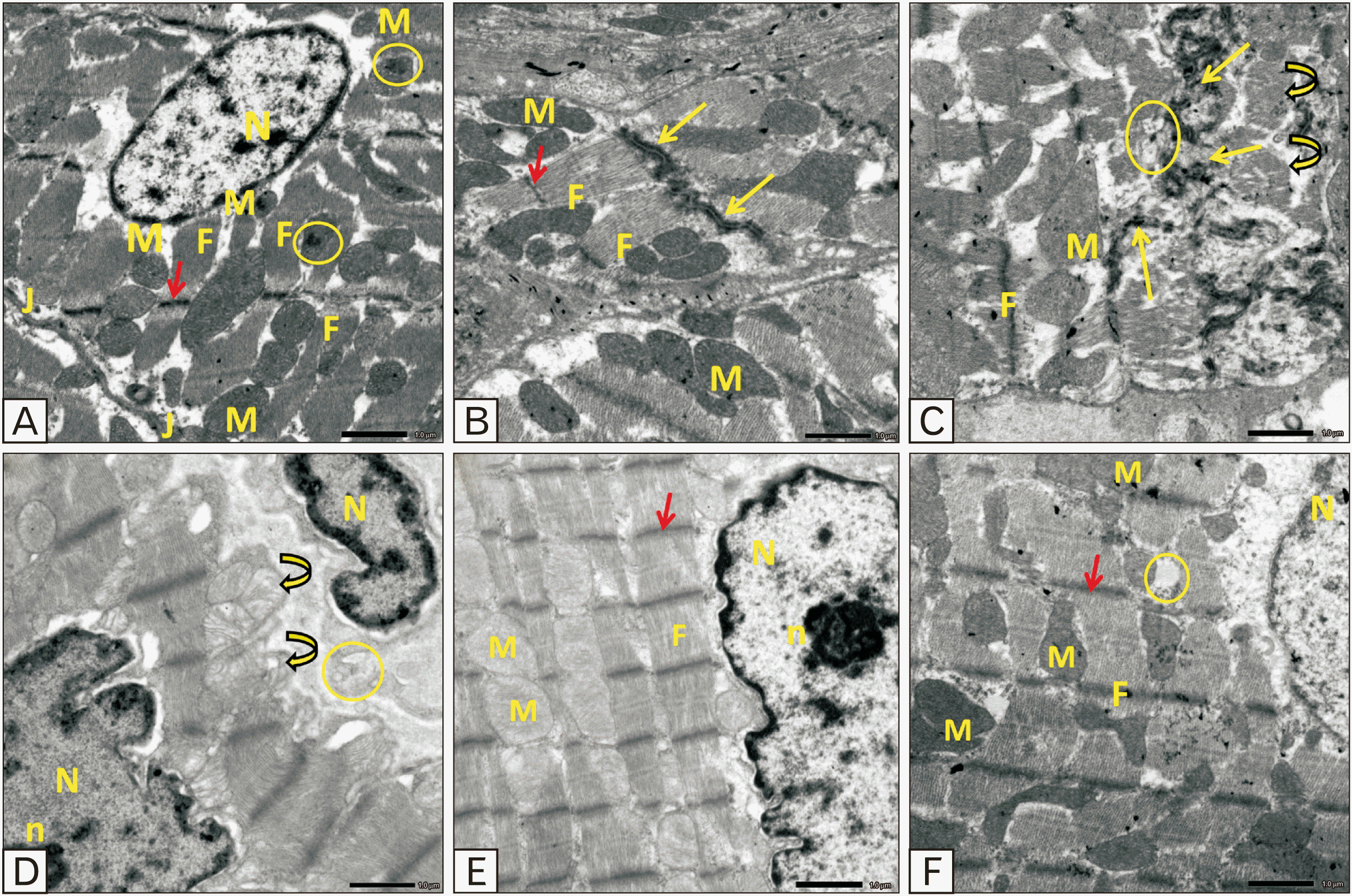
Fig. 7
Photograph of ethidium bromide-stained agarose gel was taken after electrophoresis of the control and exposed groups. Numbers (1, 2, 3, 4, and 5) represent control, sage oil, trazodone, protective, and curative groups, respectively. M, marker.
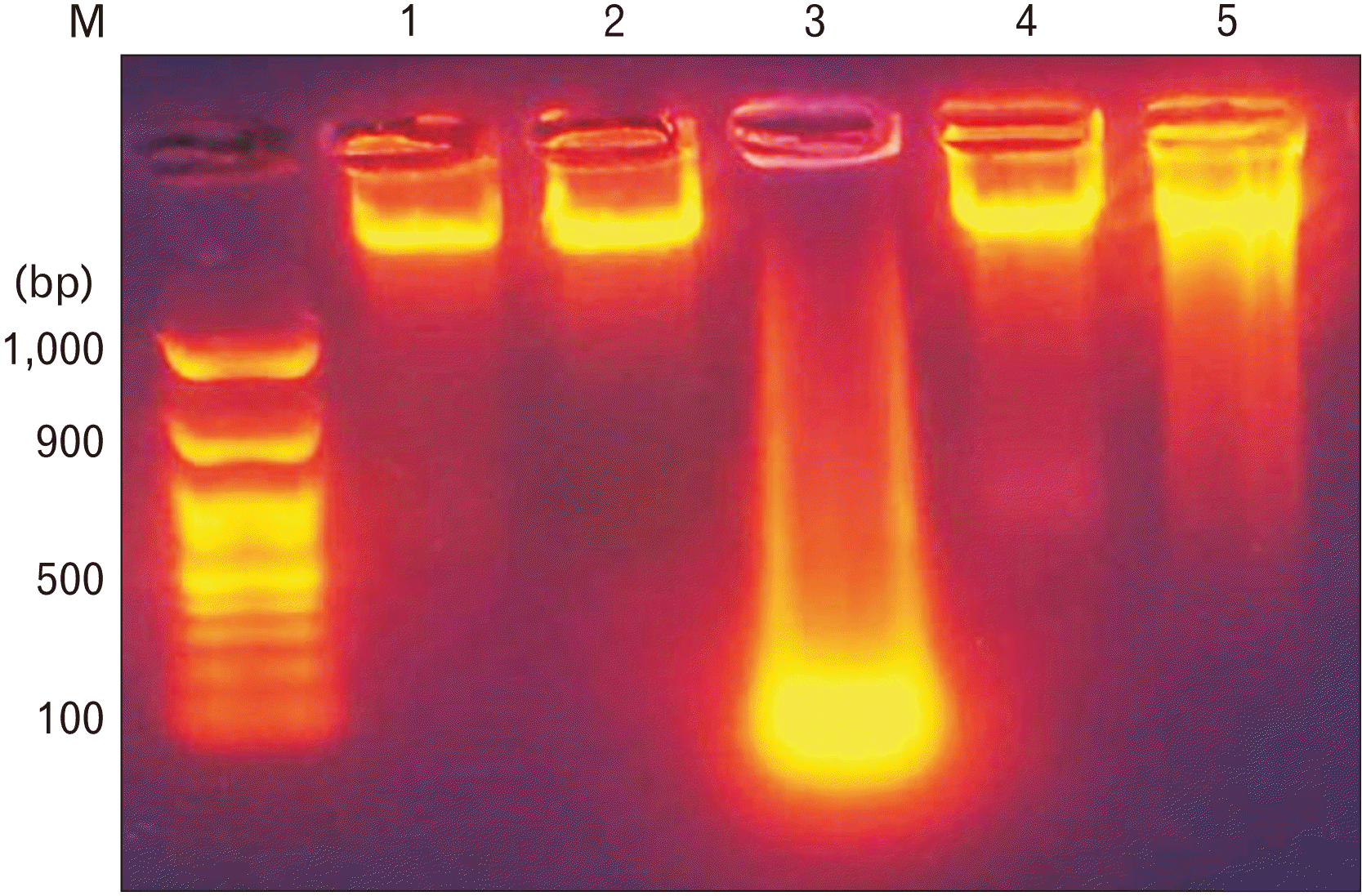
Fig. 8
Representative micro-photographs (A–E) showing the morphological changes that were assessed using a dual ethidium bromide/fluorescent acridine orange staining technique (×400) representing control, sage oil, trazodone, protective, and curative groups, respectively.
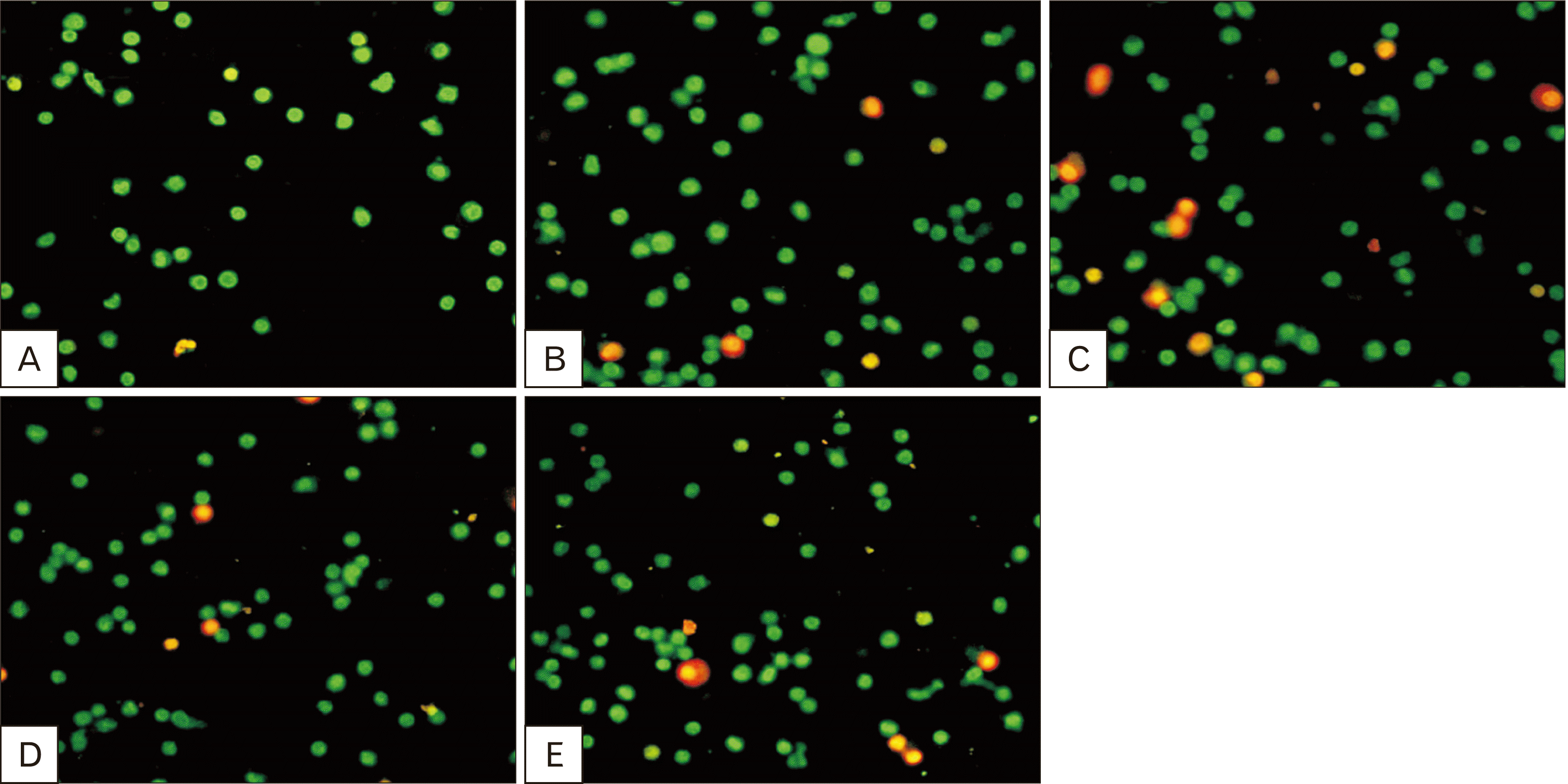
Fig. 9
Representative micro-photographs (A–E) of ethidium bromide-stained single-cell gel electrophoresis (comet assay) representing control, sage oil, trazodone, protective, and curative groups, respectively.
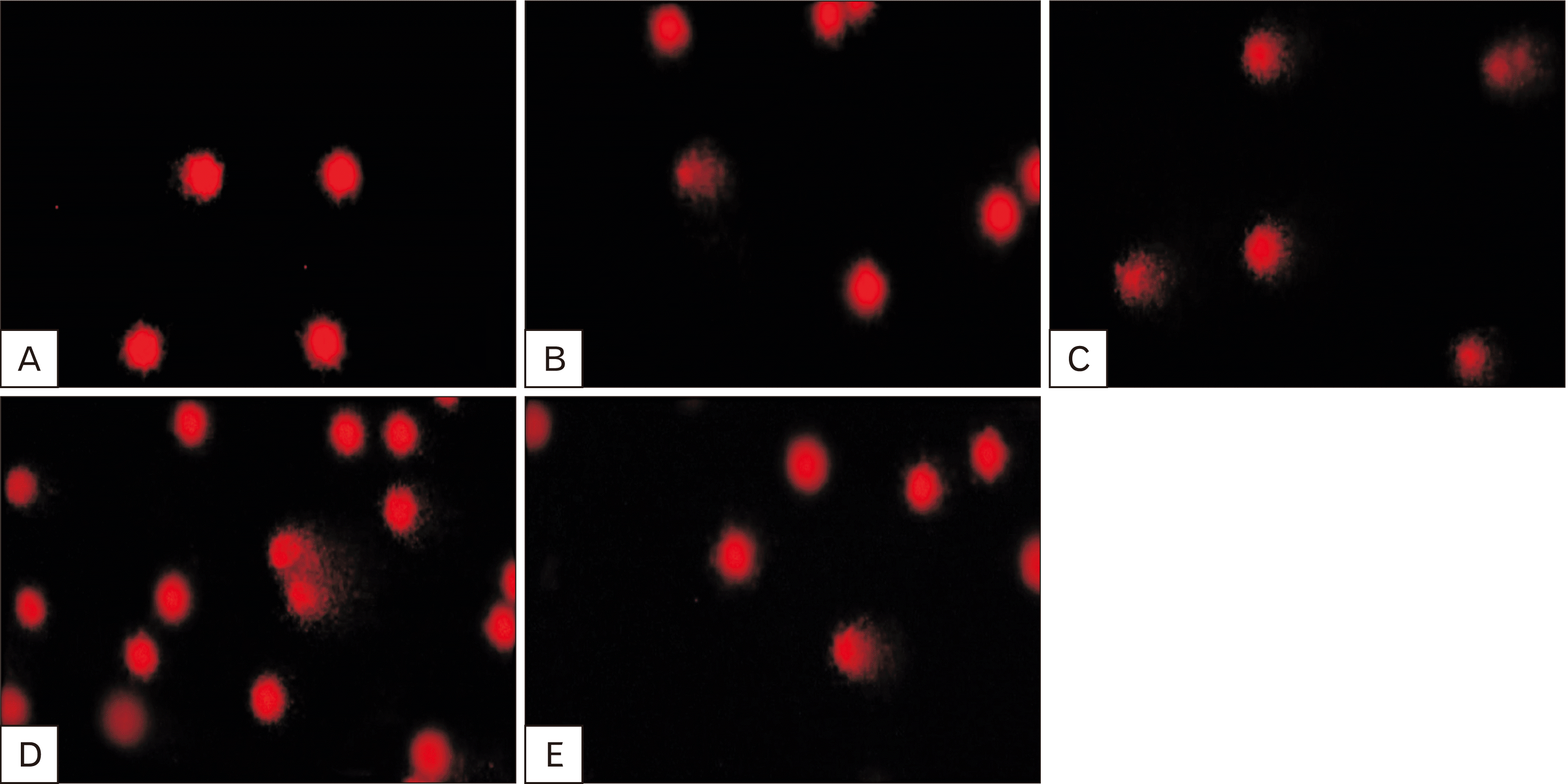
Table 1
Body weight, heart weight heart/body weight ratio of rat groups
| Parameter | Control | Sage oil | TRZ | Protective | Curative |
|---|---|---|---|---|---|
| Initial body weight | 165.0±7.694 | 162.3±8.406 | 163.0±6.164 | 168.5±7.918 | 163.7±11.31 |
| Final body weight | 193.8±5.193 | 190.7±10.82 | 185.7±7.285 | 193.5±7.583 | 189.7±10.63 |
| Heart weight | 0.8417±0.0546 | 0.8083±0.0527 | 1.152±0.3393a) | 0.8250±0.0351c) | 0.8417±0.0527c,e) |
| Heart/body weight ratio | 0.4373±0.0150 | 0.4302±0.0139 | 0.6155±0.1592b) | 0.4264±0.0077d) | 0.4564±0.0177d,f) |




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download