Abstract
Knowledge of the superficial radial nerve (SRN) relationship and anatomic variations of the first extensor compartment (1st EC) will contribute to a better outcome of de Quervain tenosynovitis treatment. We dissected 87 embalmed cadaveric wrists to determine the relationship of the SRN, the 1st EC length, distance from the proximal and distal 1st EC borders to radial styloid process (RSP), abductor pollicis longus (APL) and extensor pollicis brevis (EPB) tendon slip numbers, and the presence of septum. Our results revealed SRN crossing over the 1st EC in 59.5%. The lateral branch of the superficial radial nerve to the 1st EC midline in most cases (61.9%) except for one specimen, where lateral antebrachial cutaneous nerve was the closest. Distances from proximal and distal 1st EC borders to the RSP were 19.7±4.1 mm and 7.6±1.8 mm, respectively. Extensor retinaculum (ER) width over 1st EC (1st EC length) was 14.8±3.2 mm. Complete and incomplete septa were found in 17.2%, and 42.5%, respectively. The most frequent APL tendon slip number in the compartment was two in overall 47 specimens (54.0%). Almost all compartments (85 specimens; 97.7%) contained one EPB tendon slip. We detected bilateral EPB absence in one cadaver. Moreover, we recorded a tendon slip from extensor pollicis longus traveling into 1st EC bilaterally in one cadaver and observed the EPB muscle belly extension into 1st EC in 9 wrists. Awareness of 1st EC anatomic variations would be essential for successful surgical and nonsurgical outcomes.
de Quervain tenosynovitis is a common tendon-related disease of the hand in the first extensor compartment (1st EC) of the wrist, comprising the abductor pollicis longus (APL) and extensor pollicis brevis (EPB) tendons, characterized by pain and swelling of the radial aspect of the wrist at the level of 1st EC [1, 2]. Anatomical knowledge of the 1st EC is essential for understanding the de Quervain tenosynovitis pathophysiology as well as for efficient disease treatment [3]. The anatomical variations such as the presence of intra-compartmental (intertendinous) septum or multiple APL tendon slips could be potential underlying phenomena of the disease along with trauma, biomechanical compression, friction, inflammation and increased volume state [3].
The disease treatment options consist of nonsurgical (e.g., corticosteroid injection, oral nonsteroidal anti-inflammatory drugs and splinting), or surgical approaches [4], the former being considered the first-line treatments [2, 5, 6]. Corticosteroid injection into the 1st EC could potentially modify the symptoms [3, 5, 7-10]. Accurate steroid injections are reportedly important for successful treatment results [5, 11]. However, inaccurate injections and anatomic variations (e.g., septa in the 1st EC) lead to treatment failure [5, 11]. Therefore, detailed knowledge of the 1st EC location and structural arrangement is key for successful treatment.
If the nonsurgical treatments yield unsatisfying results, surgical approaches, with potentially better outcomes, might be required [2-4, 6, 7, 10, 12]. However, unawareness of the sub-compartments within the 1st EC could result in an incomplete surgical release of the compartment and treatment failure [12-14]. The surgical treatment to release the 1st EC could also bear undesired complications, e.g., hypertrophic scar or superficial radial nerve (SRN) branch injury [15, 16]. SRN injury, ranging from neurapraxia to complete nerve transection [3], could cause radial sensation deficit, hyper sensation and a painful neuroma [16-18]. Such undesirable complications could be avoided by appropriate surgical techniques along with insightful knowledge of SRN location and course around the surgical area.
In this study, we aimed at describing the 1st EC anatomy, along with the contents of the 1st EC, and its relationship with the SRN to improve the de Quervain tenosynovitis treatment. We described the relationship between SRN and the 1st EC, determined the exact 1st EC location relative to the palpable bony landmark, the radial styloid process (RSP), and assessed septal prevalence and types within the compartment. Finally, we investigated the internal compartment contents, i.e., the APL and EPB tendons, and described unusual anatomical tendon variations inside the1st EC.
We included 87 upper extremities in our study of 44 embalmed cadavers (22 males and 22 females) from the Department of Anatomy, Faculty of Medicine, Chulalongkorn University with the approval of the Institutional Ethics Committee (IRB no. 0474/66). We excluded specimens with any pathology-related altered wrist anatomy. Therefore, one specimen was excluded from the study due to previous surgery of the wrist. We received cadaveric demographic data (e.g., age, weight, height, and sex) from the registry of the Department of Anatomy.
We palpated and marked the RSP at its tip (point R, Fig. 1), then reflected the skin over the radial aspect of the wrist and dissected it to the subcutaneous tissue with care to avoid any SRN and ER damage. After outlying the 1st EC border, we observed the SRN branch appearance over the 1st EC and recorded the shortest distance from the 1st EC midline to the closest SRN branch (distance d, Fig. 1). We marked the proximal and distal borders of the ER over the 1st EC (points 2 and 1, respectively, Fig. 1), measured the length between them, and defined it as the 1st EC length. Moreover, we measured the distances from the RSP tip to the proximal and distal ER borders to identify the 1st EC location.
Next, we cut the ER close to the APL tendon and parallel the midline of the compartment from its proximal toward its distal border to expose the 1st EC. We measured the ER thickness at the cut, then carefully dissected the APL and EPB tendons until their number of slips presented that we recorded. We examined the potential presence of septa by investigating whether any osteo-fibrous tissue could be detected that prevented immediate access to the EPB tendon after the APL tendons were retracted out of the compartment. If the septa were present, we classified them based on their morphology as incomplete or complete (Fig. 2A, B, respectively). We performed all measurements twice using a digital Vernier caliper (resolution 0.01 mm; Mitutoyo®; Mitutoyo Co.) by one investigator (V.C.) and calculated the average.
We performed the statistical analysis using IBM SPSS Statistics, Version 29.0.0.0 (IBM Co.). We calculated the average value and standard deviation (SD) of each measurement, represented as the mean±SD. Paired t-test, independent t-test and chi-square analysis were used to assess the mean difference in the scale variables between sides, the mean difference in the scale variables between sex, and the difference in sex and sides of the ordinal variables, respectively. We considered P-values statistically significant at P<0.05.
The cadaver-related demographic data is demonstrated in Table 1. The average age of death of the donors was 72.1 years (ranging between 14–103 years).
Among the 84 specimens with an intact SRN, 50 specimens (59.5%) exhibited an SRN lying over the 1st EC (Fig. 3A) with no sex- and side-related differences. The shortest distance from the 1st EC midline to the closest SRN branch was 1.9±2.2 mm, which appeared to be longer in males (2.5±2.3 mm) than in females (1.3±1.9 mm) (P=0.013). The frequency of the closest SRN branch to the compartment was the lateral (SR3), medial (SR1 or SR2 or its common trunk), and both the lateral and medial branches in 52 (61.9%), 30 (35.7%), and 1 (1.2%) specimen, respectively. In one specimen (1.2%) the lateral antebrachial cutaneous nerve (LACN) that had a communicating branch from SRN was the closest branch (Fig. 3B). We observed no statistically significant sex- and side- based differences in the distance from the 1st EC midline to the closest SRN branch.
The mean distances from the proximal and distal 1st EC borders to the RSP were 19.7±4.1 mm and 7.6±1.8 mm, respectively. The proximal distance was statistically longer in males than in females (P=0.045) but showed no side-related difference, while the distal distance displayed neither sex nor side-based differences.
The ER width over the 1st EC was 14.8±3.2 mm (15.5±3.5 in males and 14.1±2.7 in females). We defined the ER width as the length of the 1st EC, being statistically greater in males than in females (P=0.044). The ER thickness over the 1st EC was 0.49±0.14 mm and displayed no sex-related difference, although it was higher on the left side than on the right side (P=0.004).
Among the 87 specimens, we recorded incomplete septa within the 1st EC (Fig. 2A) in 37 specimens (42.5%). The remaining 15 (17.2%) and 35 (40.2%) specimens contained complete septa (Fig. 2B) and no septum (Fig. 2C), respectively. The intra-compartmental septal presence and types displayed no sex- and side-related differences. Table 2 summarizes the details of the intra-compartmental septal prevalence. Bilateral presence of the same type of septum and no septum was found in 30 cadavers (69.8%) which were incomplete, complete and no septum as 13 (30.2%), 5 (11.6%), and 12 (27.9%) cadavers, respectively.
The most frequent APL tendon slip number was two in the compartment, recorded in 47 specimens (54.0%). Distal to the compartment, 42 specimens (48.3%) also exhibited two APL slips. The maximal APL tendon slip numbers within and distal to the compartment were three and five, respectively. Within and distal to almost all compartments, only one EPB slip could be observed (85 and 84 specimens; 97.7% and 96.6%, respectively). Table 3 lists the details of APL and EPB tendon slip numbers in the 1st EC of the wrist. In the 1st EC, the most common pattern was 2 APL+1 EPB and was observed in 47 specimens (54.0%) (23 males and 24 females). The number of this pattern found in the left and right sides was 23 and 24, respectively. Therefore, no statistically significant in side and sex was detected.
We detected bilateral EPB absence in a single cadaver (Fig. 4). In the aforementioned case, one of the APL slips was inserted into the proximal phalanx and responsible for extending the first metacarpophalangeal joint instead of the EPB tendon (Fig. 4).
Finally, we observed unusual phenomena in two specimens from the same cadaver that demonstrated three sub-compartments in the 1st EC (Fig. 5). We observed a split EPL tendon traveling deep to the EPB tendon into one sub-compartment of the 1st EC and remerging with the main EPL slip distal to the 1st EC, before getting inserted into the base of the distal phalanx of the thumb. The detailed course of the split EPL tendon is illustrated in Fig. 5. Moreover, we described the EPB muscle belly extension into the 1st EC in 9 (5 right and 4 left) wrists (10.34%) as a novel finding with bilateral presentations in 3 cadavers (Fig. 6).
A detailed knowledge of SRN relationship as well as anatomic variations of the 1st EC was evaluated to provide essential data to improve de Quervain tenosynovitis treatment outcome. The most important finding of this study emphasized that SRN and the 1st EC are in very close proximity. Since SRN injury could occur during the surgical treatment of de Quervain tenosynovitis [15-19], the surgeon should possess insightful knowledge about the SRN location and its relationship with the compartment. Few studies have previously focused on the relationship between the SRN (and its branches) and the 1st EC. Cheong et al. [20] demonstrated high SRN incidence (59%) over the APL tendon. Gurses et al. [21] documented that the distances between SR3 and the proximal, middle, and distal midpoints of the 1st EC are approximately 2 mm. These findings were consistent with our results. However, Ikiz and Uçerler [22] reported that the distance of the closest branch of SRN to the center of the 1st EC compartment was 5.4±3.0 mm, which is beyond the values described in our study. Moreover, the latter authors also demonstrated that the SRN was present across the compartment in 16.7% of the specimens, being significantly below our results (59.5%). The result related to LACN crossing over the 1st EC should be emphasized in the field.
Commonly preferred incision techniques include transverse, oblique, or longitudinal [12]. To avoid SRN injuries, sharp incisions should be made only through the dermis and blunt dissection should be used beyond the subcutaneous tissue [12]. Although Poublon et al. [23] described that none of these techniques would represent a significantly lower risk of iatrogenic nerve damage, certain studies indicated that the longitudinal incision carried a lower complication risk compared with transverse incision [24-26]. Based on our results, we would recommend the longitudinal skin incision in the surgical treatment for de Quervain tenosynovitis.
Concerning the 1st EC location and morphometries, Hazani et al. [27] reported that the RSP tip was 3.2±5.7 mm distal to the distal border of the compartment, which is shorter than what we measured in our study (7.6±1.8 mm). The proximal ER edge was approximately 25.1 mm proximal to the RSP, being longer than the values of our results in this study (19.7±4.1 mm). Since the ER width could represent the 1st EC length, its related values are similar to those described in previous studies [28, 29] with rather different thicknesses (Table 4) [28], potentially due to the different measurement methods and sites. Taken together, our results support the recommendation that the corticosteroids should be injected in the range of 0.76–1.97 cm, or approximately 1.5 cm proximal to RSP to ensure steroid delivery into the compartment.
We demonstrated a high septal prevalence within the 1st EC (59.8%). The presence of intra-compartmental septa was attributed to the failure of conservative treatment (corticosteroid injection) for de Quervain tenosynovitis [5, 11]. Several studies indicated that ultrasound-guided corticosteroid injection resulted in a superior accuracy over blind injections when sub-compartments were present within the 1st EC [4, 8, 30, 31]. Therefore, we recommend ultrasound-guided corticosteroid injection over blind injection for conservative de Quervain tenosynovitis treatment. Another injection technique with a high efficacy rate (89%) for cases of septa with two-point injections is one and another over the EPB and APL tendons [32]. In addition, several studies focused on septal prevalence inside the 1st EC (we listed some of them in Table 5) [33-45]. Kulthanan and Chareonwat [33] investigated septal prevalence in patients with de Quervain compared to cadavers of assumably physiological states. The authors reported that septa were more commonly found in patients with de Quervain than in cadavers, which was in good agreement with systemic reviews of Lee et al. [1], Bonczar et al. [34] and Abi-Rafeh et al. [31]. Therefore, the presence of an intra-compartmental septum within the 1st EC might also be associated with de Quervain tenosynovitis etiology or pathophysiology. In addition, bilateral presence of the same type of septum reported in this study should be considered during treatment.
We described the common tendon number pattern in the 1st EC as 2 APL and 1 EPB tendon slip, which was in line with several previous studies [1, 34-37, 46]. However, certain authors reported different results. Shiraishi and Matsumura [38] reported ≥4 APL and 1 EPB tendons in 60 specimens. Kulthanan and Chareonwat [33] documented 2 APL and 1 EPB tendons in 82 cadavers as the most common pattern, although in 66 patients with de Quervain diseases, 1 APL and 1 EPB tendon was the most common. This inconsistency in the results might arise from an asynchronous definition of the tendon number, whether it is counted within or distal to the compartment. In our study, we observed that the 3 APL tendon slip prevalence has changed dramatically from 2.3% within the compartment to 36.8% distal to the compartment (we listed certain studies about the APL and EPB tendon number patterns in Table 6). Since proper recognition of supernumerary tendons and the presence of the septum in the 1st EC were central to complete tendon release [13] and our results demonstrated the high prevalence of both aspects, we recommended that the surgeon should look for the presence of sub-compartments and supernumerary tendons in the 1st EC in all cases to ensure the complete release and successful surgery.
Our discovery of a split EPL tendon traveling deep to the EPB tendon into one sub-compartment of the 1st EC and remerging with the main EPL slip distal to the 1st EC, before inserting into the base of the distal phalanx of the thumb has not been reported previously. Rosa et al. [47] described a case with the bilateral contribution of a tendon slip from EPL that crossed laterally under the ER, entered the 1st EC, and merged with the EPB tendon which was different from our observation. Interestingly, this finding occurred bilaterally in one cadaver. Our other novel finding was the EPB muscle belly extension into the 1st EC in 9 wrists. The clinical significance of this discovery should be further elucidated in the future.
Our study also has certain limitations. The first limitation is the relatively small number of cadavers. Second, we used specimens from formalin-embalmed cadavers which could alter the length measurements compared to those of living patients. However, this should not affect SRN presence over the 1st EC, that of septa within the compartment or tendon slip numbers. Third, the history of the cadavers with de Quervain tenosynovitis was unknown. Therefore, a comparison between the presence of the septum and tendons slip numbers in the 1st EC between control participants and patients with de Quervain tenosynovitis could not be evaluated.
In conclusion, understanding SRN, 1st EC, and its content is crucial for the treatment of de Quervain tenosynovitis. SRN location should be known both for corticosteroid injections and surgical approaches to avoid any potential nerve injury that may occur during the treatment. We recommended corticosteroid injections approximately 1.5 cm proximal to RSP under ultrasound guidance for an accurate procedure. In the case of surgical treatment, the skin should be incised longitudinally to avoid complications, especially SRN injury. Tendon numbers and septal presence inside the compartment should be investigated during the surgical release of the 1st EC to ensure complete compartment release. The anatomical variations we described in this study could also significantly contribute to successful disease treatment.
Acknowledgements
The authors would like to express their gratitude to those who have donated their body for medical study and research. Special thanks are extended to the technical staff of the Department of Anatomy, Faculty of Medicine, Chulalongkorn University for their support.
Notes
References
1. Lee ZH, Stranix JT, Anzai L, Sharma S. 2017; Surgical anatomy of the first extensor compartment: a systematic review and comparison of normal cadavers vs. De Quervain syndrome patients. J Plast Reconstr Aesthet Surg. 70:127–31. DOI: 10.1016/j.bjps.2016.08.020. PMID: 27693273.

2. Currie KB, Tadisina KK, Mackinnon SE. 2022; Common hand conditions: a review. JAMA. 327:2434–45. Erratum in: JAMA 2023;330:772. DOI: 10.1001/jama.2022.8481. PMID: 35762992.
3. Ilyas AM, Ast M, Schaffer AA, Thoder J. 2007; De quervain tenosynovitis of the wrist. J Am Acad Orthop Surg. 15:757–64. Erratum in: J Am Acad Orthop Surg 2008;16:35A. DOI: 10.5435/00124635-200712000-00009. PMID: 18063716.

4. Larsen CG, Fitzgerald MJ, Nellans KW, Lane LB. 2021; Management of de Quervain tenosynovitis: a critical analysis review. JBJS Rev. 9:e21.00069. DOI: 10.2106/JBJS.RVW.21.00069. PMID: 34506345.
5. Harvey FJ, Harvey PM, Horsley MW. 1990; De Quervain's disease: surgical or nonsurgical treatment. J Hand Surg Am. 15:83–7. DOI: 10.1016/S0363-5023(09)91110-8. PMID: 2299173.

6. Gurses IA, Coskun O, Gayretli O, Kale A, Ozturk A. 2015; The anatomy of the fibrous and osseous components of the first extensor compartment of the wrist: a cadaveric study. Surg Radiol Anat. 37:773–7. DOI: 10.1007/s00276-015-1439-2. PMID: 25645546.

7. Ilyas AM. 2009; Nonsurgical treatment for de Quervain's tenosynovitis. J Hand Surg Am. 34:928–9. DOI: 10.1016/j.jhsa.2008.12.030. PMID: 19410999.

8. McDermott JD, Ilyas AM, Nazarian LN, Leinberry CF. 2012; Ultrasound-guided injections for de Quervain's tenosynovitis. Clin Orthop Relat Res. 470:1925–31. DOI: 10.1007/s11999-012-2369-5. PMID: 22552767. PMCID: PMC3369070.

9. Güleç A, Türkmen F, Toker S, Acar MA. 2016; Percutaneous release of the first dorsal extensor compartment: a cadaver study. Plast Reconstr Surg Glob Open. 4:e1022. DOI: 10.1097/GOX.0000000000001022. PMID: 27826460. PMCID: PMC5096515.

10. Saaiq M. 2021; Management outcome of de Quervain's disease with corticosteroid injection versus surgical decompression. Arch Bone Jt Surg. 9:167–73. DOI: 10.22038/abjs.2020.47822.2359. PMID: 34026933. PMCID: PMC8121040.
11. Zingas C, Failla JM, Van Holsbeeck M. 1998; Injection accuracy and clinical relief of de Quervain's tendinitis. J Hand Surg Am. 23:89–96. DOI: 10.1016/S0363-5023(98)80095-6. PMID: 9523961.

12. Weller WJ. Azar FM, Beaty JH, editors. Stenosing tenosynovitis of the wrist and hand. Campbell's Operative Orthopaedics. 14th ed. Elsevier;2021. p. 3850–6.
13. Giles KW. Anatomical variations affecting the surgery of de Quervain's disease. J Bone Joint Surg Br. 1960; 42-B:352–5. DOI: 10.1302/0301-620X.42B2.352. PMID: 13850049.

14. Leslie BM, Ericson WB Jr, Morehead JR. 1990; Incidence of a septum within the first dorsal compartment of the wrist. J Hand Surg Am. 15:88–91. DOI: 10.1016/S0363-5023(09)91111-X. PMID: 2299174.

15. Visuthikosol V, Chanyasawat S. 1988; Surgical treatment of de Quervain's diseases: a clinical review of 42 cases. J Med Assoc Thai. 71:637–9.
16. Suresh SS, Zaki H. 2009; De quervain disease: Ibri technique to avoid superficial radial nerve injury. Tech Hand Up Extrem Surg. 13:113–5. DOI: 10.1097/BTH.0b013e31819f6cdd. PMID: 19516139.
17. Arons MS. 1987; de Quervain's release in working women: a report of failures, complications, and associated diagnoses. J Hand Surg Am. 12:540–4. DOI: 10.1016/S0363-5023(87)80204-6. PMID: 2956316.

18. Huanmanop T, Agthong S, Luengchawapong K, Sasiwongpakdee T, Burapasomboon P, Chentanez V. 2007; Anatomic characteristics and surgical implications of the superficial radial nerve. J Med Assoc Thai. 90:1423–9. PMID: 17710987.
19. Chhabra AB, Freilich AM. Miller MD, Chhabra AB, Browne JA, Park JS, Shen FH, Weiss DB, editors. Wrist and hand. Orthopaedic Surgical Approaches. 2nd ed. Elsevier;2015. p. 105–59.
20. Cheong IY, Rhyu IJ, Kim KH, Chung PW, Kim D, Park BK, Kim DH. 2016; Anatomical basis for injection around first dorsal compartment of the wrist: a fresh cadaveric study. Pain Physician. 19:E893–900. DOI: 10.36076/ppj/2016.19.E893. PMID: 27454280.
21. Gurses IA, Coskun O, Gayretli O, Kale A, Ozturk A. 2014; The relationship of the superficial radial nerve and its branch to the thumb to the first extensor compartment. J Hand Surg Am. 39:480–3. DOI: 10.1016/j.jhsa.2013.12.004. PMID: 24495622.

22. Ikiz ZA, Uçerler H. 2004; Anatomic characteristics and clinical importance of the superficial branch of the radial nerve. Surg Radiol Anat. 26:453–8. DOI: 10.1007/s00276-004-0256-9. PMID: 15365770.

23. Poublon AR, Kleinrensink GJ, Kerver A, Coert JH, Walbeehm ET. 2018; Optimal surgical approach for the treatment of Quervains disease: a surgical-anatomical study. World J Orthop. 9:7–13. DOI: 10.5312/wjo.v9.i2.7. PMID: 29468135. PMCID: PMC5807885.

24. Abrisham SJ, Karbasi MH, Zare J, Behnamfar Z, Tafti AD, Shishesaz B. 2011; De qeurvian tenosynovitis: clinical outcomes of surgical treatment with longitudinal and transverse incision. Oman Med J. 26:91–3. DOI: 10.5001/omj.2011.23. PMID: 22043391. PMCID: PMC3191679.
25. Kumar K. 2016; Outcome of longitudinal versus transverse incision in de Quervain's disease and its implications in Indian population. Musculoskelet Surg. 100:49–52. DOI: 10.1007/s12306-015-0388-6. PMID: 26645452.

26. Mangukiya HJ, Kale A, Mahajan NP, Ramteke U, Manna J. 2019; Functional outcome of De Quervain's tenosynovitis with longitudinal incision in surgically treated patients. Musculoskelet Surg. 103:269–73. DOI: 10.1007/s12306-018-0585-1. PMID: 30600438.

27. Hazani R, Engineer NJ, Cooney D, Wilhelmi BJ. 2008; Anatomic landmarks for the first dorsal compartment. Eplasty. 8:e53. PMID: 19092992. PMCID: PMC2586286.
28. Massaki AN, Tan J, Huang BK, Chang EY, Trudell DJ, Resnick DL. 2013; Extensor retinaculum of the wrist: gross anatomical correlation with MR imaging after ultrasound-guided tenography with emphasis on anatomical features in wrist dorsiflexion responsible for tendon impingement. Skeletal Radiol. 42:1727–37. DOI: 10.1007/s00256-013-1739-8. PMID: 24085470.

29. Sugiura S, Matsuura Y, Suzuki T, Nishikawa S, Kuniyoshi K, Ohtori S. 2019; Histological assessment of a septum in the first dorsal compartment: a fresh cadaver study. J Hand Surg Eur Vol. 44:805–9. DOI: 10.1177/1753193419838204. PMID: 30917737.

30. Leversedge FJ, Cotterell IH, Nickel BT, Crosmer M, Richard M, Angermeier E. 2016; Ultrasonography-guided de Quervain injection: accuracy and anatomic considerations in a cadaver model. J Am Acad Orthop Surg. 24:399–404. Erratum in: J Am Acad Orthop Surg 2016;24:565. DOI: 10.5435/JAAOS-D-15-00753. PMID: 27128027.
31. Abi-Rafeh J, Mojtahed Jaberi M, Kazan R, Alabdulkarim A, Boily M, Thibaudeau S. 2022; Utility of ultrasonography and significance of surgical anatomy in the management of de Quervain disease: a systematic review and meta-analysis. Plast Reconstr Surg. 149:420–34. DOI: 10.1097/PRS.0000000000008792. PMID: 35077418.

32. Sawaizumi T, Nanno M, Ito H. 2007; De Quervain's disease: efficacy of intra-sheath triamcinolone injection. Int Orthop. 31:265–8. DOI: 10.1007/s00264-006-0165-0. PMID: 16761148. PMCID: PMC2267566.

33. Kulthanan T, Chareonwat B. 2007; Variations in abductor pollicis longus and extensor pollicis brevis tendons in the Quervain syndrome: a surgical and anatomical study. Scand J Plast Reconstr Surg Hand Surg. 41:36–8. DOI: 10.1080/02844310600869720. PMID: 17484184.

34. Bonczar M, Walocha J, Pasternak A, Depukat P, Dziedzic M, Ostrowski P, Bonczar T, Warchoł Ł, Koziej M. 2023; Anatomical variations in the first dorsal compartment of the wrist: meta-analysis. Folia Morphol (Warsz). 82:766–76. DOI: 10.5603/FM.a2022.0081. PMID: 36165900.

35. Bahm J, Szabo Z, Foucher G. 1995; The anatomy of de Quervain's disease. A study of operative findings. Int Orthop. 19:209–11. DOI: 10.1007/BF00185223. PMID: 8557414.
36. Roy AJ, Roy AN, De C, Banerji D, Das S, Chatterjee B, Halder TC. 2012; A cadaveric study of the first dorsal compartment of the wrist and its content tendons: anatomical variations in the Indian population. J Hand Microsurg. 4:55–9. DOI: 10.1007/s12593-012-0073-z. PMID: 24293951. PMCID: PMC3509286.

37. Coşkun O, Ok F, Şahin B, Gürses İA. 2023; First extensor compartment morphology and clinical significance: a cadaver series study. Anat Cell Biol. 56:328–33. DOI: 10.5115/acb.23.008. PMID: 36987785. PMCID: PMC10520861.

38. Shiraishi N, Matsumura G. 2005; Anatomical variations of the extensor pollicis brevis tendon and abductor pollicis longus tendon--relation to tenosynovectomy. Okajimas Folia Anat Jpn. 82:25–9. DOI: 10.2535/ofaj.82.25. PMID: 15934601.
39. Mahakkanukrauh P, Mahakkanukrauh C. 2000; Incidence of a septum in the first dorsal compartment and its effects on therapy of de Quervain's disease. Clin Anat. 13:195–8. DOI: 10.1002/(SICI)1098-2353(2000)13:3<195::AID-CA6>3.0.CO;2-V.

40. Gousheh J, Yavari M, Arasteh E. 2009; Division of the first dorsal compartment of the hand into two separated canals: rule or exception? Arch Iran Med. 12:52–4. PMID: 19111030.
41. Nayak SR, Hussein M, Krishnamurthy A, Mansur DI, Prabhu LV, D'Souza P, Potu BK, Chettiar GK. 2009; Variation and clinical significance of extensor pollicis brevis: a study in South Indian cadavers. Chang Gung Med J. 32:600–4. DOI: 10.1007/s00276-009-0526-7. PMID: 19554250.
42. Opreanu RC, Wechter J, Tabbaa H, Kepros JP, Baulch M, Xie Y, Lackey W, Katranji A. 2010; Anatomic variations of the first extensor compartment and abductor pollicis longus tendon in trapeziometacarpal arthritis. Hand (N Y). 5:184–9. DOI: 10.1007/s11552-009-9234-3. PMID: 19834771. PMCID: PMC2880676.

43. Sugiura S, Matsuura Y, Kuniyoshi K, Nishikawa S, Toyooka T, Mori C, Suzuki T. 2017; Anatomic study of the first extensor compartment and the relationship between the extensor tendon width and its distal insertion. Surg Radiol Anat. 39:1223–6. DOI: 10.1007/s00276-017-1867-2. PMID: 28484860.

44. Ravi PK, Tewari J, Mishra PR, Tripathy SK, Nanda SN, Gantaguru A. 2019; Variations of extensor pollicis brevis tendon in Indian population: a cadaveric study and review of literature. J Clin Orthop Trauma. 10:278–81. DOI: 10.1016/j.jcot.2018.02.008. PMID: 30828193. PMCID: PMC6383140.

45. Pahlavansabagh A, Soleimani M, Ghaznavi A, Roshanipour M, Yousefi F, Taheri SN. 2022; Prevalence of septated first dorsal compartment among Iranian patients with de Quervain tenosynovitis. Med J Islam Repub Iran. 36:130. DOI: 10.47176/mjiri.36.130. PMID: 36620472. PMCID: PMC9805808.

46. Chang CY, Kheterpal AB, Vicentini JRT, Huang AJ. 2017; Variations of anatomy on MRI of the first extensor compartment of the wrist and association with DeQuervain tenosynovitis. Skeletal Radiol. 46:1047–56. DOI: 10.1007/s00256-017-2639-0. PMID: 28389821.

47. Rosa RC, de Oliveira KM, Léo JA, Elias BA, dos Santos PR, de Santiago HA. 2016; Anomalous bilateral contribution of extensor pollicis longus and muscle fusion of the first compartment of the wrist. Rev Bras Ortop. 51:235–8. DOI: 10.1016/j.rbo.2015.04.030. PMID: 27069895. PMCID: PMC4812036.

Fig. 1
The diagram of the right wrist demonstrates landmarks and structures investigated in this study. 1, distal border of ER; 2, proximal border of ER; d, distance to the closest branch of superficial radial nerve; APL, abductor pollicis longus; EPB, extensor pollicis brevis; ER, extensor retinaculum; R, radial styloid process; SR, branches of the superficial radial nerve.
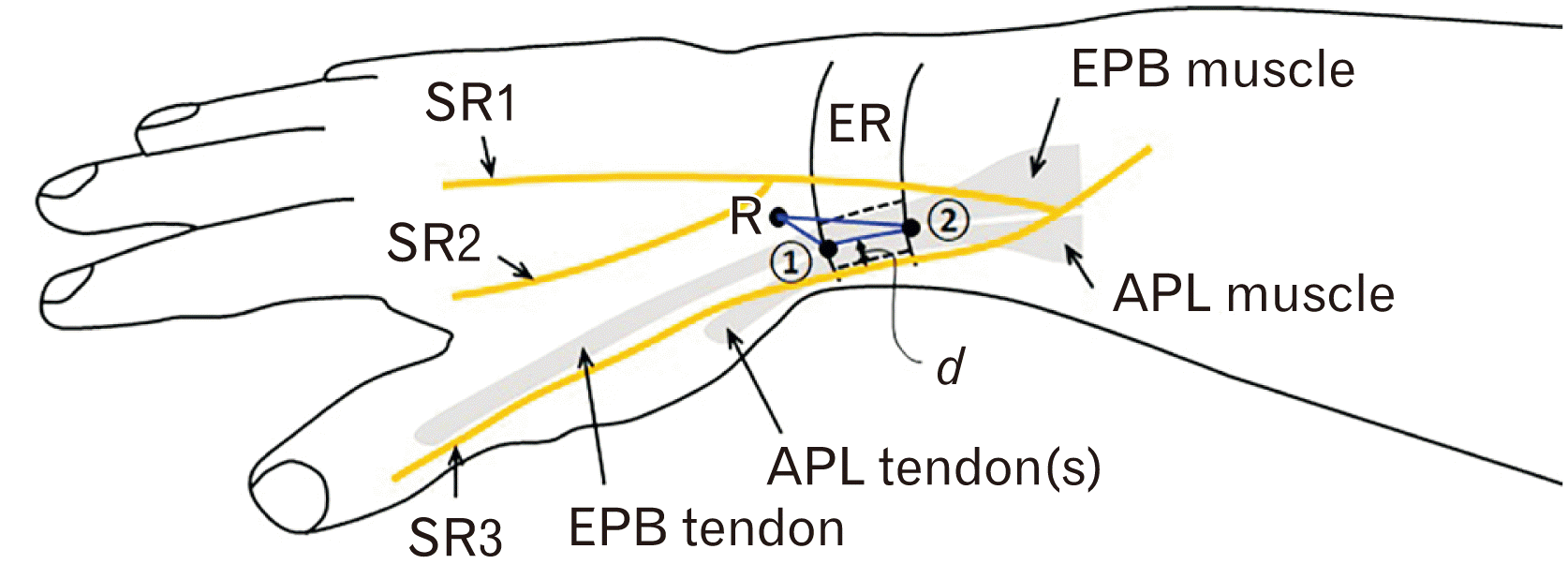
Fig. 2
Types of the first extensor compartment septa. (A) An incomplete septum separating EPB and APL tendons. (B) A complete septum. (C) No septum. 1, distal border of ER; 2, proximal border of ER; 3, proximal border of the incomplete septum; APL, abductor pollicis longus; EPB, extensor pollicis brevis; ER, extensor retinaculum; SR, branches of superficial radial nerve.
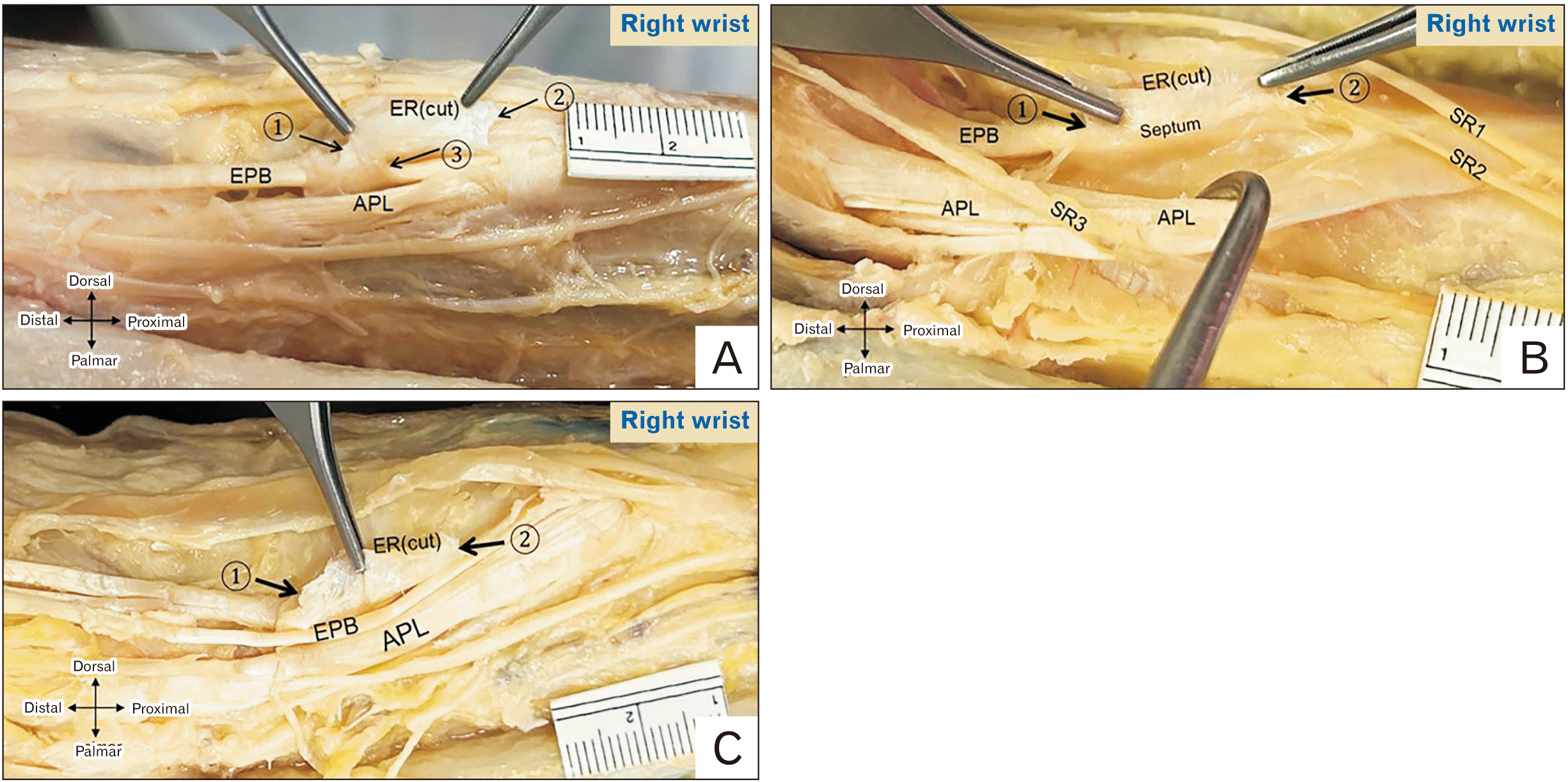
Fig. 3
Relation of the SRN and first EC. (A) Right cadaveric wrist showing two branches of SRN lying on the ER over the first EC. (B) Left cadaveric wrist showing the LACN lying on the ER over the first EC and a communicating branch between SRN and LACN. 1, distal border of ER; 2, proximal border of ER; APL, abductor pollicis longus; ER, extensor retinaculum; EPB, extensor pollicis brevis; LACN, lateral antebrachial cutaneous nerve; SRN, superficial radial nerve; EC, extensor compartment.
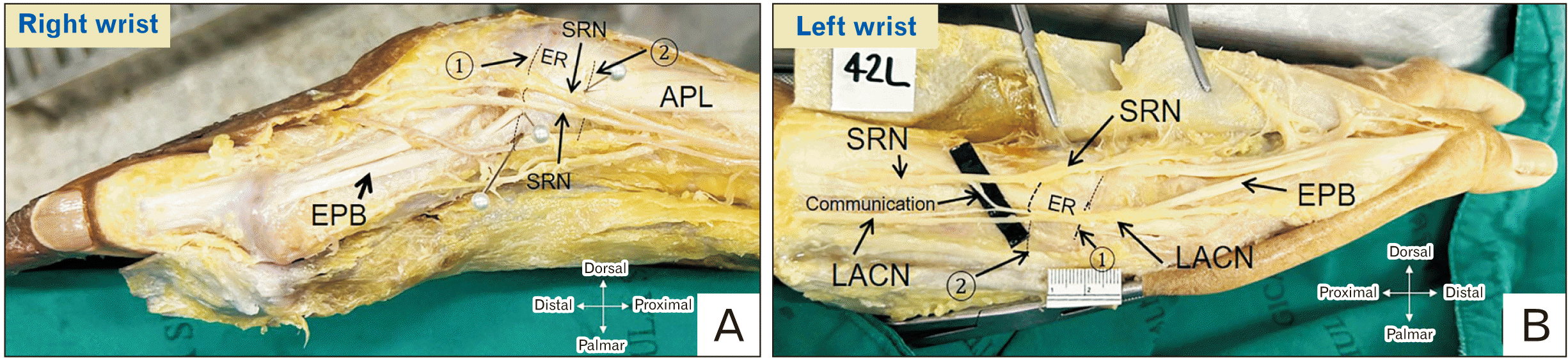
Fig. 4
Absence of EPB in the first extensor compartment. A slip from APL (asterisk) inserting at the proximal phalanx. EPB, extensor pollicis brevis; APL, abductor pollicis longus; EPL, extensor pollicis longus; ER, extensor retinaculum; 1, distal border of ER; 2, proximal border of ER.
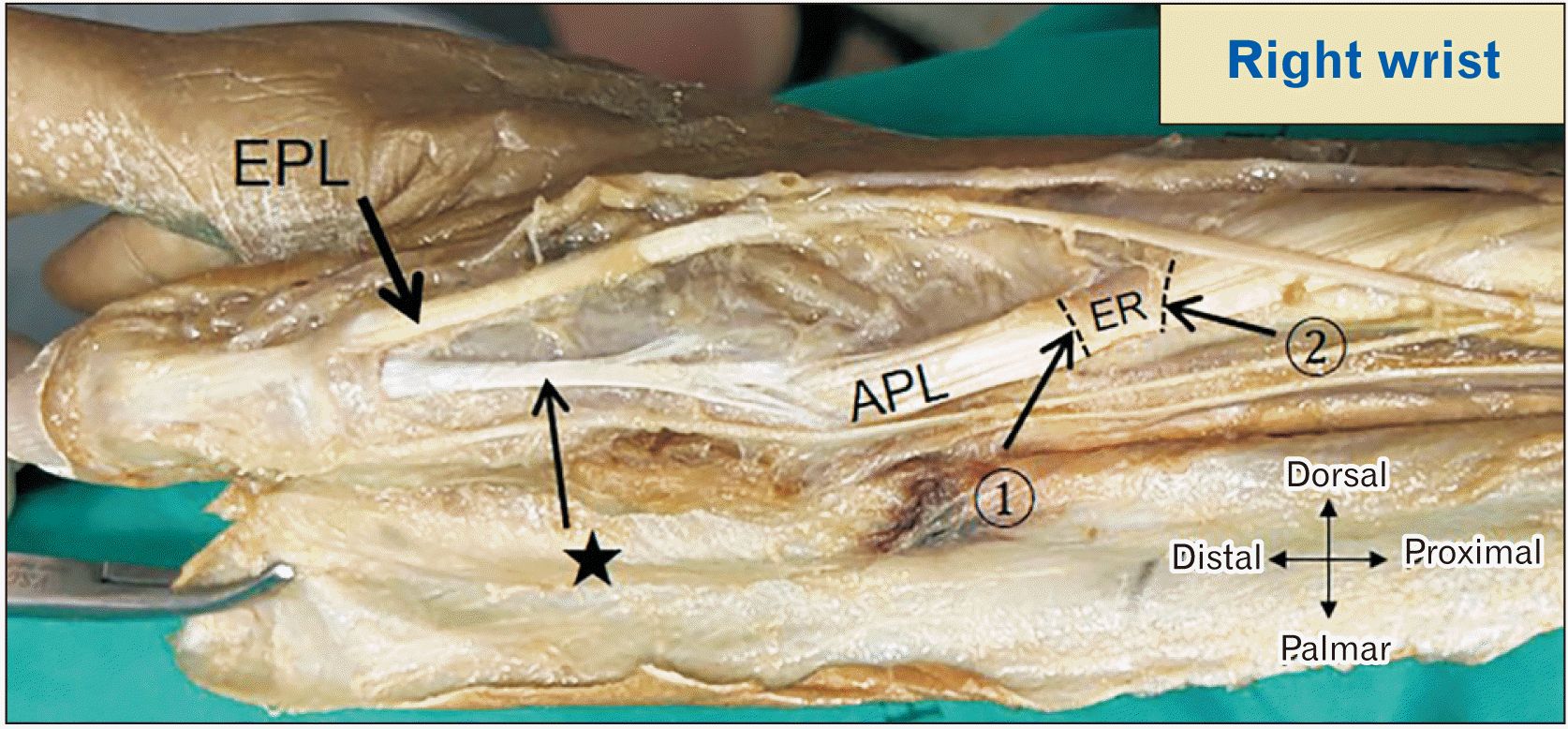
Fig. 5
Photographs and diagrams illustrating an EPL split tendon and three sub-compartments within the first EC. (A) Before ER cut, two tendons distal to the first EC. (B) After the ER cut, one EPB tendon and a split tendon from EPL (asterisk). (C) Course of the split EPL tendon. (D) Cross-section of the 1st EC showing three sub-compartments and contents. 1, distal border of ER; 2, proximal border of ER; asterisk, split EPL tendon; #common insertion of EPB and EPL at distal phalanx of thumb. APL, abductor pollicis longus tendon; EPB, extensor pollicis brevis tendon; EPL, extensor pollicis longus tendon; ER, extensor retinaculum; EC, extensor compartment.
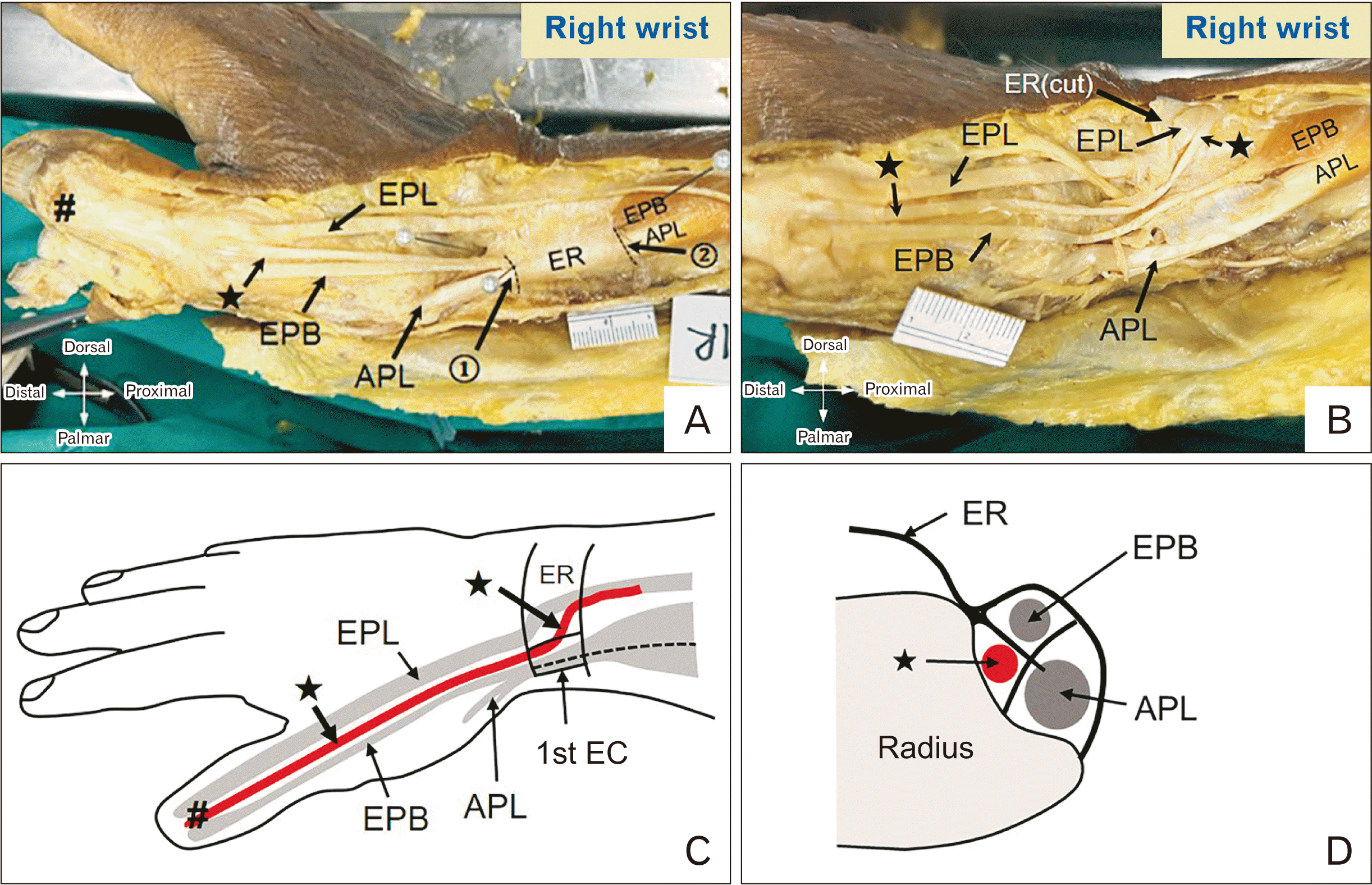
Fig. 6
Illustration of the extension of EPB muscle belly into the first EC. (A) Right cadaveric wrist with the extension of the EPB muscle belly into the first EC. (B) Right cadaveric wrist without extension of EPB muscle belly. 1, distal border of the 1st EC; 2, proximal border of the 1st EC; APL, abductor pollicis longus; EPB, extensor pollicis brevis; EPL, extensor pollicis longus; ER, extensor retinaculum; EC, extensor compartment.
Table 1
Demographic data of the cadavers in current study
| Sex | Number | Age (yr) | Height (cm) | Body weight (kg) |
|---|---|---|---|---|
| Male | 22 | 76.3±12.2 (49–103) | 166.3±5.2 | 57.9±6.3 |
| Female | 22 | 67.9±19.5 (14–98) | 156.9±4.6 | 50.5±3.9 |
| Total | 44 | 72.1±16.6 (14–103) | 161.6±6.8 | 54.2±6.4 |
Table 2
Prevalence of intra-compartmental septum
Table 3
Number of abductor pollicis longus and extensor pollicis brevis tendon slip in the first extensor compartment of the wrist in current study
Table 4
Comparison of the width and thickness of extensor retinaculum with literature
| Study | Country (yr) | Type | Number of specimens | ER width (mm) | ER thickness(mm) |
|---|---|---|---|---|---|
| Hazani et al. [27] | United States (2009) | C | 32 | 21.9±3.7 | - |
| Massaki et al. [28] | United States (2013) | C | 10 | 13.6±3.1a) | 1.67±0.39a) |
| Güleç et al. [9] | Turkey (2016) | C | 48 | 26.6±3.0 b) | - |
| Sugiura et al. [29] | Japan (2019) | C | 24 | 14.0±1.7 | - |
| Present study | Thailand (2024) | C | 87 | 14.8±3.2 | 0.49±0.14 |
Table 5
Comparison of the presence of septum in the first extensor compartment with literature
| Study | Country (yr) | Type of the study | No. of specimens | No. of specimens that septum was presented (%) | Note |
|---|---|---|---|---|---|
| Leslie et al. [14] | United States (1990) | C | 100 | 20 (20.0) | Bilateral presence in 14 specimens (70.0%) |
| Bahm et al. [35] | France (1995) | D | 70 | 42 (60.0) | |
| Mahakkanukrauh and Mahakkanukrauh [39] | Thailand (2000) | C | 200 | 155 (77.5) | Sixty-one complete septa (39.4%), 94 incomplete septa (60.6%) |
| Shiraishi and Matsumura [38] | Japan (2005) | C | 159 | 70 (44.0) | 49 complete septa (70.0%), 21 incomplete septa (30.0%) |
| Kulthanan and Chareonwat [33] | Thailand (2007) | C & D | 82 (C) | 30 (36.6) | |
| 66 (D) | 38 (57.6) | ||||
| Gousheh et al. [40] | Iran (2009) | D | 50 | 43 (86.0) | |
| Hazani et al. [27] | United States (2008) | C | 32 | 11 (34.7) | |
| Nayak et al. [41] | India (2009) | C | 156 | 54 (34.6) | |
| Opreanu et al. [42] | United States (2010) | C | 50 | 36 (72.0) | Bilateral presence in 71.4% of the cases |
| Roy et al. [36] | India (2012) | C | 86 | 32 (37.2) | |
| Massaki et al. [28] | United States (2013) | C | 10 | 1 (10.0) | |
| Gurses et al. [6] | Turkey (2015) | C | 50 | 27 (54.0) | Twelve complete septa (44.4%), 15 incomplete septa (55.6%) |
| Güleç et al. [9] | Turkey (2016) | C | 48 | 16 (33.3) | |
| Lee et al. [1] | United States (2017) | R | 1,857 (C) | 797 (42.9) | |
| 470 (D) | 279 (59.4) | ||||
| Sugiura et al. [43] | Japan (2017) | C | 45 | 37 (82.2) | |
| Ravi et al. [44] | India (2019) | C | 77 | 45 (58.4) | |
| Sugiura et al. [29] | Japan (2019) | C | 24 | 12 (50.0) | Bilateral presence in 5 specimens (41.7%) |
| Bonczar et al. [34] | Poland (2023) | R | 2,635 (C) | 45.7 | |
| 1,592 (D) | 64.0 | ||||
| Pahlavansabagh et al. [45] | Iran (2022) | D | 37 | 23 (62.2) | Two complete septa (8.7%), 21 incomplete septa (91.3%) |
| Coşkun et al. [37] | Turkey (2023) | C | 87 | 53 (60.8) | Twenty-two complete septa (41.5%), 31 incomplete septa (58.5%) |
| Present study | Thailand (2024) | C | 87 | 52 (59.8) |
Fifteen complete septa (28.8%), 37 incomplete septa (71.2%) Bilateral presence of incomplete and complete in 26 (50%) and 10 (19.2%) of 52 specimens, respectively |
Table 6
Comparison of the prevalence of abductor pollicis longus (APL) and extensor pollicis brevis (EPB) tendon slips number with the literature
| Study | Country (yr) | Type of the study | Number of specimens | Number of APL tendon slip(s) | Number of EPB tendon slip(s) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | ≥4 | 0 | 1 | 2 | ≥3 | |||||
| Bahm et al. [35] | France (1995) | D | 70 | 17 (24.3) | 31 (44.3) | 14 (20.0) | 8 (11.4) | 1 (1.4) | 67 (95.8) | 1 (1.4) | 1 (1.4) | |
| Shiraishi and Matsumura [38] | Japan (2005) | C | 60 | 1 (1.7) | 8 (13.3) | 23 (38.3) | 28 (46.7) | 0 (0) | 51 (85.0) | 6 (10.0) | 2 (3.4) | |
| Kulthanan and Chareonwat [33] | Thailand (2007) | C & D | 82 (C) | 9 (11.0) | 52 (63.4) | 21 (25.6) | 0 (0) | 0 (0) | 80 (97.6) | 2 (2.4) | 0 (0) | |
| 66 (D) | 34 (51.5) | 28 (42.4) | 4 (6.1) | 0 (0) | 0 (0) | 62 (93.9) | 4 (6.1) | 0 (0) | ||||
| Hazani et al. [27] | United States (2008) | C | 32 | 9 | 30 | 43 | 26 | 0 | 100 | 0 | 0 | |
| Nayak et al. [41] | India (2009) | C | 156 | - | - | - | - | 0 (0) | 133 (85.3) | 17 (10.9) | 6 (3.8) | |
| Opreanu et al. [42] | United States (2010) | C | 50 | 1 (2.0) | 36 (72.0) | 12 (24.0) | 1 (2.0) | - | - | - | - | |
| Roy et al. [36] | India (2012) | C | 86 | 33 (38.4) | 50 (58.1) | 2 (2.3) | 1 (1.2) | 1 (1.2) | 72 (83.7) | 13 (15.1) | 0 (0) | |
| Lee et al. [1] | United States (2017) | R | 1,096 (C) | 200 (18.3) | 408 (37.3) | 196 (17.9) | 145 (13.2) | - | - | - | - | |
| 320 (D) | 87 (27.2) | 156 (48.8) | 50 (15.6) | 15 (4.7) | - | - | - | - | ||||
| 1,519 (C) | - | - | - | - | 28 (1.8) | 1,402 (92.3) | 89 (5.9) | |||||
| 307 (D) | - | - | - | - | 10 (3.3) | 288 (93.8) | 9 (2.9) | |||||
| Ravi et al. [44] | India (2019) | C | 77 | - | - | - | - | 1 (1.3) | 73 (94.8) | 2 (2.6) | 1 (1.3) | |
| Bonczar et al. [34] | Poland (2023) | R | 1,749 (C) | 16.9 | 46.0 | 19.2 | 8.4 | - | - | - | - | |
| 608 (D) | 23.8 | 45.3 | 22.7 | 6.8 | - | - | - | - | ||||
| 1,814 (C) | - | - | - | - | 1.2 | 94.9 | 3.5 | 0.8 | ||||
| 608 (D) | - | - | - | - | 2.4 | 92.0 | 4.7 | 0.8 | ||||
| Pahlavansabagh et al. [45] | Iran (2022) | D | 37 | 7 (18.9) | 23 (62.2) | 7 (18.9) | 0 (0) | 0 (0) | 34 (91.9) | 3 (8.1) | 0 (0) | |
| Coşkun et al. [37] | Turkey (2023) | C | 87 | 23 (26.4) | 49 (56.3) | 11 (12.6) | 4 (4.6) | 0 (0) | 87 (100) | 0 (0) | 0 (0) | |
| Present study | Thailand (2024) | C | 87 | 38a) (43.7) | 47a) (54.0) | 2a) (2.3) | 0a) (0) | 2a) (2.3) | 85a) (97.7) | 0a) (0) | 0a) (0) | |
| 8b) (9.2) | 42b) (48.3) | 32b) (36.8) | 5b) (5.7) | 2b) (2.3) | 84b) (96.6) | 1b) (1.1) | 0b) (0) | |||||




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download