Introduction
The coracobrachialis muscle (CBM) is one muscle of the anterior group of arm. Classically, it originates from coracoid process in common with the short head of biceps brachii muscle and inserts into the middle of anteromedial surface and medial border of humerus. Mostly, it receives its nerve supply from the musculocutaneous nerve (MCN). The MCN is the continuation of the lateral cord (LC) of brachial plexus. It supplies CBM before piercing then it descends between the biceps brachii and brachialis muscles, innervating both of them, to continue in the forearm as lateral cutaneous nerve that gives the sensory supplies of the lateral aspect of the forearm down to the wrist joint [
1].
Different variants in the origin, insertion and nerve supply of CBM have been reported in literatures [
2-
5]. Also, relation of MCN to CBM is mentioned in few studies, where MCN is commonly piercing CBM, but in few cases it passes close to CBM without piercing [
6,
7]. Moreover, the association of MCN and median nerve (MN) with CBM has been studied [
8]. In addition, separate cases of additional band, heads, bellies, or accessory slips of CBM, inserting into the medial supracondylar ridge or medial epicondyle of humerus are reported [
7]. Three main variable morphological types of CBM are identified according to its proximal attachment: type I (single muscle belly), type II (two-headed CBM), and three-headed CBM in type III [
5,
6,
9].
CBM has great clinical significance, where it can be used in the blockade of the brachial plexus as a mark for the axillary artery [
3,
4,
10], or as a graft in the reconstruction after mastectomy [
2], in some axillary malformations and transplantation for treatment of the neglected facial palsy [
3,
4].
This study was created to identify the different morphological types of CBM according to the number of heads and the anatomical variations of its distal attachment. In addition, the relation of CBM with MCN and the different innervation patterns of CBM were reported as well. Also, the original level and length of the different muscular branches to CBM originating from MCN were morphometrically measured in all cadaveric specimens as well to provide an accurate plan for surgical or endoscopic interventions in the arm.
Results
Four main morphological types of CBM were identified according to the number of heads (
Fig. 1). The commonest type was the two-headed CBM (type II) which was recorded in 63.0% of cases. This type revealed no significant difference (
P>0.5) between right (62.0%) and left (64.0%) limbs as well as between male (63.3%) and female (62.5%) cadaveric specimens, while the single belly CBM (type I) was observed in 22.0% of all, 24.0% of right and 20.0% of left limbs. However, the three-headed CBM (type III) was identified in 12.0% of all, 10.0% of right and 14.0% of left limbs. Meanwhile, the four-headed CBM (type IV) was seen in 3.0% of all, two (4.0%) of right and one (2.0%) of left limbs. Generally, no sex or side significant difference (
P>0.5) was reported in the distribution of different CBM morphological types (
Table 1).
In type I, the single head of CBM originated from the tip of coracoid process, in common with the short head of biceps, and inserted into the middle of anteromedial surface and medial border of humerus. In this type, MCN did no pierce CBM and passed superficial to it (
Fig. 1A). However, in type II, CBM exhibited superficial and deep heads (two-headed form). According to the origin of these heads, this type could be differentiated into three subtypes, where the superficial head might originate from the coracoid process or from the medial side of short head of biceps. While the origin of its deep head was the coracoid process either directly or through a common tendon with the short head of biceps or from the deep aspect of short head of biceps. In all variants of this type, MCN passed between its two heads (
Fig. 1B). In type III, CBM had two superficial and one deep heads (three-headed form). The origin of superficial heads was the short head of biceps or the coracoid process, while its deep head originated only from the coracoid process. MCN of this type passed between the superficial and deep heads (
Fig. 1C). In few cases of type III, CBM showed one superficial head originating from the short head of biceps and two deep heads originating from the coracoid process. Moreover, in type IV, where CBM having four-heads (four-headed type), one superficial and three deep. The coracoid process of this limb is bifid into lateral and medial parts. Its lateral part gives a common tendon that divided later into superficial lateral slip which continued as short head of biceps and deep medial slip that gave the origin of 3rd, and 4th heads of CBM in addition to a deep sling to the short head of biceps, while the medial part of coracoid process gives the origin of 1st superficial head of CBM only. The first three heads of CBM merged with each other distal to MCN and inserted onto the middle of the medial surface of humeral shaft, while its 4th head deviated laterally and fused with the deep surface of the common tendon of biceps muscle.
The origin of two of them was the coracoid process, while the origin of the other two heads was the short head of biceps (
Fig. 1D).
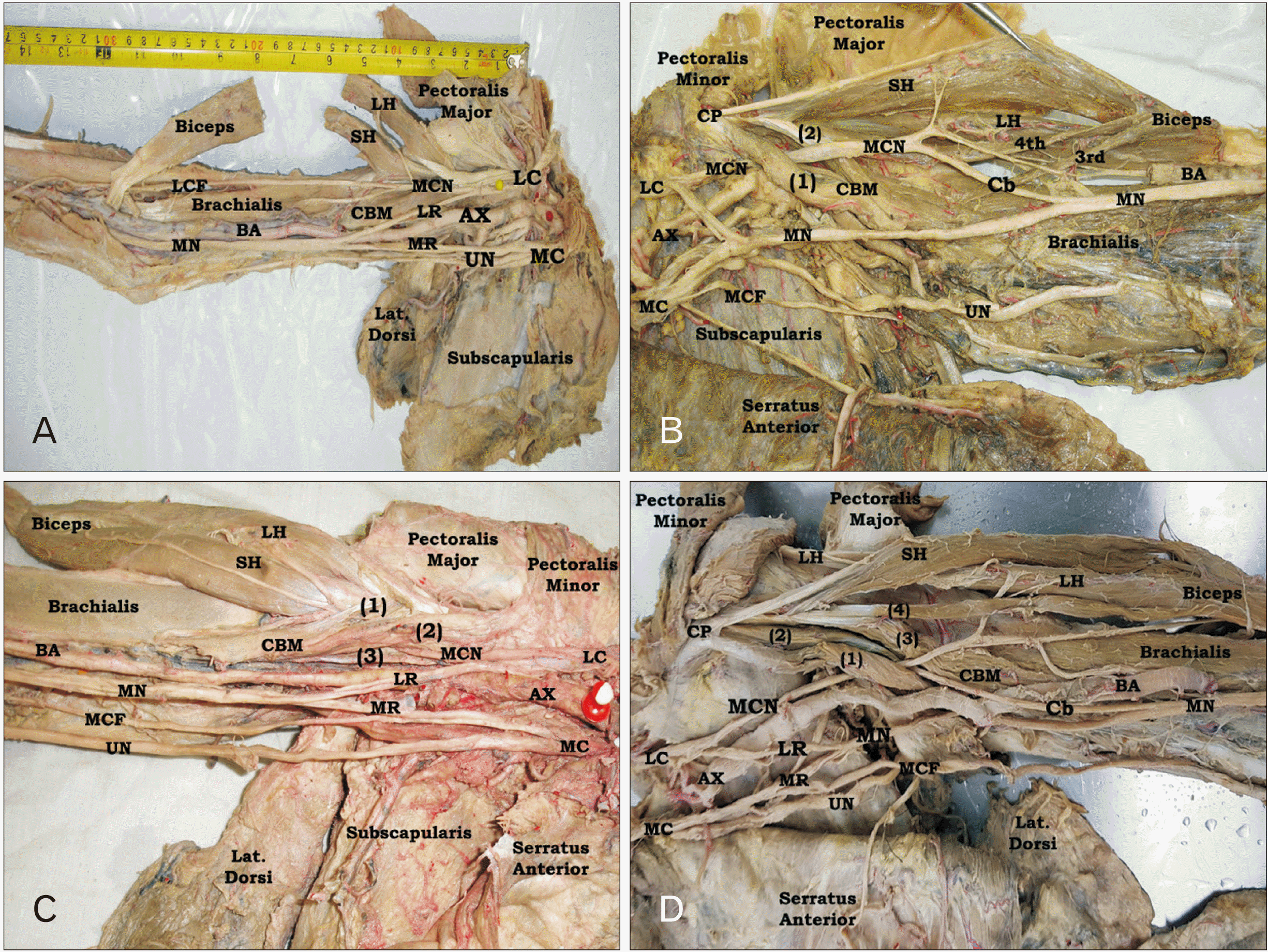
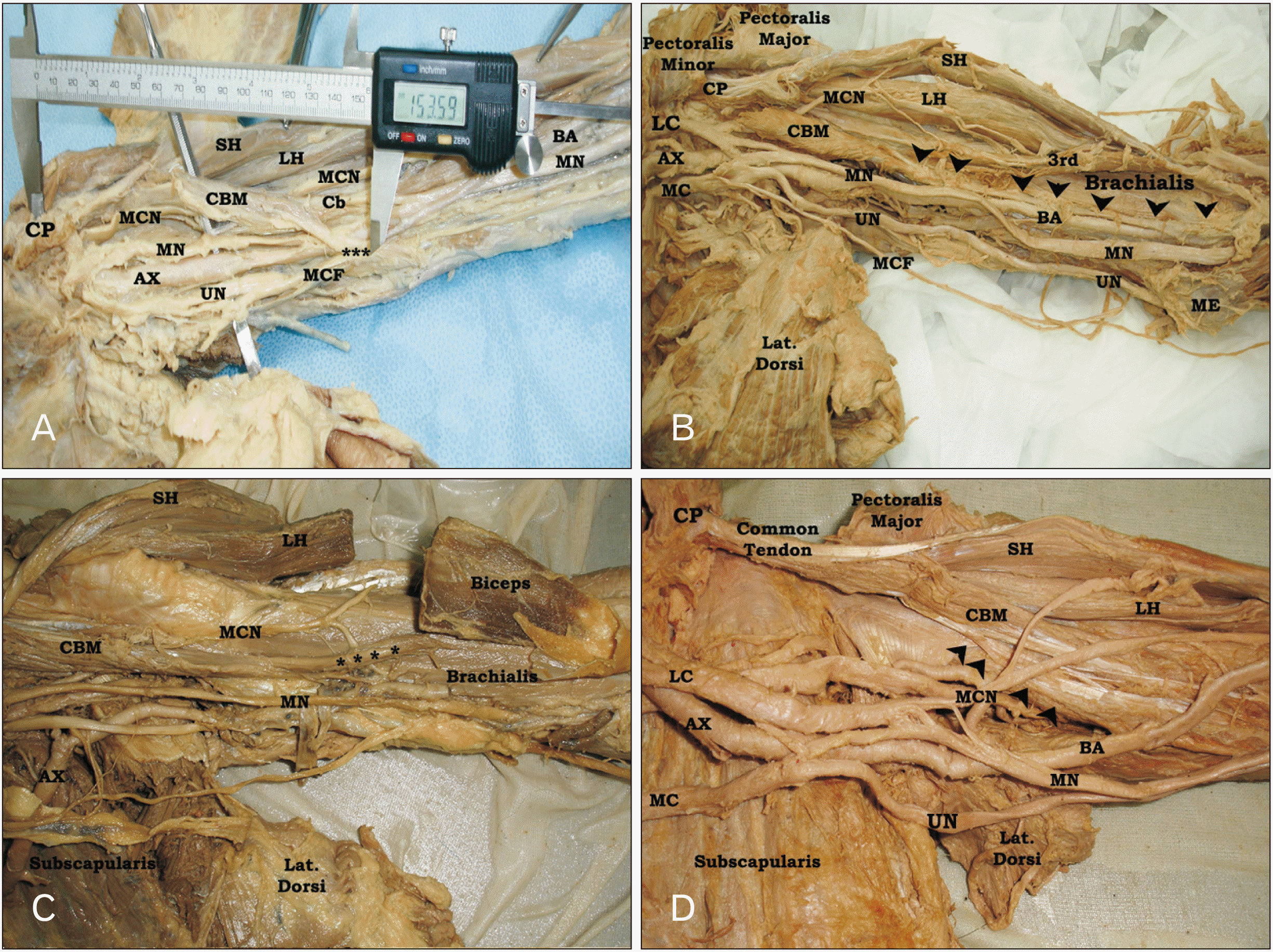
Variant insertion of CBM was observed in four left limbs (
Fig. 2), two male and two female. In case (1), CBM had one superficial and one deep heads originating from short head of biceps and coracoid process respectively and inserted at the middle of anteromedial surface of humerus. However, at 112.4 mm distal to the coracoid process, a muscular slip originating from the medial aspect of the insertion extended downward and medially crossing the MN, brachial artery (BA) and its vena commitant, ulnar nerve (UN), and medial cutaneous nerve of forearm (MCF) superficially to end at the medial border of the humerus at a distance 153.6 mm from the coracoid process and 131.5 mm from the medial humeral epicondyle. The length of this muscular band measured 50 mm and its breadth was 10 mm. After piercing CBM, MCN divided into medial and lateral divisions. The lateral one passed between biceps and brachialis muscles to continue as lateral cutaneous nerve of forearm (LCF) as usual, while its medial division passed along the lateral border of the CBM insertion downward to unite with the MN at a distance 18 cm from the acromion. At a distance of 116.6 mm from the mid-interepicondylar line, MN of this limb crossed the BA deeply from the lateral to medial aspect down to the cubital fossa (
Fig. 2A). Also, in second variant case; CBM exhibited one superficial and one deep heads with passage of MCN between them and classic insertion at the middle of medial border of the humerus. But a variant muscular sling extended from the insertion downward to the medial epicondyle of humerus where it attached. This sling crossed the BA just above the medial humeral epicondyle superficially. This form of the muscle was called CBM longus. The biceps of this limb had four heads (
Fig. 2B). In third limb, CBM of this limb was normally inserted into the middle of anteromedial surface of humerus. But, a variant muscular prolongation, extending from the lower lateral aspect of the classic insertion of the muscle downward and laterally superficially to brachialis muscle and MCN to attach into the deep surface of the common tendon of biceps muscle, was observed (
Fig. 2C). However, CBM of the 4th limb had single head originating from the coracoid process in common with the short head of biceps, while the distal attachment of this muscle divided into large lateral muscular part extending downward to the middle of the anteromedial surface of humerus where it attached and a small medial short sling attached to upper third of the medial border of humerus just below the lower border of latissimus dorsi muscle. The MCN of this limb passed superficial to CBM without piercing (
Fig. 2D).
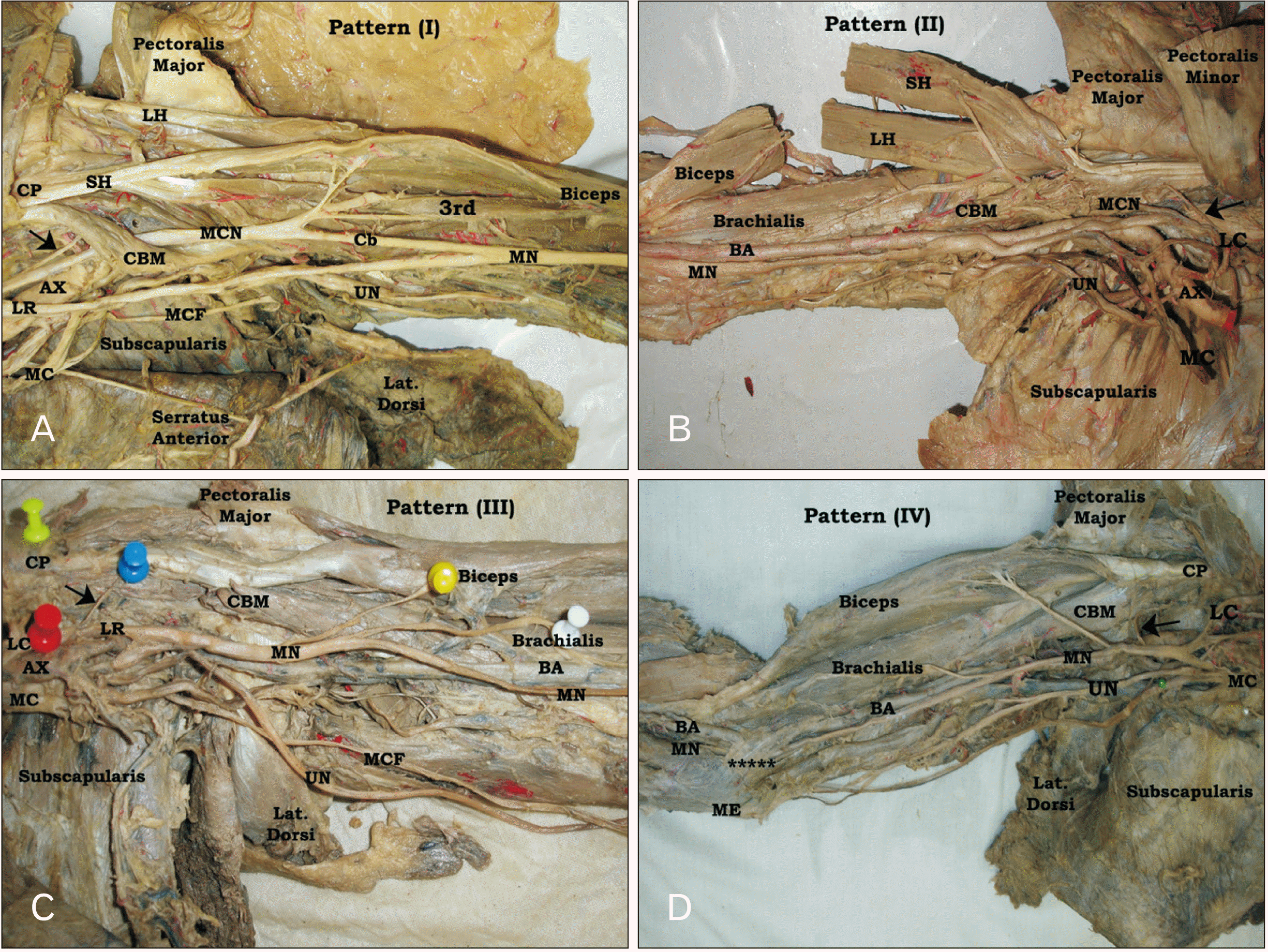
Four different innervation patterns of CBM were determined (
Fig. 3). In type I, the CBM was supplied by MCN, where this pattern was reported in 80.0% of all, 76.0% of right and 84.0% of left limbs whether piercing or not piercing CBM (
Fig. 3A). The incidence of this pattern showed left side predominance without sex significant difference. However, in pattern (II) CBM was supplied by the LC of brachial plexus (
Fig. 3B). This type was seen in 14.0% of all, 18.0% of right and 10.0% of left limbs. Moreover, in pattern (III), the CBM was supplied by the lateral root (LR) of MN where MCN was absent in the limbs of this pattern (
Fig. 3C). This pattern was identified in 4.0% of the limbs with no side difference (two right and two left), but it was seen in three male (two right and one left) and one left female limbs. Meanwhile, in type IV CBM was supplied by MN itself with absence of MCN also (
Fig. 3D). This pattern was determined in 2.0% of limbs (one left male and one right female). Overall, no sex significant difference (
P>0.05) was reported regarding the distribution and incidence of the different innervation patterns of CBM (
Table 2).
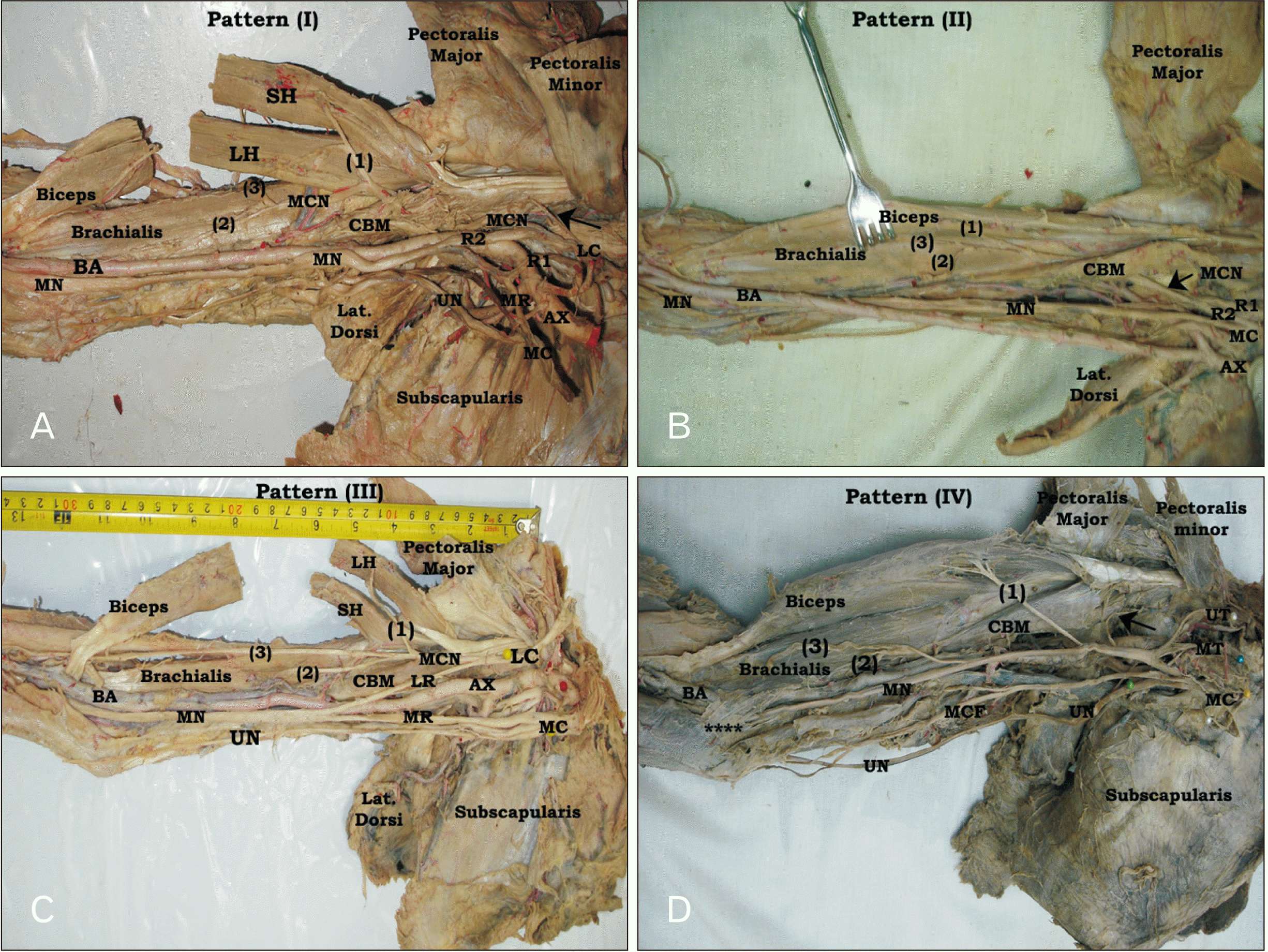
The MCN was present in 90.0% of all, 86.0% of right, and 94.0% of left limbs with no sex difference (90.0% of each). MCN showed normal formation as the continuation of the LC of brachial plexus in all limbs (
Fig. 4A), except in one right female upper limb only, where MCN was abnormally formed from two roots originating from the lateral and medial cords of brachial plexus respectively but with normal distribution within the arm, where it supplied CBM before its piercing then it passed between the biceps brachii and brachialis muscles and continued as LCF. In addition, the MN of this limb exhibited one root only originating from the medial cord, then it passed on the lateral side of axillary artery to cross deep to the BA from lateral to medial and descent downward to the cubital fossa (
Fig. 4B).
The course of MCN revealed two different patterns in relation to CBM. In the first and common pattern, MCN pierced CBM or passed between its superficial and deep heads (
Fig. 4A). This pattern was seen in 71.0% of all, 70.0% of right and 72.0% of left limbs with no sex significant difference (
P>0.05). However, in the second pattern, MCN passed medial (superficial) to CBM without piercing and descent between the brachialis and biceps muscles (
Fig. 4C). This form was determined in 19.0% of all, 16.0% of right, and 22.0% of left limbs with no sex significant difference, where it seen in 20.0% of male limbs and 17.5% of female limbs. Meanwhile, MCN was absent (
Fig. 4D) in 10.0% of all, 14.0% of right- and 6.0% of left limbs with no sex significant difference (10.0% each). In these limbs, CBM was supplied by a branch from LC, or lateral root of MN or from MN itself. In all limb having MCN, both biceps and brachialis muscles received muscular branches from it. Generally, no sex significant difference was determined regarding the formation, distribution, connection and branching pattern of MCN, but there was left-side predominance in MCN presence and CBM supplying. However, connection and fusion of MCN with MN revealed right-side predominance (
Table 3).
The morphometric measurements were done using the tip of the coracoid process and medial epicondyle of humeral as reference points for the arm length. The mean length of the right arm measured 290.7±19.1 mm and the length of left arm was 288.1±16.4 mm with no significant difference (
P>0.05), but its mean length was 302.1±12.02 mm in male limbs and 275.5±11.1 mm in female limbs with extreme significant difference (
P<0.001). Moreover, the distance between the coracoid process and the original level of the different muscular branches of CBM originating from MCN was measured, where the first muscular branch of CBM originated from the MCN at a mean distance of 43.9±9.7 mm from the coracoid process in right limbs and 34.3±11.3 mm in left limbs with no sex significant difference (
P>0.05), where it originated at a mean distance of 44.2±8.8 mm in male and 42.1±7.9 mm in female. However, the second muscular branch of CBM originated at a mean distance of 46.0±6.8 mm in right limbs, 42.0±7.6 mm in left limbs, 43.8±6.6 mm in male limbs, and 43.7±8.9 mm in female limbs with no side or sex significant difference (
P>0.05). Moreover, the mean of the first muscular branch length of CBM measured 36.1±13.3 mm in male, 32.9±8.7 mm in female, 35.0±11.6 mm in right arms, and 34.3±11.3 mm in left arms with no side or sex significant difference. But the mean of the second muscular branch length of CBM measured 31.5±10.8 mm in male, 27.5±11.0 mm in female, 28.1±10.3 mm in right, and 30.6±12.1 mm in left limbs (
Table 4).
Discussion
A good understanding of the morphological variations, innervation patterns and anatomical relations provide an accurate radiological interpretation and proper planning of the different surgical or endoscopic interventions in the arm and shoulder regions [
11], where possible compression of the nerves or blood vessels could be occurred in some variations [
7]. Based on the morphological classification of Piagkou et al. [
9], the results of the current study revealed four morphological types of CBM. The two-headed CBM (type II) was the commonest (63.0%), followed by the single belly (type I) CBM (22.0%), then three-headed (type III) CBM (12.0%) and four-headed CBM (3.0%). Such morphological types mostly showed no side or sex significant difference. Similarly, Piagkou et al. [
9] identified four morphological types of CBM; two-headed in 63.0%, three-headed in 22.2%, single belly in 11.1%, and four-headed in 3.7%. Similar to the results of this study, Olewnik et al. [
7] observed a unilateral four-headed CBM with a split coracoid process. Also, a unilateral six-headed CBM [
12] and a bilateral asymmetrical multiplication of CBM heads [
13] were reported. Moreover, unilateral variants of CBM were identified by Georgiev et al. [
3,
4] and Zielinska et al. [
11]. In most cases, the addition heads or accessory muscles could be correlated with some kind of neurovascular compression, between the heads of CBM [
7,
11].
However, Ilayperuma et al. [
10] identified two main forms of CBM; two-headed CBM in 83.3% with passage of MCN between them and one-headed CBM with medial course of MCN without piercing in 16.7%. Contrariwise, CBM were categorized into three main morphological types, with some subtypes: single muscle belly (type I) in 49.5%, two-headed CBM (type II) in 42.6% with two subtypes, and three-headed CBM (type III) in 7.9%. The two heads of two-headed CBM originated from the coracoid process and short head of biceps in the first subtype (type II A), while coracoid process provided the origin to the both heads in the second subtype (type II B). However, two heads originated from the coracoid process and the third one originated from the short head of biceps in type III (three-headed) CBM. The authors added that, MCN pierced CBM in most of the CBM exhibiting two-heads or more [
5]. It is worth noting that the three-headed CBM was not previously described in other studies [
6,
10]. In addition, almost all cases were of the two-headed CBM. The origin of the superficial head was the medial border of the short head of biceps, while the origin of the deep head was the coracoid process and the lateral border of short head of biceps [
6].
Moreover, relative to the tendon of the short head of biceps, three different sites of the proximal attachment of the single belly CBM were described, one from the lateral side of the tendon, one from the medial aspect of the tendon, and the third one from the deep surface of the tendon [
10]. Recently, a new classified was done based on the relationship between CBM and short head of biceps [
11]. Through this study three main different types of relationship were described. In type 1 (54.0%), both CBM and short head of biceps attached the coracoid process by a common tendon. Type II was divided into two main subtypes. An independent attachment of CBM and short head of biceps to the coracoid process was described in subtype II a (10.0%), while in subtype II b (5.0%) the two heads of CBM attached to the coracoid process. Meanwhile, in type III (31.0%) the two heads of CBM originated from a bifurcated coracoid process with the short head of biceps in-between.
Similar to the results of this study, CBM was mostly inserting into the middle of the anteromedial surface and medial border of humerus [
6,
10]. However, two main patterns of CBM insertion were reported: one similar to the previous pattern and was reported in 60.4% of limbs, while the other type was into the distal third of humeral shaft and was observed in 39.6% limbs [
5]. In other study, three forms of CBM insertion were reported: one into the medial humeral epicondyle which was called CBM superficialis or longus, the other form inserting into the shaft of humerus and was called CBM medius, and the third type inserting into the neck of humerus and was called CBM brevis or profundus [
3,
4]. Based on the later classification of CBM insertion, 96.0% of CBM in this study was of the CBM medius type, while CBM longus and CBM brevis were seen in one limb each (1.0%). Moreover, a variant insertion of CBM was observed in another two left limbs (2.0%); one of them showed an abnormal fibromuscular sling extending from the medial side of the common insertion of CBM downward and medially crossing MN, UN, MCF, and BA superficially to attach into the medial border of humerus, while the insertion of CBM in the other limb extended downward and laterally to attach in the deep surface of the common tendon of biceps brachii muscle. Similar to the results of this study, rare case of CBM longus was found [
2-
4,
7], where CBM longus was observed in one left limb only. Also, a tendinous insertion and aponeurotic expansion of CBM was reported by El-Naggar and Al-Saggaf [
2]. Such previous cases of a tendinous insertion and aponeurotic expansion of CBM insertion were observed in two left limbs of this study. However, Zielinska et al. [
11] observed CBM longus in 11.0% of all cases. The later authors were classified these cases into two main subtype according to its distal attachment based on the description stated by Georgiev et al. [
3] and Olewnik et al. [
7] respectively.
Regarding the incidence of the commonest variant of CBM morphological types, a remarkable difference was recorded between the present study (two-headed CBM) and that was done by Szewczyk et al. [
5] (single belly CBM). Such difference might be related to the examined populations, where the cadavers belonged to a Central European population in Szewczyk et al. [
5] study, cadavers of Greek population in Piagkou et al. [
9] study, cadavers of Sri Lankan individuals in Ilayperuma et al. [
10] study, cadavers of Colombian origin in Larrotta et al. [
14] study, and the cadavers of the current study were of Arabian origin. Thus, the incidence of the difference of the anatomical topography might be related to the examined populations.
The anatomical variations of CBM attachments could be explained based on the developmental background, where the primordia of the arm muscles were formed from the migration of the mesenchymal cells of the myotome into the limb buds during the 4th to 6th week of the embryo. Thereafter, the muscle primordia fuse to form a single pre-muscular mass that later can be identified as separate muscles in 14–16 mm embryo length. The proximal end of the common mass differentiates earlier than of the distal end in an embryo of 11–19 mm in length [
15]. Not all muscle primordia fuse, but normally disappear through cell death. Failure or premature termination of this process results in accessory structures such as coracocapsularis, CBM brevis, or CBM longus muscle [
3,
4]. So, the morphological variations of CBM might be related to the premature termination of the regression process of the muscle primordia [
7]. Moreover, formation of the muscles is completed before nerves’ formation. So, occurrence of an abnormal muscle differentiation may lead to atypical innervation. Thus, the variable patterns of CBM innervation could be explained through this mechanism [
14].
In this study, the piercing of MCN to CBM was observed in 71.0% of limbs, while in 19.0% of limbs, no piercing of MCN to CBM was seen, where MCN revealed a medial course on the single-headed CBM. However, perforation of MCN to CBM was observed in 88.9%, while the medial (superficial) course of MCN to CBM was recorded in 11.1% [
9]. Moreover, piercing MCN to CBM was observed in 83.4% and its passage without piercing CBM was determined in 16.6% [
10]. Also, no piercing MCN to CBM was observed in 11.1% of limbs [
16]. However, MCN of medial course was identified in 22.0% in ultrasound study [
17]. Similar to the results of the current study, in 100% of the two-headed (or more) CBM, MCN passed between the heads of CBM, while MCN showed superficial (medial) course to CBM in 100% of the single-headed CBM [
9]. However, MCN pierced CBM instead of passing between the heads in one case of two-headed CBM [
5]. So, such anatomical variation of CBM could be considered during the diagnostic and surgical interventions in the arm to avoid the iatrogenic injuries [
7,
18].
The previous thought supposed unimportant function of CBM. However recently, CBM seem to be of great clinical significance, where effective flexion of the shoulder joint and stabilization with minimal anterior dislocation by CBM were proved clinically [
19]. Moreover, CBM could be used as a guide for axillary artery during brachial plexus block in surgery and anaesthesia. Also, the insertion of CBM could be used as a mark for the site of the humeral nutrient artery [
7,
10]. In addition, CBM could be used for grafting in treatment of the long-standing facial palsy, reconstruction in post-mastectomy and in both axillary and infraclavicular deformities due to its higher vascularity [
2]. In contrary, presence of supernumerary heads of CBM in association with atypical course of MCN could be leads to an abnormal entrapment syndrome or vascular compression [
5,
7]. Also, anatomical variants of CBM could cause confusion during imaging evaluation and surgery, therefore meticulous clinical examination and preoperative imaging must be done before performing surgical or endoscopic procedures in the arm to avoid the iatrogenic injury of MCN.
In conclusion, CBM revealed high morphological variations, where four types of proximal attachment, four types of distal attachment, two forms of MCN relationships, and four different innervation patterns of CBM were identified. These variations had vital significance in both basic and clinical practice, where they can influence surgical approaches, interpretation of imaging studies, and rehabilitation strategies. So, better understanding of the morphological variations of CBM is essential for physicians and surgeons especially in planning and executing physiotherapy, radiological, orthopedic and endoscopic interventions in the arm to obtain an accurate diagnosis, effective treatment and better outcome.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download