Abstract
Notes
References
Table 1
| Source | Cell | GF | Endpoint | Reference (yr) |
|---|---|---|---|---|
| Goat | SSCs with Sertoli | FBS, KSR, GDNF, EGF, bFGF, GFRα1 |
1. Melatonin regulated the proliferation of dairy goat SSCs through GDNF. 2. Melatonin promoted proliferation and inhibit differentiation of goat SSCs. |
Niu et al. (2016) [15] |
| Sheep | Testicular cells (SSCs c, Sertoli cells, peritubular myoid cells and Leydig cells) | FBS, SCF, bFGF, EGF, GDNF | Melatonin, promotes production of cAMP level. So, increases testosterone production and the level of meiotic anaphase marker genes Dnmt3a and Bcl2. | Deng et al. (2016) [16] |
| Mice | SSCs | FBS, GDNF, LIF | Melatonin as an antioxidant increases viability of SSCs by reducing oxygen free radicals and improves culture. | Navid et al. (2017) [13] |
| Mice | Testes | 10% KSR |
1. Melatonin supplement along with Glutamax has a positive effect on the maturation of male germ cells. 2. The concentration of at least 10% KSR plays a decisive role in the maturation of germ cells and testosterone production. |
Reda et al. (2017) [17] |
| Mice | Testes | FBS | Melatonin maintains demethylation in these cells by maintaining H3K9me1 and preserve the normal process of spermatogenesis and meiosis. | Zhang et al. (2018) [18] |
| Sheep | Leydig and Sertoli cells | None |
1. Melatonin reduces the expression of SCF and insulin-like growth factor-1 and estrogen synthesis in Sertoli cells. 2. The increase in the expression of SCF is through the retinoic acid receptor-RORα, which is dependent on the MEK/extracellular signal-regulated kinase pathway. 3. Melatonin increases the expression of testosterone in Leydig cells. |
Deng et al. (2018) [19] |
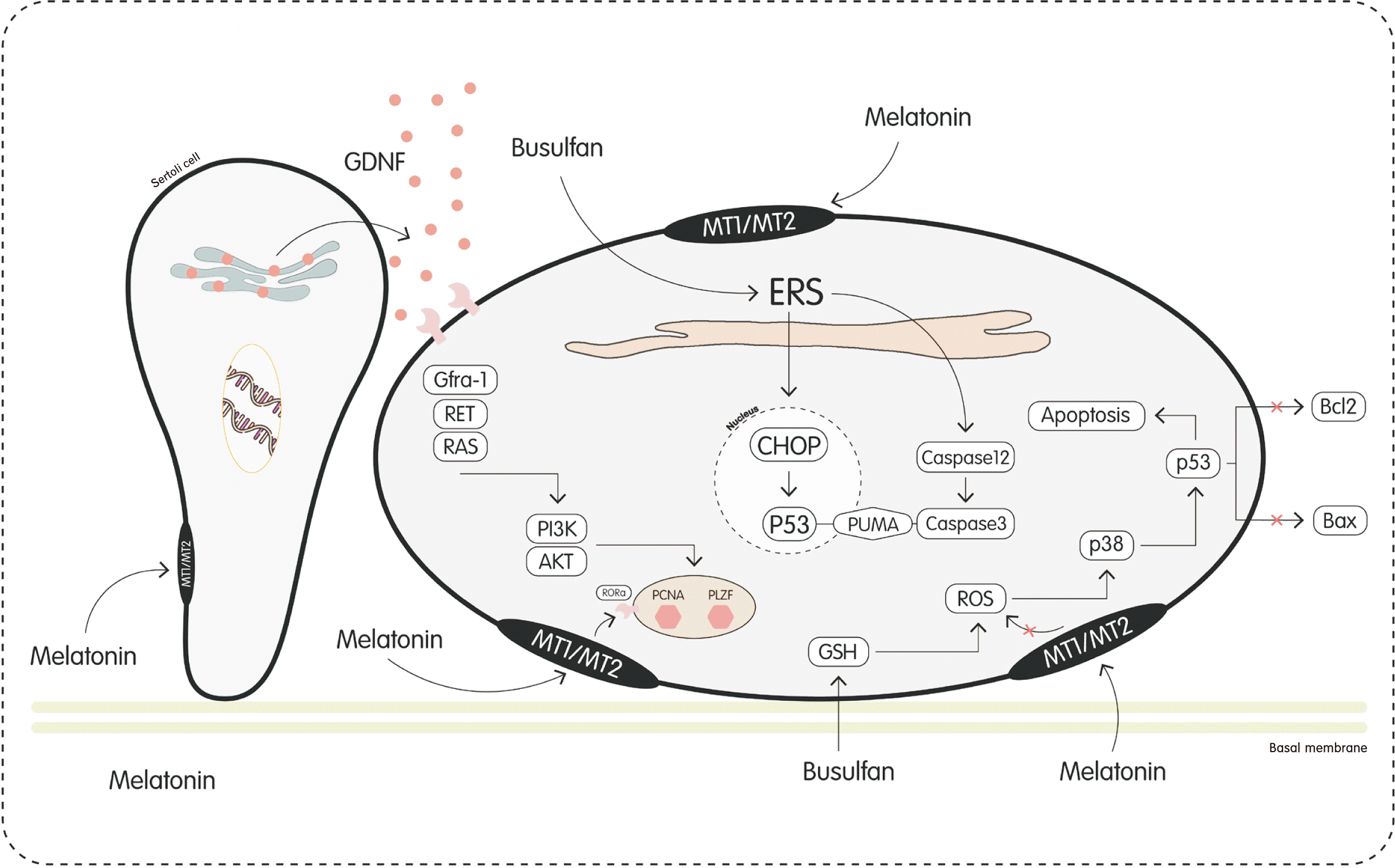
| Mice | SSCs | None | Melatonin, by activating SIRT1, reduces oxidative stress, ERS, mitochondrial dysfunction, and DNA damage in SSCs. | Xu et al. (2020) [20] |
| Mice | SSCs | FBS | Melatonin, in addition to its role as an antioxidant and destroying ROS, inhibits the function of autophagy by the expression of METTL3-mediated RNA m6A proteins. | Lv et al. (2021) [21] |
| Sheep | SSCs | FBS | Melatonin has anti-apoptotic properties in SSCs. | Alinezhad et al. (2023) [23] |
GF, growth factor; SSC, spermatogonial stem cell; FBS, fetal bovine serum; KSR, knockout serum replacement; GDNF, glial cell line-derived neurotrophic factor; EGF, epidermal growth factor; bFGF, basic fibroblast growth factor; GFRα1, glial cell line-derived neurotrophic factor family receptor α 1; SCF, stem cell factor; LIF, leukemia inhibitory factor; H3K9me1, histone 3 lysine 9 trimethylation; RORα, related orphan receptor-α; SIRT1, sirtuin type 1; ERS, endoplasmic reticulum stress; ROS, reactive oxygen species.
Table 2
| Source | Cells | GF | Feeders/substrate | Endpoint | Reference (yr) |
|---|---|---|---|---|---|
| Mice | SSCs | FBS, GDNF, LIF | 3D SACS | Melatonin as an antioxidant increases viability of SSCs by reducing oxygen free radicals and improves culture of SSCs. | Navid et al. (2017) [13] |
Table 3
| Source | Cell in freezing medium | GF culture | Endpoint | Reference (yr) |
|---|---|---|---|---|
| Mice | Testes | None | Melatonin as an antioxidant increases viability of SSCs by reducing oxygen free radicals and induces apoptosis in damaged cells. | Gholami et al. (2014) [24] |
| Goat | SSCs | FBS, GDNF |
1. Melatonin has an anti-apoptotic function. By regulating Bax-Bcl2 gene expression and caspase-3. 2. Melatonin has an antioxidant function by stimulating the secretion of SOD, CAT, and GSH-Px enzymes. 3. Melatonin has an anti-autophagy function by regulating the secretion of LC3-I, LC3-II, P62, Beclin1, and ATG7 proteins. |
Feng et al. (2020) [27] |
| Mice | SSCs | FBS, GDNF, bFGF, LIF | Melatonin as an antioxidant improve viability of SSCs by reducing oxygen free radicals and improves transplanting SSCs into adult male into mature azoospermic mice. | Kazemzadeh et al. (2022) [28] |
| Mice | SSCs | FBS, GDNF, bFGF, LIF | Melatonin as an antioxidant and anti-apoptotic can be useful in the long-term preservation of SSCs in cryogenic environment. | Nazeri et al. (2022) [29] |
Table 4
| Source | Cell | GF | Endpoint | Reference (yr) |
|---|---|---|---|---|
| Mice | Testes | - | Melatonin improve the degenerative and apoptotic changes in the basement membrane and epithelial cells of the spermatogenic tubules in testes of mice. | Hemadi et al. (2013) [30] |
| Rat | Testes | - | Melatonin decrease in the average number of caspase-3 positive cells in the seminiferous tubules is a sign of the increase of germ cells. | Abd El Aziz and Metwally (2013) [31] |
| Mice | SSCs | FBS |
1. Melatonin block the secretion of proteins caspase-3, caspase-12, and CHOP. 2. Melatonin decrease the secretion of PUMA and P53 proteins. |
Cui et al. (2017) [32] |
| Goat | Testes (SSCs c, Sertoli cells, peritubular myoid cells and Leydig cells) | FBS, SCF, fibroblast growth factor, insulin-like growth factor, GDNF |
1. Melatonin promoted proliferation and inhibit differentiation of goat SSCs. 2. Melatonin up-regulates the expression of steroidogenesis-related genes via the nuclear receptor RORα. |
Deng et al. (2017) [33] |
| Mice | SSCs | FBS | Melatonin increases MnSOD and SIRT1 expression, which decrease cell ROS and p53, respectively. | Li et al. (2018) [34] |
| Mice | SSCs, Leydig cells | FBS, EGF, bFGF, LIF, GDNF |
1. Melatonin inhibited the induction of apoptosis on Leydig cells. 2. Leydig cells secretes CSF-1 and induces the self-renewal process of SSCs. |
Du et al. (2018) [35] |
| Mice | Testes | - | Melatonin destroys the ROS due to the production of Sod, Gpx, and Cat. So, it brings the production of P-ATM and P53 to the normal level and stops cell arrest and apoptosis in SSCs. | Zhang et al. (2019) [36] |
| Mice | Testes | None | Melatonin alleviates LPS‐induced ERS and inflammation in spermatogonial stem cells. | Yang et al. (2021) [37] |
| Mice (C57BL/6 J) | Testes | - | Melatonin protect SSCs from Cr(VI)-induced damage by scavenging ROS, by suppressing ATM-p53 phosphorylation and the MAPK pathway, as well as by restoring H3K9me3 levels in cell cycle promoter and apoptosis-related regions. | Li et al. (2022) [38] |
GF, growth factor; SSC, spermatogonial stem cell; FBS, fetal bovine serum; CHOP, C/EBP homologous protein; PUMA, p53 upregulated modulator of apoptosis; SCF, stem cell factor; RORα, related orphan receptor-α; MnSOD, manganese superoxide dismutase; SIRT1, sirtuin type 1; EGF, epidermal growth factor; bFGF, basic fibroblast growth factor; LIF, leukemia inhibitory factor; GDNF, glial cell line-derived neurotrophic factor; ROS, reactive oxygen species; LPS, lipopolysaccharide; ERS, endoplasmic reticulum stress; Cr(VI), chromium; H3K9me3, histone 3 lysine 9 trimethylation; -, not applicable.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download