Introduction
Materials and Methods
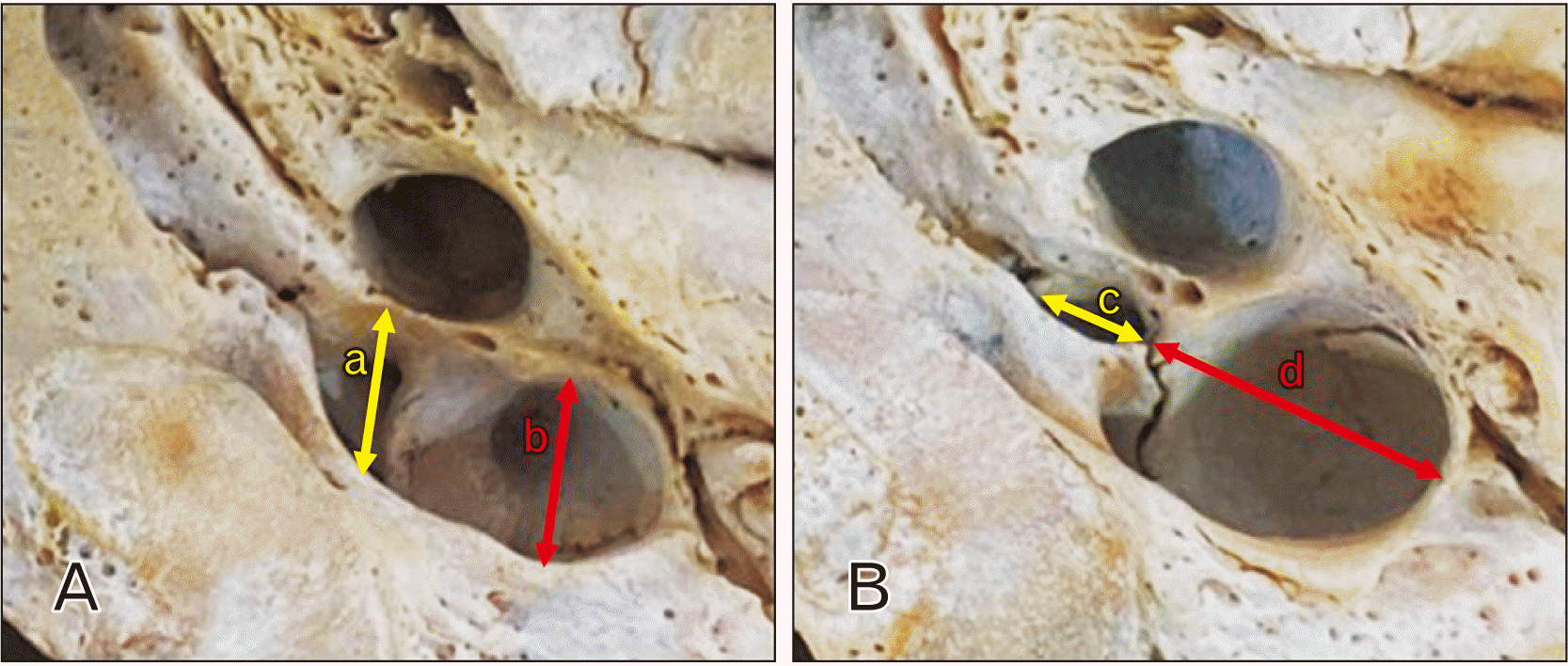 | Fig. 1Linear measurements of the jugular foramen (JF). (A) a: Anteroposterior diameter (APD) of the anteromedial compartment of the JF; b: APD of the posterolateral compartment of the JF. (B) c: Lateromedial diameter (LMD) of the anteromedial compartment of the JF; d: LMD of the posterolateral compartment of the JF. Total LMD=c+d. |
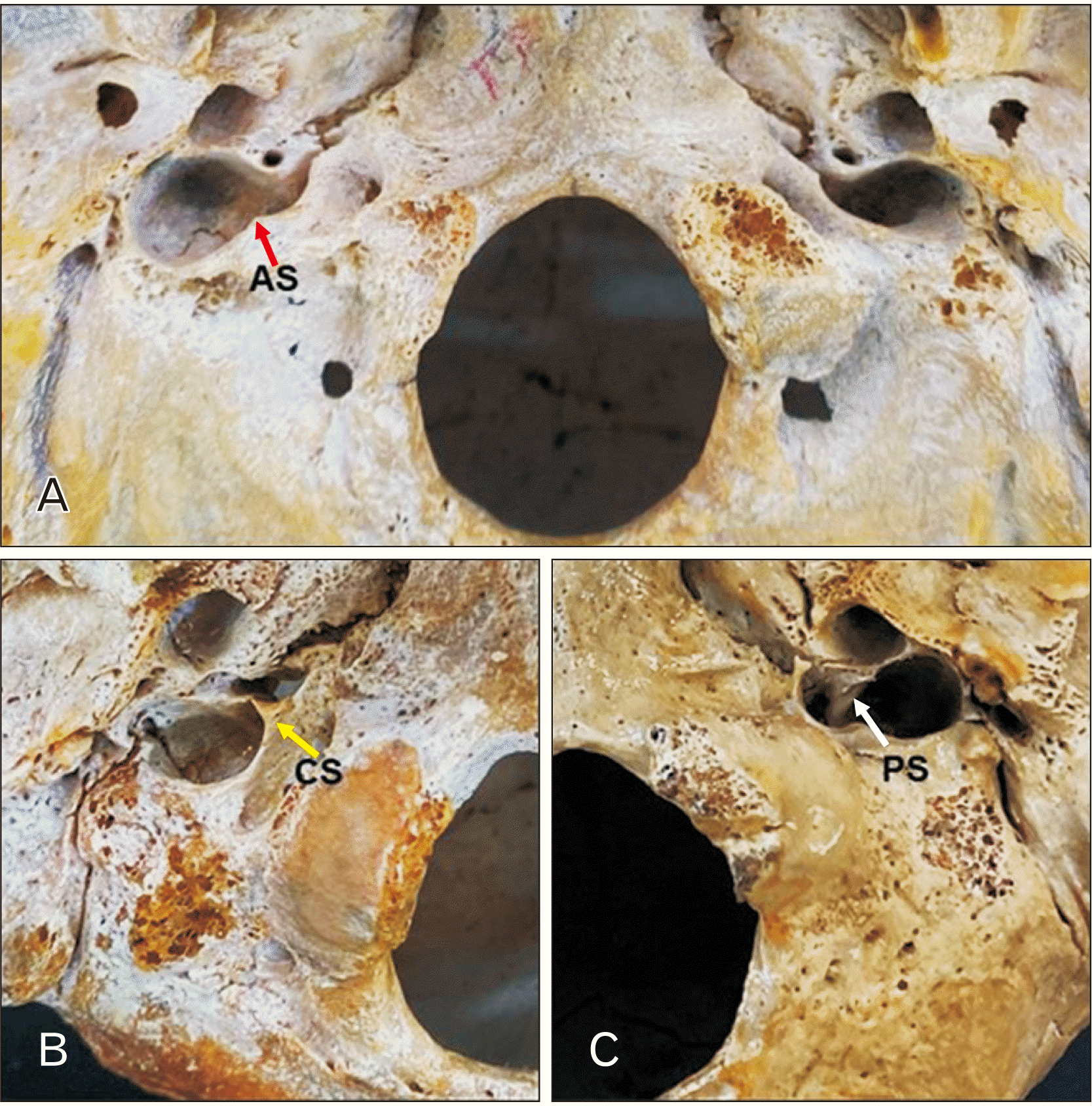
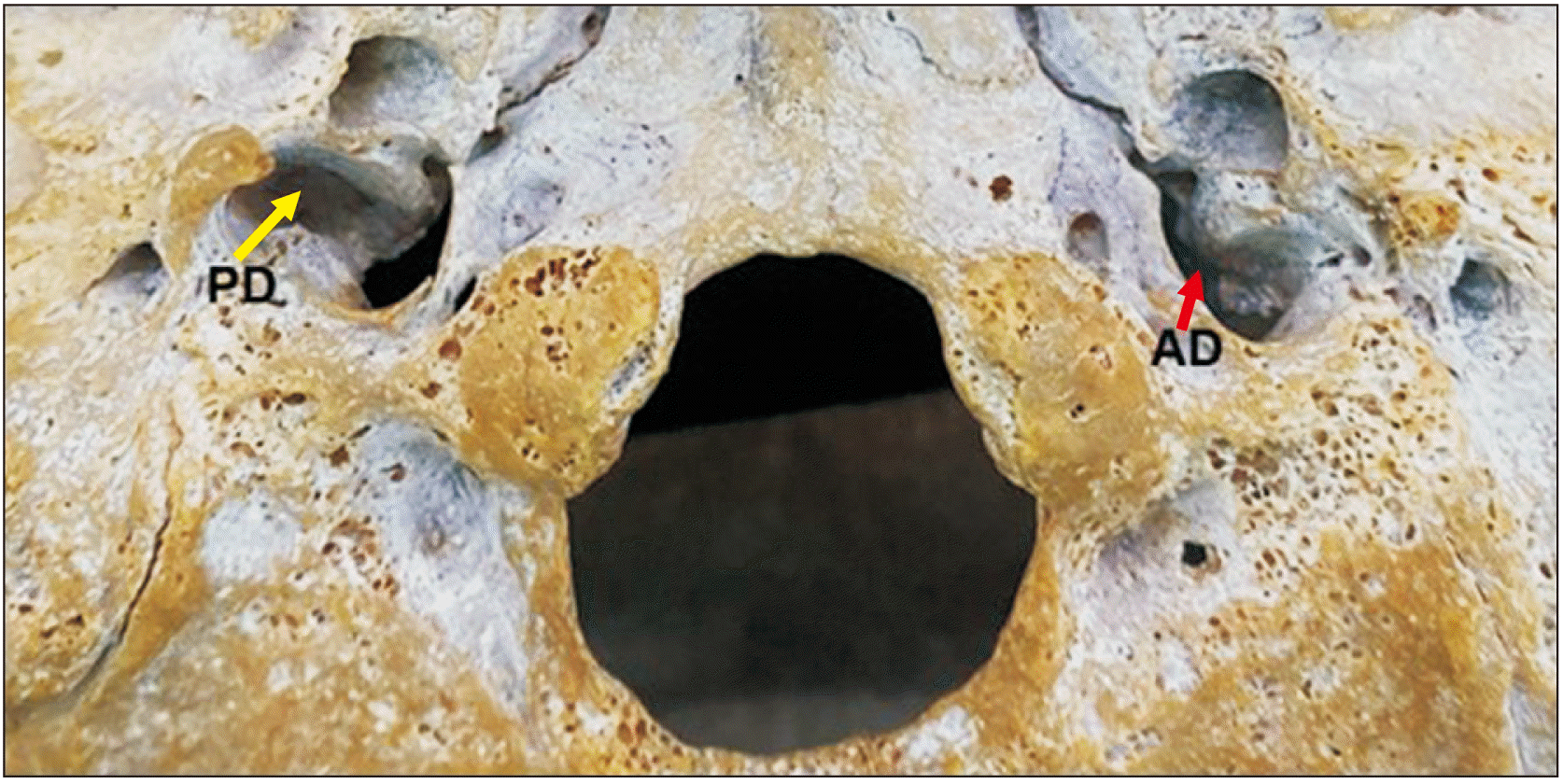
Results
Table 1
Table 3
| Measurements | Total sample (n=256) | Male (n=128) | Female (n=128) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Right | Left | P-value | Right | Left | P-value | Right | Left | P-value | |||
| APD (posterolateral compartment) (mm) | 9.05±2.62 | 8.00±2.40 | <0.001 | 9.10±2.63 | 7.80±2.15 | 0.007 | 9.19±1.77a) | 8.16±2.10a) | 0.003 | ||
| LMD (posterolateral compartment) (mm) | 11.90±3.03 | 11.00±3.20 | <0.001 | 12.00±3.07 | 11.30±3.63 | 0.003 | 11.50±1.91a) | 10.40±2.06a) | 0.001 | ||
| APD (anteromedial compartment) (mm) | 5.30±1.73 | 5.00±1.55 | 0.011 | 5.69±1.24a) | 5.34±1.13a) | 0.058 | 5.10±1.65 | 4.60±1.42 | 0.123 | ||
| LMD (anteromedial compartment) (mm) | 6.67±1.65a) | 6.87±1.44a) | 0.186 | 6.86±1.70a) | 7.14±1.44a) | 0.191 | 6.48±1.58a) | 6.59±1.40a) | 0.586 | ||
| TLMD (mm) | 17.00±4.05 | 16.30±3.92 | 0.001 | 17.80±3.10a) | 17.00±2.70a) | 0.054 | 16.90±2.37a) | 16.00±2.55a) | 0.005 | ||
| Area (mm) | 121.00±46.60 | 106.00±45.10 | <0.001 | 125.00±56.60 | 110.00±44.30 | 0.006 | 119.00±38.90 | 103.00±42.30 | <0.001 | ||
Number is total number of jugular foramina analyzed in the study. APD, anteroposterior diameter; LMD, latero-medial diameter; TLMD, total latero-medial diameter (a sum of LMD posterolateral part and LMD anteromedial part). a)Values described as mean±SD. The other linear measurements were described as median±interquartile range (IQR).
Table 4
| Antimers | Sex | APD (posterolateral compartment) (mm) | LMD (posterolateral compartment) (mm) |
APD (anteromedial compartment) (mm) |
LMD (anteromedial compartment) (mm) | TLMD (mm) | Area (mm) |
|---|---|---|---|---|---|---|---|
| Right (n=128) | Male (n=64) | 9.10±2.63 | 12.00±3.07 | 5.69±1.24a) | 6.86±1.70a) | 17.80±3.10a) | 125.00±56.60 |
| Female (n=64) | 8.90±2.55 | 11.80±2.63 | 5.07±1.14a) | 6.48±1.58a) | 16.90±2.37a) | 119.00±38.90 | |
| P-value | 0.977 | 0.202 | 0.004 | 0.188 | 0.082 | 0.276 | |
| Left (n=128) | Male (n=64) | 7.80±2.15 | 11.00±2.46a) | 5.45±1.43 | 7.14±1.44a) | 17.00±2.70a) | 110.00±44.30 |
| Female (n=64) | 8.10±2.63 | 10.40±2.06a) | 4.60±1.42 | 6.59±1.40a) | 16.00±2.55a) | 103.00±42.30 | |
| P-value | 0.941 | 0.152 | 0.002 | 0.031 | 0.029 | 0.252 | |
| Total (n=256) | Male (n=128) | 8.60±2.40 | 11.50±2.29a) | 5.52±1.20a) | 7.01±1.58a) | 17.40±2.92a) | 114.00±51.30 |
| Female (n=128) | 8.55±2.60 | 11.00±2.05a) | 4.91±1.14a) | 6.54±1.49a) | 16.50±2.49a) | 111.00±38.90 | |
| P-value | 0.962 | 0.084 | <0.001 | 0.014 | 0.005 | 0.139 |
Number is total number of jugular foramina analyzed in the study. APD, anteroposterior diameter; LMD, latero-medial diameter; TLMD, total latero-medial diameter (a sum of LMD posterolateral part and LMD anteromedial part). a)Values described as mean±SD. The other linear measurements were described as median±interquartile range (IQR).




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download