Abstract
The corona of the glans clitoris is a clinically important yet poorly understood anatomical structure. There has been longstanding confusion regarding the prevalence of the corona of the glans clitoris and, moreover, its very existence. Therefore, this anatomical study assesses the prevalence of the corona of the glans clitoris and the gross anatomy of the proximal glans clitoris. Anatomy was assessed in 104 female donor bodies ranging in age from 50 to 102 years with an average age-at-death of 78.1±10.9 years (mean±SD). All clitorises (100%; 104:104 dorsums and 100%; 208:208 sides) were found to have a well-defined clitoral corona. Three of 104 (2.9%) coronas possessed grossly visible, outward-projecting, bluntly rounded papillae. Some donors possessed a coronopreputial frenulum. Clitoropreputial adhesions were common and associated with clitoral pearls. Clitoral pearls were identified in 37.8% (14:37) of unembalmed donors and observed to create clitoral craters, structural deformations in the surface of the corona and glans. The results of this study suggest that the corona of the glans clitoris is a ubiquitous anatomical structure. The clitoral coronal papillae and coronopreputial frenulum are novel, previously undescribed, anatomical structures. This study identifies that the corona of the glans clitoris is prone to pathological processes such as clitoral pearl formation and clitoral deformation. In addition to novel anatomical findings, the results of this study call attention to the need for life-long clitoral examinations. Furthermore, the corona of the glans clitoris should be regularly included in anatomical texts and accurately depicted in anatomical illustrations.
The corona of the glans clitoris (Latin: corona glandis clitoridis) (CGC) has been utilized as an anatomical landmark for varied urologic and gynecologic surgeries (e.g., clitoroplasty) [1-6], assessment and management of clitoral adhesions [7, 8], measurement of the clitoral index for the determination of clitoromegaly and, accordingly, the identification of endocrinopathies including congenital adrenal hyperplasia, androgen-producing tumors, and polycystic ovarian syndrome [9, 10]. However, despite the purported clinical importance of the CGC, there has been longstanding confusion regarding its prevalence and, moreover, its very existence.
The CGC was first documented in German anatomical texts written in the late-18th to middle-19th century [11-14]. A century later, references to the CGC appeared in the English language; however, the reports mentioning the CGC were sparse and inconsistent. For example, in an 1899 dissection manual, Eckley and Eckley [15, 16] instruct to, “cut the skin along the dorsum of the clitoris for an inch upward. Then you can see the corona glandis and the glans clitoris in full view” [15, 16]. Yet, in 1914, Pratt [17] unambiguously notes that “the glans clitoridis has no corona.”
In modern times, confusion regarding the CGC continues. A 1992 in vivo anatomical study detailed the length of the glans clitoris in a sample of 200 females by measuring from the “tip to the back of the corona” [9]. The study makes no mention of any absence of a corona from which to measure, thus implying that the CGC is a typical anatomical structure [9]. Yet, in 2014, Di Marino and Lepidi [18] explicitly note that the corona of the glans clitoris exists as part of some, but not all, clitorises.
Perhaps due to the longstanding confusion regarding the presence of the CGC or the dearth of its mention in anatomical texts, the term corona glandis clitoridis has not been included in Terminologia Anatomica (TA), the compendium of anatomical terminology formally accepted by the Federative International Programme for Anatomical Terminology (FIPAT) of the International Federation of Associations of Anatomists (IFAA) [19]. Likewise, the CGC was not mentioned in the recent recommendations for standardized anatomic terms for the anatomy of the female pelvis and vulva by the Society for Gynecologic Surgeons (SGS) Pelvic Anatomy Group (PAG) [20-24]. Consequently, the absence of the corona glandis clitoridis from TA and SGS PAG recommendations for standardized anatomical terminology might imply that the corona is not typical anatomy and that it is, perhaps, an anatomical variation, a result of developmental dysmorphogenesis, a pathological finding, a nonexistent body part, or that it is not important enough to be listed.
Despite the confusion regarding the presence of the CGC, and despite the lack of formally recognized terminology specific to the anatomy of the glans clitoris, the terms corona and coronal sulcus continue to be used in the modern-day clinical literature as they relate to the clitoris, especially in the context of disorders of sex development (DSD) (also known as differences in sex development, variations in sex characteristics, and diverse sex development) [1-10, 25-28].
A lack of anatomical study regarding the glans clitoris has resulted in confusion regarding the corona. Therefore, morphological study of the glans clitoris and assessment of the CGC is warranted. Hence, this research aims to identify the prevalence and gross morphology of the CGC.
The research was approved by the West Virginia Anatomical Board. A total of 104 female donor bodies, accessed through the West Virginia Human Gifts Registry, and ranging in age-at-death from 50 to 102 years were included in the study. The average age-at-death of the population sample was 78.1±10.9 years (mean±SD). Of the 104 donors, 64 were embalmed (age-at-death ranging from 50–100; average age 77.4±10.9 years) and 40 were not embalmed (age-at-death ranging from 53–102; average age 79.2±11.0 years) and assessed shortly after death. Prior to death, two donors self-reported their race as “black,” and 102 donors self-reported their race as “white” on donation forms.
The anatomy of the glans clitoris was assessed in order to detail the proximal aspect of the glans clitoris and adjacent structures. The assessments were performed with donor bodies supine. Thighs of the donors were abducted to facilitate visualization. If the prepuce of the clitoris was obscured by the mons pubis, the mons pubis was bisected to the extent necessary to reveal the prepuce. Retraction of the prepuce was attempted in order to visualize underlying anatomy. The proximal-most aspect of the glandopreputial sulcus (balanopreputial sulcus) was identified with a blunt probe. Then, the prepuce was bisected, distal-to-proximal, to its reflection (i.e., the proximal-most aspect of the glandopreputial sulcus). Substances that occupied the glandopreputial sulcus, such as smegma, were removed, and tissue adhesions were broken to visualize native tissues. To improve visualization, the skin overlying the body of the clitoris was also bisected from distal to proximal, as needed. Upon clear visualization, the clitoris was assessed around the full extent of the glans (104 dorsal aspects, 104 left sides, and 104 right sides). The prevalence of clitoral pearls was documented in the sample of recently deceased unembalmed donors (n=40). In situ macrophotography was performed with a digital camera (Canon PowerShot SX50 HS, 12.1 Megapixel). Ex situ stereomicroscopy was performed in the sample of embalmed donors with a Leica S8AP0 microscope and Leica EC3 camera. Stereoscopic images were captured with LAS EZ software.
In 104 of 104 (100%) donor bodies, a CGC was present at the proximal-most aspect of the glans (Fig. 1). The corona was identified at 104 of 104 dorsums and along each side of each clitoris (208:208; 100% of sides). Likewise, a coronal sulcus (retroglandular sulcus) was able to be visualized immediately proximal to each corona. In all cases, the corona and coronal sulcus were oriented in an oblique plane relative to the axis of the body of the clitoris (corpus clitoridis).
In all donors, the corona was crescentic and oblong; its surface structure did not form a complete ellipse. Accordingly, each corona did not encompass the entirety of the glans. The maximum of the convexity of the incomplete ellipse was always at the midsagittal point, the posterior-most aspect of the glans. At the midsagittal point, a dorsal frenulum was occasionally observed to connect the inner aspect of the prepuce (i.e., hood) with the corona (Fig. 2).
The crescentic coronas tended to taper, to varied degrees, at their distal-ventral-most extremities where they seemingly blended and embedded into surrounding structures. The periphery of each corona protruded outward and was, thus, convex like that of the periphery of a torus. The peripheral convexities of some coronas were exaggerated by a concavity immediately distal to the corona.
Three of 104 (2.9%) coronas possessed outward-projecting bluntly rounded papillae (Fig. 3). Papillae were organized inline or in parallel rows along the corona (Fig. 3). Some papillae had pearly, pale-colored apices (Fig. 3). Such grossly visible papillae resembled so-called pearly penile papules in relative location (corona), shape (bluntly rounded), arrangement (denticulate and in linear rows with papillae separated at their bases), and color (uniform, some with pearly, pale-colored tips) (Fig. 3). Conical papillae were also observed (Fig. 3C).
All donors were found to have an intact glans and prepuce, and there was no evidence of genital mutilation including clitoridectomy, prior preputial surgery, or developmental variations such as aposthia analogous to that which is rarely identified in males [29-31]. Accordingly, the prepuce abutted the glans clitoris in all cases.
Clitoropreputial adhesions (CPAs) were commonly observed. After retraction and separation of the prepuce from the glans, a crescentic fringe of remnant tissue that delineated the junction between the lining of the clitoris and the inner lining of its overlying prepuce allowed for localizing the CPA relative to the corona (Fig. 4). The distal margin of the CPA was most often located along the distal-most aspect of the corona and formed a well-delineated boundary between the corona and the distal glans. However, CPAs that were relatively more distal were also observed and were often accompanied by proximally located clitoral pearls (Figs. 4–6). In such cases, the CPA margins were often irregular or crenulated.
Upon separation of the prepuce from the entirety of the glans (i.e., proximal to the corona) the zone of prior adhesion could be easily visualized. The surface area of the glans that was adherent to the prepuce was often observed to be greater than the surface area of the non-adherent, easily observable glans (Figs. 4–6).
Among the unembalmed, recently deceased sample, clitoral pearls were observed in 14 of 37 (37.8%) (three of the total sample of 40 were excluded from assessment because the extent of dissection necessary to visualize the corona was enough to potentially disrupt clitoral pearls and, thus, influence observations of the pearls). Clitoral pearls were rarely seen among individuals without CPAs. Conversely, among donors who possessed CPAs, clitoral pearls were relatively common in the zone of prior adhesion, proximal to the distal-most CPA margin (Figs. 4–6). Relatively more pronounced clitoral pearls tended to be observed in those with more distal CPAs (Figs. 4, 5). Relatively less pronounced clitoral pearls, sometimes approximating the size of the coronal papillae, were more often observed in individuals with more proximal CPAs (Fig. 6B).
Upon removal of clitoral pearls, it was observed that they often produced craters in the clitoris and, less commonly, craters in the abutting preputial tissue (Fig. 6). The clitoral craters were resistant to tissue manipulation and appeared to be a result of structural change to native tissues (Fig. 6). Craters were most commonly located in the corona and immediately distal to the corona (Fig. 6).
Gross anatomical study of the glans clitoris has been largely neglected throughout history. Indeed, aside from the assessment of clitoral dimensions, this anatomical study is the first to focus specifically on the surface anatomy of the proximal glans clitoris and corona of the glans. This study is also the first to document the prevalence of the CGC—finding the presence of a CGC in all individuals studied.
This study documents the presence of papillae along the surface of the clitoral corona—a novel anatomical variation with clinical implications. Likewise, this report is the first to identify dorsal frenulae spanning from the hood of the clitoris to the corona.
The common identification of CPAs, the identification of clitoral pearls in 37.8% of individuals, and the presence of clitoral deformations is especially noteworthy. Such findings stress that this study represents underrepresented anatomy (the clitoris), in an underrepresented age group (>50 years of age, mostly geriatric, and primarily post-menopausal), of underrepresented people (females). Accordingly, the results of this study emphasize the need for life-long clitoral examinations.
Historically, the CGC has been thought to be non-existent, an anatomical variant, or a characteristic of a pathologic process [9, 10, 17, 18]; however, the results of this study suggest that the CGC is typical non-variant anatomy.
The results of this study suggest that the CGC is ubiquitous anatomy; though, in some, the CGC may not be easily apparent and may be obscured by the crescentic adherence of tissues that tether the prepuce to the glans. Thus, the commonality of coronopreputial adhesions may be implicated in the widespread and longstanding confusion regarding the prevalence and existence of the CGC.
The adherence between the corona of the glans and the prepuce may be likened to that which is generally well-known and commonly seen between the glans and prepuce in the neonate male [32-36], whose glans is approximate in size to that of the adult female [37]. In the neonate male, the glans and prepuce are typically adherent and, over time, the production of smegma facilitates the breaking of adhesions and delamination [32-34]. The smegma-facilitated process of delamination in the neonate male is not always successful and may result in smegma entrapment and pearl production [32-34].
The same process that is common to the neonate male may be occurring in adult females (and likely females of any age, including neonates), though without the same typical result of complete delamination and full exposure of the corona and coronal sulcus, which, in males, is facilitated by pronounced growth of the phallus. Thus, the intentional breaking of CPAs may represent another means of delamination (though, like in neonatal males, intentional breaking of adhesions may cause inflammation and promote subsequent adhesions). Yet, upon successful delamination (or breaking of adhesions), the full corona may be visualized, as evidenced in this study.
The gross observations of this report demonstrate that the corona of the glans may be easily observable in the absence of adhesions, or otherwise obscured to varied degree by adherence between the prepuce and the glans. These observations are consistent with the histological study of glandopreputial sulci in adult females by van der Putte and Sie-Go [38] that identified 1) a completely opened sulcus lined by stratified squamous epithelium with complete horny layers on both the glans and prepuce or alternatively having a non-cornifying epithelium at the deep prepuce; 2) a partially open and partially “solid” (i.e., partially closed) sulcus; and 3) a “solid” glandopreputial lamella resulting in a closed glandoopreputial sulcus. The completely open glandopreputial lamella, described by van der Putte and Sie-Go [38], corresponds to the grossly visible CGC that requires neither delamination nor breaking of adhesions to view, such as those seen in Fig. 1 of this report. Likewise, the partially “solid” or “solid” glandopreputial lamellae described by van der Putte and Sie-Go [38] correspond to those grossly obscured CGC that do require delamination or breaking of adhesions in order to view, such as those seen in Figs. 4–6 of this report.
Considering the findings of this study alongside histological findings [38] and with consideration for the analogous, well-established condition of preputial adhesions that occurs among males during a transient developmental stage when homologous structures are approximately similar in size to those of adult females [32-37], the author posits that the distalmost aspect of the semi-circumferential clitoropreputial “adherence” may otherwise represent the periphery of a persistent preputial lamella which has not fully delaminated, or at least has not remained delaminated over time. Thus, the black arrowheads seen in Figs. 4–6, may indicate persistent preputial lamellar margins and, accordingly, the post-adhesion remnants of tissue that identify clitoropreputial delamination margins.
Due to its approximation with the prepuce and glandopreputial sulcus, the clitoris is predisposed to adhesion development and phimosis with sequestration of desquamated epithelial cells, smegma, and keratin pearls which can result in vulvovaginal irritation and clitorodynia/vulvodynia that has been described akin to “having a grain of sand in the eye” [39, 40]. Further, clitoral adhesions may be indicative of local trauma, dermatologic disorders (e.g., lichen sclerosis), and varied infections [8, 28, 40, 41]. Adhesions also predispose the development of infection [28]. Likewise, adhesions have been associated with a history of dyspareunia, blunt perineal or genital trauma, decreased free testosterone, menopause, long-term oral contraceptive use, and sexual dysfunctions including persistent genital arousal disorder/genitopelvic dysesthesia [8]. Accordingly, it is important to differentiate normal anatomy, or normal contact between the clitoris and the prepuce, from pathological adhesion.
Clinicians must be aware of anatomical variations regarding the depth of the glandopreputial sulcus. This study agrees with Aerts et al. [7] who have suggested that adhesions should be suspected if the corona is not fully visualized. Likewise, Radke and Stockdale [28] as well as Winter and Rubin [42] note that an inability to visualize the corona with retraction of the prepuce is diagnostic for clitoral adhesions. Additionally, the author suggests that what may be considered a preputial “adhesion” may likewise be a condition of incomplete delamination.
The lack of full exposure of the glans in the setting of CPAs or incomplete delamination would undoubtedly affect the function of the local tissues and thus affect sensory functions implicated in sexual sensation and stimulation. It is important to emphasize that the surface area of the adhesion zone is oftentimes greater than that of the non-adherent surface area of the glans (Figs. 4–6). The pronounced lack of surface exposure of the glans and prepuce as well as hinderance of free movement between the glans and prepuce likely reflects a diminished capacity for optimal glans and prepuce sensory function relative to the non-adherent, fully exposed, and freely translating glans and prepuce.
Likewise, the presence of adhesions, along with clitoral pearls, cysts, and craters, would likely affect sensory functions implicated in sexual sensation and stimulation. Adhesions and subsequent sequestration of smegma and development of clitoral pearls (including smegmaliths) may predispose one to dyspareunia. Further, CPAs are associated with pain and difficulties with arousal and orgasm [43]. Thus, in the setting of sexual dysfunction, it is prudent to recognize and assess the interface between the prepuce and glans including the anatomy surrounding the CGC. Moreover, sexual dysfunction may be prevented by prophylactic measures including regular anatomical screenings throughout life and education with regard to the anatomy of the clitoris that includes knowledge of the corona of the glans and coronal sulcus.
The assessment of clitoral anatomy is important to appreciate from an endocrinological perspective. With regard to CPAs, hypoestrogenism is thought to be implicated [40]. Likewise, the clitoral index, calculated by measurement of the glans clitoris, is reflective of both endogenous and exogenous androgen exposure [9, 10]. Accordingly, the clitoral index is used to identify clitoromegaly and myriad endocrinopathies including congenital adrenal hyperplasia, androgen-producing tumors, polycystic ovarian syndrome, and masculinizing neoplasms of the ovary or adrenal glands, as well as utilization of masculinizing steroids [9, 10].
The clitoral index measures the size of the glans clitoris [9, 44, 45]. Thus, the clitoral index would be difficult to measure in the clinical setting among those with obscured coronas. Therefore, this research brings into question the accuracy of the methodology and the interpretation of the clitoral index. Clitoral index measurements have likely misrepresented the actual size of the glans since the clitoral index cannot be properly ascertained without recognition and visualization of the corona.
Like the adage of measuring the full size of an iceberg by only measuring its dimensions above the water, the mensuration of the glans in vivo, without full view of the corona, likely under-measures the true size of the glans among a given population. Thus, clinically, visual inspection of the glans clitoris is only revealing the “tip of the iceberg” in some cases. Indeed, the glans itself is often considered the “tip of the iceberg” with regard to the clitoris; thus, to continue with the idiom—the assessment of the glans without visualization of the corona only reveals the “tip of the tip of the iceberg.” And, apparently, anatomical research and clinical assessment regarding the clitoris have advanced at a glacial pace.
Vestibular papillomatosis has been described as a condition homologous to pearly penile papules [46, 47]. However, this study suggests that clitoral coronal papillae or “pearly clitoral papules” might better fit such a comparison, at least with regard to location.
Condyloma acuminatum is common at the site of the corona of the glans penis [48, 49]. Perhaps the same is true of the corona of the glans clitoris. However, due to the widespread lack of knowledge regarding even the existence of the corona of the glans clitoris, it is likely that condyloma acuminatum is under-recognized at this location. Furthermore, the coronal sulcus may serve as a pathogenic reservoir for human papillomavirus and other pathogens which include those with oncogenic potential. Thus, in addition to a potentially under-recognized location for benign neoplasia, the corona of the glans clitoris, the coronal sulcus, neck of the clitoris, and the extent of the body of the clitoris identifiable per glandopreputial sulcus may also be an under-recognized site of malignant neoplasm. Additionally, lesions at the corona of the glans clitoris or coronal sulcus and nearby under-recognized sites represent a mode of ingress and egress of other pathogens including human immunodeficiency virus, for example. Such pathogens may be transferred during sexual contact and childbirth. Thus, thorough assessment of the corona and the coronal sulcus is important in limiting the spread of infection.
From the perspective of masquerading as a pathology and the establishment of a differential diagnosis, clitoral papillae should not be mistaken for genital warts (condyloma acuminata), molluscum contagiosum, lichen nitidus, and sebaceous hyperplasia [50]. Likewise, clitoral papillae should not be mistaken for clitoral pearls which, as identified in this study, can be particularly small (Fig. 6).
It must also be noted that, in all cases where coronal papillae were identified in this study, there was an absence of clitoral pearls and clitoral craters. Therefore, clitoral papillae might afford the corona with an irregular surface that promotes delamination, confers resistance to preputial adherence, and promotes the free movement between the prepuce and the clitoris.
This report is the first to identify the presence of clitoral coronal papillae. The language used to describe similar structures of the penis includes pearly penile papules, papillomatosis corona penis, corona capillitia, hirsuties coronas glandis, papillae coronis glandis, and hirsutoid papillomas [50]. Thus, if female-specific language is desired, terms such as pearly clitoral papules and papillomatosis corona clitoris may be preferred. Otherwise, terms such as hirsuties coronas glandis may be applied regardless of sex or phallus phenotype including among individuals with DSD. Without regard for sex, the author recommends describing the structures as coronal papillae and the condition of having such papillae as papillomatosis of the corona. With regard for sex, the author recommends describing the structures as clitoral coronal papillae and the condition of having such papillae as papillomatosis of the clitoral corona.
The presentation of clitoral papillae seen in this study suggests that coronal papillae are generally smaller in females than those typically found among males; however, more anatomical research is necessary to determine similarities and differences regarding sexual dimorphism as well as to assess whether papillae are allometrically proportionate to glans or CGC size regardless of sex. Moreover, in addition to further gross anatomical study, histologic study of clitoral coronal papillae is warranted.
Bringing awareness of the existence of the corona of the glans clitoris may increase its examination in the clinical setting and, therefore, improve diagnostics. Awareness may be enhanced by including the corona of the glans clitoris (corona glandis clitoridis) in the anatomical language espoused and described by, for example, the FIPAT of the IFAA, the SGS PAG, and textbooks of anatomy. It is time to formally coronate the clitoris.
Illustrations of the clitoris rarely include the corona and are, accordingly, incorrect and misleading. Indeed, the proximal aspect of the glans clitoris resembles that of the glans penis, a homologous anatomical structure that is regularly and accurately represented in illustrations of male anatomy. Henceforth, illustrations of the clitoris should include the corona of the glans and the coronal sulcus.
This report suggests that the corona of the glans clitoris is typical anatomy, and its ubiquitous prevalence is similar to that of the corona of the glans penis. However, in some individuals, a semi-circumferential adhesion, or region of non-delamination, between the lining of the clitoris and the inner aspect of the prepuce may obscure the view of the corona. Also, in some individuals, papillae may be present along the corona of the glans clitoris. Care should be taken to differentiate papillae along the corona of the glans clitoris from pathologies including clitoral pearls that have the potential to deform the corona and glans, in general. This research provides novel anatomical knowledge, including the first description of coronal papillae of the glans clitoris and the coronopreputial frenulum and remedies the longstanding historical confusion regarding the corona of the glans clitoris.
Acknowledgements
The author would like to acknowledge the West Virginia Anatomical Board and the West Virginia University Human Gift Registry. For assistance with laboratory and donor access, the author wishes to acknowledge Robert J. Bolyard and Anthony Cress of the West Virginia University Human Gift Registry, Holly Vopal, PhD of West Liberty University, and Amanda Troy, PhD of Duquesne University College of Osteopathic Medicine. Most importantly, the author would like to acknowledge the individuals who donated their bodies for the advancement of science and healthcare, without whom, this work would not have been possible.
Notes
REFERENCES
1. Allen LE, Hardy BE, Churchill BM. 1982; The surgical management of the enlarged clitoris. J Urol. 128:351–4. DOI: 10.1016/S0022-5347(17)52923-7. PMID: 7109108.

2. Farkas A, Chertin B, Hadas-Halpren I. 2001; 1-Stage feminizing genitoplasty: 8 years of experience with 49 cases. J Urol. 165(6 Pt 2):2341–6. DOI: 10.1016/S0022-5347(05)66199-X. PMID: 11371974.

3. Perovic SV, Djordjevic ML. 2003; Metoidioplasty: a variant of phalloplasty in female transsexuals. BJU Int. 92:981–5. DOI: 10.1111/j.1464-410X.2003.04524.x. PMID: 14632860.

4. Lesma A, Bocciardi A, Montorsi F, Rigatti P. 2007; Passerini-glazel feminizing genitoplasty: modifications in 17 years of experience with 82 cases. Eur Urol. 52:1638–44. DOI: 10.1016/j.eururo.2007.02.068. PMID: 17408848.

5. Kujur AR, Joseph V, Chandra P. 2016; Nerve sparing clitoroplasty in a rare case of idiopathic clitoromegaly. Indian J Plast Surg. 49:86–90. DOI: 10.4103/0970-0358.182241. PMID: 27274128. PMCID: PMC4878251.

6. Adams MC, DeMarco RT, Thomas JC. Docimo SG, Canning D, Khoury A, Salle JLP, editors. Surgery for intersex disorders and urogenital sinus. The Kelalis-King-Belman textbook of clinical pediatric urology. 6th ed. CRC Press;2018. p. 1215–31.
7. Aerts L, Rubin RS, Randazzo M, Goldstein SW, Goldstein I. 2018; Retrospective study of the prevalence and risk factors of clitoral adhesions: women's health providers should routinely examine the glans clitoris. Sex Med. 6:115–22. DOI: 10.1016/j.esxm.2018.01.003. PMID: 29559206. PMCID: PMC5960030.

8. Myers MC, Romanello JP, Nico E, Marantidis J, Rowen TS, Sussman RD, Rubin RS. 2022; A retrospective case series on patient satisfaction and efficacy of non-surgical lysis of clitoral adhesions. J Sex Med. 19:1412–20. DOI: 10.1016/j.jsxm.2022.06.011. PMID: 35869023.

9. Verkauf BS, Von Thron J, O'Brien WF. 1992; Clitoral size in normal women. Obstet Gynecol. 80:41–4. PMID: 1603495.
10. Nigam A, Prakash A, Saxena P, Yadav R, Raghunandan C. 2011; Hirsutism and abnormal genitalia. J Indian Acad Clin Med. 12:46–8.
11. Mayer JCA. Beschreibung des ganzen menschlichen körpers, mit den wichtigsten neueren anatomischen entdeckungen bereichert, nebst physiologischen erläuterungen. G. J. Decker. 1788. German.
12. Bernstein JG. Handbuch nach alphabetischer ordnung über die vorzüglichsten gegenstände der anatomie, physiologie und gerichtliche arzneigelahrtheit, für praktische wundärzte. Schwickertschen Verlage. 1794. German.
13. Hempel AF. Anfangsgründe der anatomie. Johann Christian Daniel Schneider. 1801. German.
14. Moser A. Lehrbuch der geschlechtskrankheiten des weibes, nebst einem anhange, enthaltend die regeln für die untersuchung der weiblichen geschlechtstheile. Nach den neuesten quellen und eigener erfahrung. Hirschwald. 1843. German.
15. Eckley WT, Eckley CB. Practical anatomy, including a special section on the fundamental principles of anatomy. P. Blakiston's Son & Co.;1899. DOI: 10.5962/bhl.title.29837.
16. Eckley WT, Eckley CB. A manual of dissection and practical anatomy: founded on Gray and Gerrish. Lea Brothers & Company;1903. DOI: 10.1097/00000441-190305000-00023.
17. Pratt EH. 1914; Sympathetic nerve waste. JAAOS. 2:62–6.
18. Di Marino V, Lepidi H. Di Marino V, Lepidi H, editors. External morphology of the clitoris. Anatomic study of the clitoris and the bulbo-clitoral organ. Springer;2014. p. 27–38. DOI: 10.1007/978-3-319-04894-9_4.
19. Federative International Programme for Anatomical Terminology. Terminologia anatomica. 2nd ed. International Anatomical Terminology;2019.
20. Jeppson PC, Balgobin S, Washington BB, Hill AJ, Lewicky-Gaupp C, Wheeler T 2nd, Ridgeway B, Mazloomdoost D, Balk EM, Corton MM, DeLancey J. Society of Gynecologic Surgeons Pelvic Anatomy Group. 2018; Recommended standardized terminology of the anterior female pelvis based on a structured medical literature review. Am J Obstet Gynecol. 219:26–39. DOI: 10.1016/j.ajog.2018.04.006. PMID: 29630884.

21. Hill AJ, Balgobin S, Mishra K, Jeppson PC, Wheeler T 2nd, Mazloomdoost D, Anand M, Ninivaggio C, Hamner J, Bochenska K, Mama ST, Balk EM, Corton MM, Delancey J. Society of Gynecologic Surgeons Pelvic Anatomy Group. 2021; Recommended standardized anatomic terminology of the posterior female pelvis and vulva based on a structured medical literature review. Am J Obstet Gynecol. 225:169.e1–16. DOI: 10.1016/j.ajog.2021.02.033. PMID: 33705749.

22. Zdilla MJ. 2022; Recommended standardized anatomic terminology of the posterior female pelvis and vulva. Am J Obstet Gynecol. 227:118. DOI: 10.1016/j.ajog.2022.02.018. PMID: 35183502.

23. Zdilla MJ. 2022; What is a vulva? Anat Sci Int. 97:323–46. DOI: 10.1007/s12565-022-00674-7. PMID: 35704265.

24. Hill AJ, Balgobin S, Delancey J. 2022; Reply: Further discussion and clarification of female vulvar anatomy. Am J Obstet Gynecol. 227:119. DOI: 10.1016/j.ajog.2022.02.017. PMID: 35183501.

25. Goldstein I, Meston CM, Davis S, Traish A. Women's sexual function and dysfunction: study, diagnosis and treatment. Taylor & Francis;2005. DOI: 10.1201/b14618.
26. Khatun S. Principles and practices of premalignant and malignant disorders of vulva. Jaypee Brothers Medical Publishers;2019.
27. Fahmy MAB. Normal and abnormal prepuce. Springer;2020. DOI: 10.1007/978-3-030-37621-5.
28. Radke SM, Stockdale CK. Goldstein AT, Pukall CF, Goldstein I, Krapf JM, Goldstein SW, Goldstein G, editors. Chronic clitoral pain and clitorodynia. Female sexual pain disorders: evaluation and management. 2nd ed. John Wiley & Sons;2020. p. 375–80. DOI: 10.1002/9781119482598.ch41.
29. Sadeghipour Roudsari S, Esmailzadehha N. 2010; Aposthia: a case report. J Pediatr Surg. 45:e17–9. DOI: 10.1016/j.jpedsurg.2010.05.030. PMID: 20713198.

30. Nayak SB, Pai SU, Shenoy MG, Reghunathan D. 2018; Accessory fold of skin on the ventral surface of the penis: is it a redundant prepuce? Andrologia. 50:e12941. DOI: 10.1111/and.12941. PMID: 29315704.

31. Esse I, Kincaid CM, Terrell CA, Mesinkovska NA. 2023; Female genital mutilation: overview and dermatologic relevance. JAAD Int. 14:92–8. DOI: 10.1016/j.jdin.2023.07.022. PMID: 38352964. PMCID: PMC10862004.

32. Deibert GA. 1933; The separation of the prepuce in the human penis. Anat Rec. 57:387–99. DOI: 10.1002/ar.1090570409.

33. Gairdner D. 1949; The fate of the foreskin, a study of circumcision. Br Med J. 2:1433–7. illust. DOI: 10.1136/bmj.2.4642.1433. PMID: 15408299. PMCID: PMC2051968.
34. Sonthalia S, Singal A. 2016; Smegma pearls in young uncircumcised boys. Pediatr Dermatol. 33:e186–9. DOI: 10.1111/pde.12832. PMID: 27071486.

35. Baskin L, Shen J, Sinclair A, Cao M, Liu X, Liu G, Isaacson D, Overland M, Li Y, Cunha GR. 2018; Development of the human penis and clitoris. Differentiation. 103:74–85. DOI: 10.1016/j.diff.2018.08.001. PMID: 30249413. PMCID: PMC6234061.

36. Liu X, Liu G, Shen J, Yue A, Isaacson D, Sinclair A, Cao M, Liaw A, Cunha GR, Baskin L. 2018; Human glans and preputial development. Differentiation. 103:86–99. DOI: 10.1016/j.diff.2018.08.002. PMID: 30245194. PMCID: PMC6234068.

37. Puri A, Sikdar S, Prakash R. 2017; Pediatric penile and glans anthropometry nomograms: an aid in hypospadias management. J Indian Assoc Pediatr Surg. 22:9–12. DOI: 10.4103/0971-9261.194610. PMID: 28082769. PMCID: PMC5217151.

38. van der Putte SC, Sie-Go DM. 2011; Development and structure of the glandopreputial sulcus of the human clitoris with a special reference to glandopreputial glands. Anat Rec (Hoboken). 294:156–64. DOI: 10.1002/ar.21279. PMID: 21157926.

39. Krapf J, Mautz T, Lorenzini S, Holloway J, Goldstein A. 2022; Clinical presentation of clitorodynia associated with clitoral adhesions and keratin pearls. J Sex Med. 19(8 Suppl 3):S14. DOI: 10.1016/j.jsxm.2022.05.032.

40. Bragiel RM, Umasankar N, Burgis JT, Tomlin KV. 2023; Treatment of clitoral keratin pearls with topical estrogen cream: case report. J Pediatr Adolesc Gynecol. 36:321–3. DOI: 10.1016/j.jpag.2022.10.002. PMID: 36209998.

41. Winfrey OK, Fei YF, Dendrinos ML, Rosen MW, Smith YR, Quint EH. 2022; Lichen sclerosus throughout childhood and adolescence: not only a premenarchal disease. J Pediatr Adolesc Gynecol. 35:624–8. DOI: 10.1016/j.jpag.2022.08.011. PMID: 36038010.

42. Winter AG, Rubin RS. Goldstein AT, Pukall CF, Goldstein I, Krapf JM, Goldstein SW, Goldstein G, editors. Vulvoscopic evaluation of vulvodynia. Female sexual pain disorders: evaluation and management. 2nd ed. John Wiley & Sons;2020. p. 125–31. DOI: 10.1002/9781119482598.ch15.
43. Romanello JP, Myers MC, Nico E, Rubin RS. 2023; Clitoral adhesions: a review of the literature. Sex Med Rev. 11:196–201. DOI: 10.1093/sxmrev/qead004. PMID: 36973166.

44. Tagatz GE, Kopher RA, Nagel TC, Okagaki T. 1979; The clitoral index: a bioassay of androgenic stimulation. Obstet Gynecol. 54:562–4. PMID: 503381.
45. Sane K, Pescovitz OH. 1992; The clitoral index: a determination of clitoral size in normal girls and in girls with abnormal sexual development. J Pediatr. 120(2 Pt 1):264–6. DOI: 10.1016/S0022-3476(05)80439-1. PMID: 1735824.

46. Ackerman AB, Kronberg R. 1973; Pearly penile papules. Acral angiofibromas. Arch Dermatol. 108:673–5. DOI: 10.1001/archderm.1973.01620260023007. PMID: 4356233.

47. Agharbi FZ, Kelati A, Chiheb S. 2022; The role of dermatoscopy to differentiate vestibular papillae from condyloma acuminate in a pregnant woman. Clin Med Rev Case Rep. 9:386. DOI: 10.23937/2378-3656/1410386.

48. Cook LS, Koutsky LA, Holmes KK. 1993; Clinical presentation of genital warts among circumcised and uncircumcised heterosexual men attending an urban STD clinic. Genitourin Med. 69:262–4. DOI: 10.1136/sti.69.4.262. PMID: 7721284. PMCID: PMC1195083.

49. Shah RB, Amin MB. Zhou M, Magi-Galluzzi C, editors. Diseases of the penis, urethra, and scrotum. Genitourinary pathology. Churchill Livingstone;2006. p. 419–76. DOI: 10.1016/B978-0-443-06677-1.50013-2.
50. Love LW, Badri T, Ramsey ML. Aboubakr S, Abu-Ghosh A, Adibi Sedeh P, Aeby TC, Aeddula NR, Agadi S, editors. Pearly penile papule. StatPearls. StatPearls Publishing;2022. DOI: 10.23937/2378-3656/1410386.
Fig. 1
Anatomy of the glans clitoris featuring the corona of the glans clitoris and the coronal sulcus. (A, B) Anterior and left dorsolateral views of the glans clitoris in a 90-year-old embalmed white female donor with a bisected prepuce. (C, D) Dorsal and right lateral view the glans clitoris in a 64-year-old recently deceased, unembalmed, white female donor with prepuce intact and retracted. C, corona; CS, coronal sulcus; F, frenulum/frenulae; G, glans clitoris; GPS, glandopreputial sulcus/balanopreputial sulcus; Lmaj, labium majus; Lmin, labium minus; P, prepuce; P*, prepuce bisected.
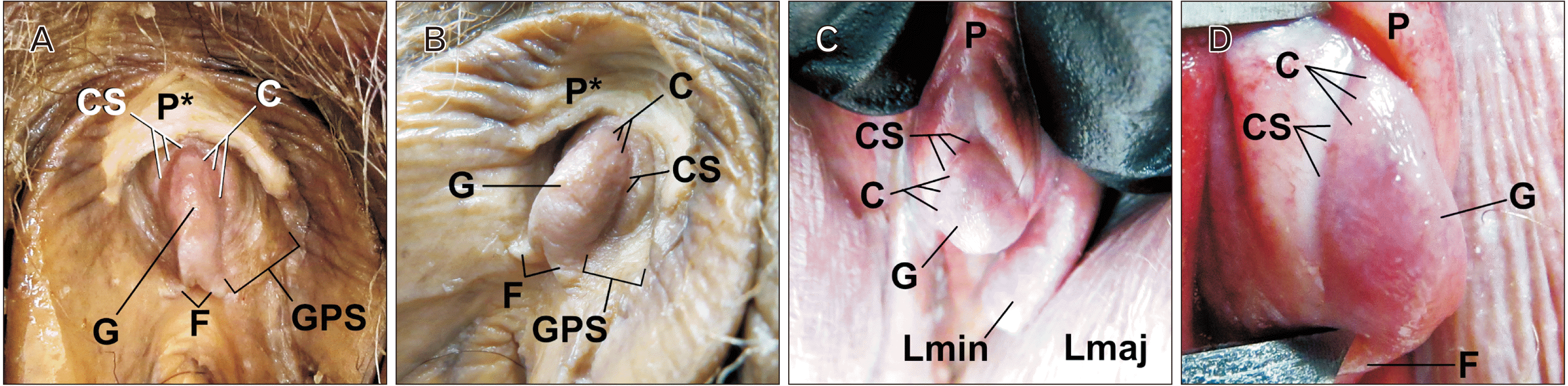
Fig. 2
Coronopreputial frenulae (white arrowheads) joining the corona of the glans clitoris (black stars) to the prepuce of the clitoris from dorsal and left lateral views. The superimposed black boxes correspond to adjacent magnified views. (A) Coronopreputial frenulum in a 95-year-old unembalmed white female donor. (B) Coronopreputial frenulum in a 71-year-old embalmed white female donor.
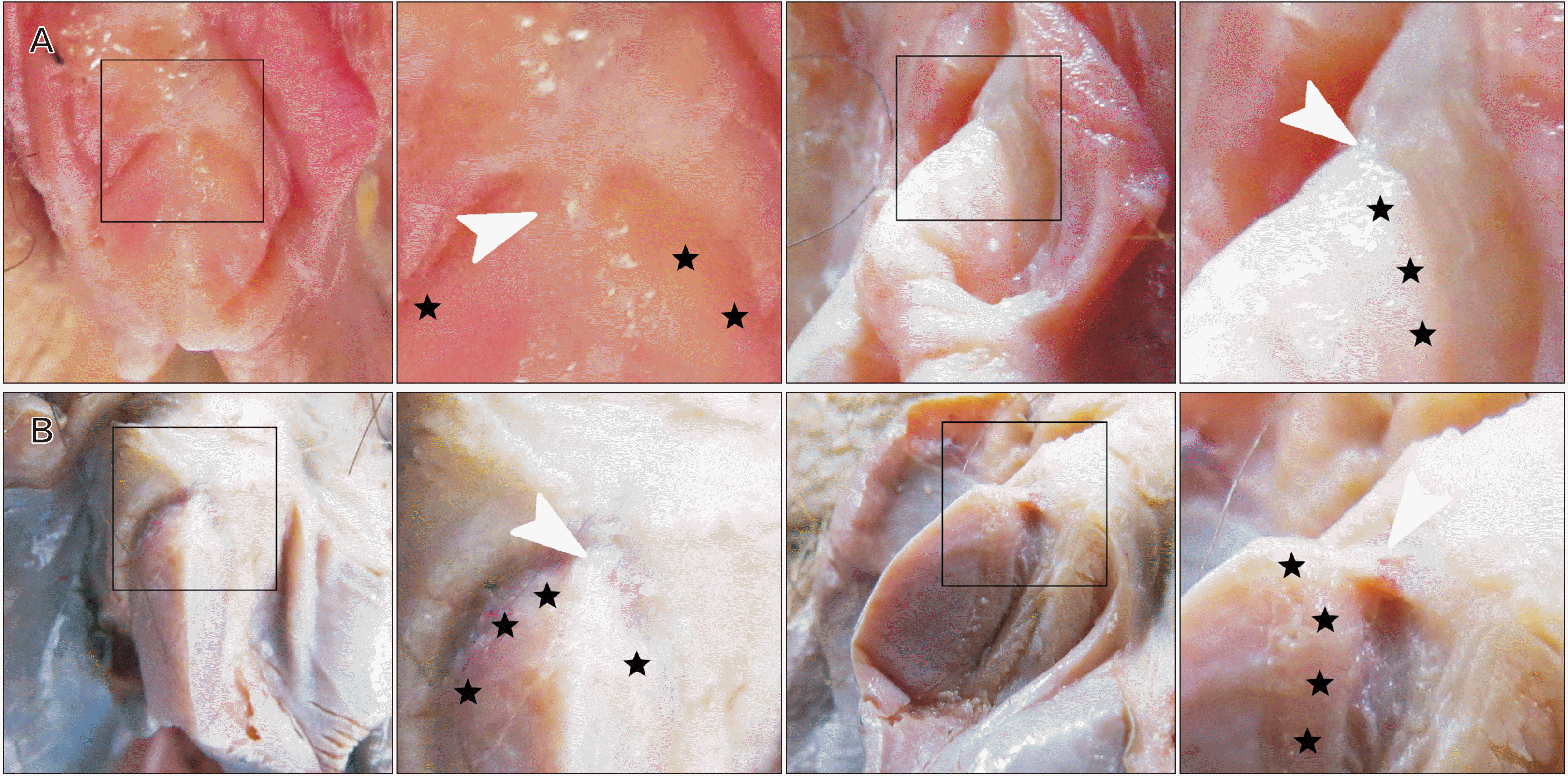
Fig. 3
Stereomicroscopy of three clitorises with denticulate papillae located along the periphery of each corona. Each row of images represents a series of increasing magnification from left to right. Superimposed black boxes correspond to adjacent magnified views. (A) Corona of an 86-year-old white female with marked papillae, arranged in-line, creating a fimbriated ridge along the distal aspect of the corona. Papillae are bluntly rounded and are separated from one another at their bases. Some papillae have pale-colored tips and independent bases, representing “pearly” papillae. (B) Corona of an 81-year-old white female which possesses papillae that are arranged in several rows, projecting distally from the corona. (C) Corona of a 75-year-old black female donor with bluntly rounded papillae with independent bases. Most papillae are bluntly rounded; however, the papilla in the upper right corner is conical.

Fig. 4
Preputial retraction and subsequent lysis of adhesions reveals occult clitoral pearls. Preputial retraction is resisted by adhesion between the glans clitoris and prepuce, thus indicating the distal-most aspect of the adherent tissues which remain upon the glans after lysis and mark the clitoropreputial delamination margin (black arrowheads). (A) Left and right lateral views of retraction and delamination of the prepuce from the glans in a 76-year-old unembalmed white female donor body revealing underlying clitoral pearls. (B) Right lateral view of retraction and delamination of the prepuce from the glans in a 72-year-old unembalmed black female donor body revealing underlying clitoral pearls.
Fig. 5
Clitoral pearls located proximal to clitoropreputial adhesions (CPAs) (black arrowheads). (A) Left-sided dorsolateral view of clitoral pearls located along the corona of a 92-year-old embalmed white female donor. (B) Clitoral pearls in a 75-year-old unembalmed white female donor are shown alongside a crenulated CPA remnant that mirrors the contour of the pearls. (C) Clitoral pearls are seen along the corona of the clitoris and proximal to a wavy-contoured remnant of a CPA in a 76-year-old embalmed white female donor. (D) Clitoral pearls are clustered proximal to a far-distally-located CPA remnant in a 73-year-old embalmed white female donor.
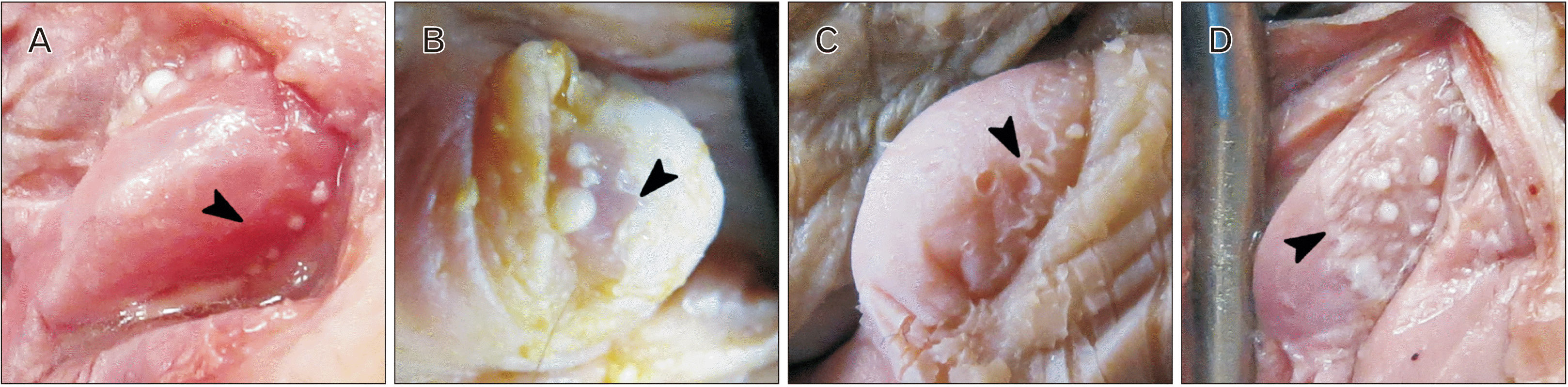
Fig. 6
Macrophotography and stereomicroscopy (images with orange hue) detailing clitoral pearls, and clitoral craters (white arrowheads), proximal to clitoropreputial adhesions (CPAs) (black arrowheads) in three example clitorises. Each row of images represents a series of increasing magnification from left to right. Superimposed black boxes correspond to adjacent magnified views. (A) A clitoral crater, formed by a clitoral pearl, is seen at the distal margin of the corona (stars) and proximal to a CPA in the clitoris of a 92-year-old white female donor body. A fringe of remnant preputial tissues remains adherent to the glans (yellow arrowheads). (B) Small clitoral pearls located proximal to a CPA are seen leaving clitoral craters post-removal in the clitoris of a 71-year-old white female donor body. Scale bars are found in stereomicroscopic images to illustrate the relatively small size of the clitoral pearls and the remnant craters. (C) Clitoral craters and remnants of the tissues covering the cavities which occupied clitoral pearls (blue arrowheads) are seen interspersed with numerous, relatively smaller, clitoral pearls in the clitoris of a 76-year-old white female donor body. Preputial craters (green arrowheads) are also seen on the face of the prepuce immediately opposing the clitoral crater.





 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download